Molecular and Cellular Mechanisms of Sperm-Oocyte Interactions Opinions Relative to in Vitro Fertilization (IVF)
Abstract
:1. Sperm Migration into the Human Female Oviduct
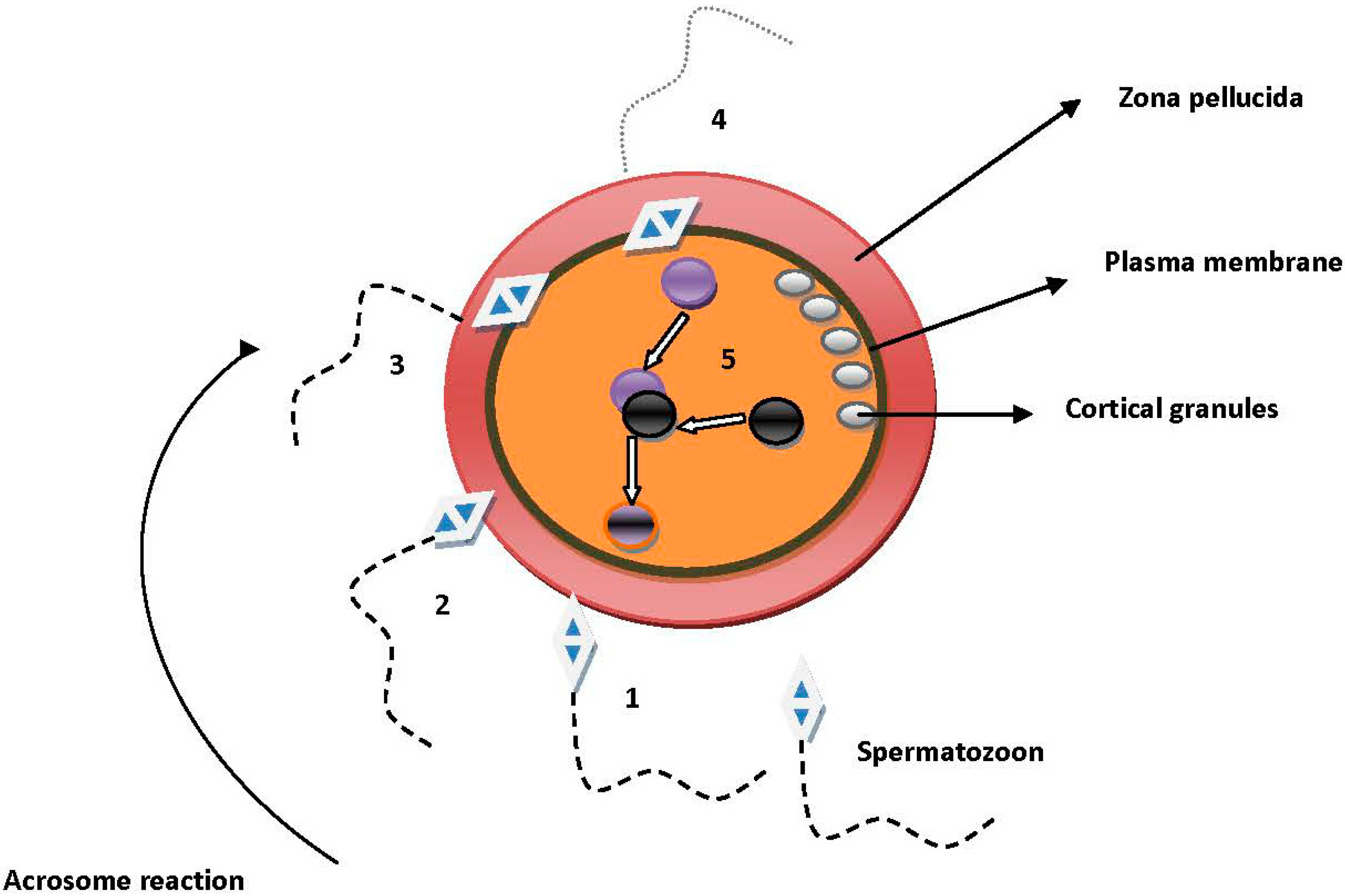
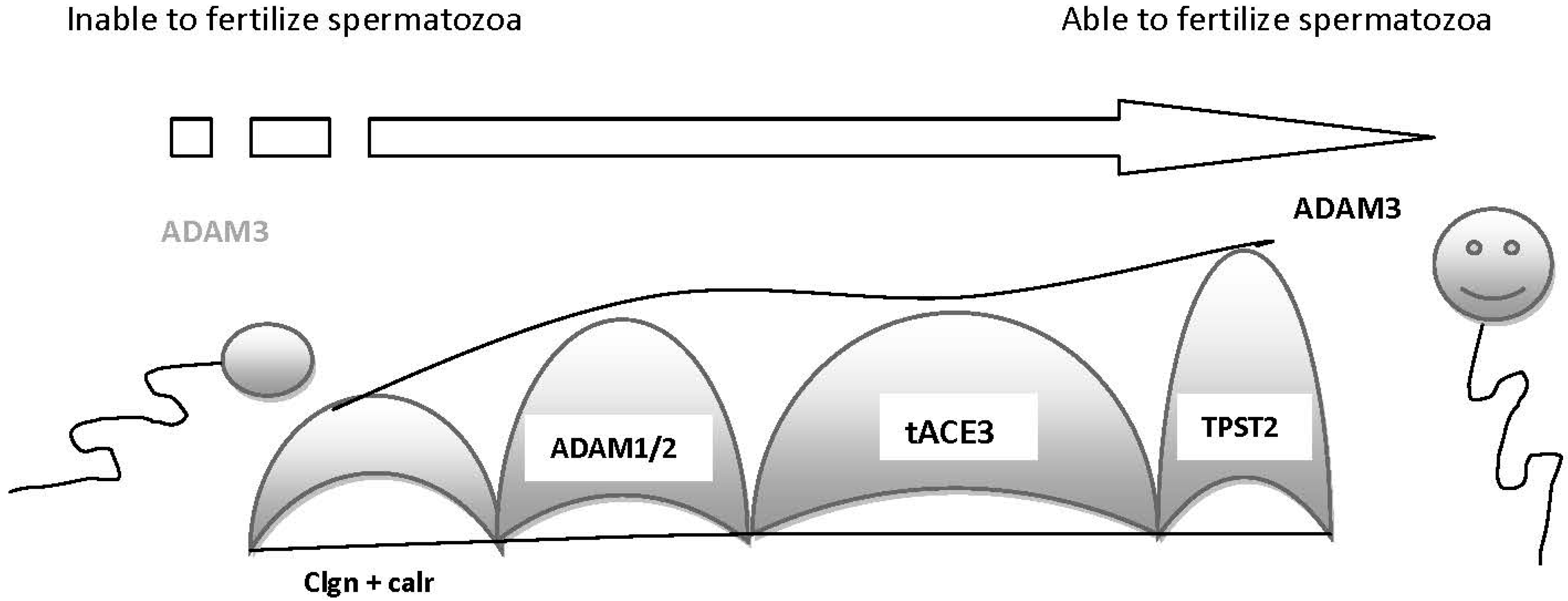
2. Molecules Involved in Sperm–Oocyte Interactions
2.1. Sperm and Cumulus Mass Interactions
2.2. Sperm Binding, Adhesion and Penetration of the Zona Pellucida (ZP)
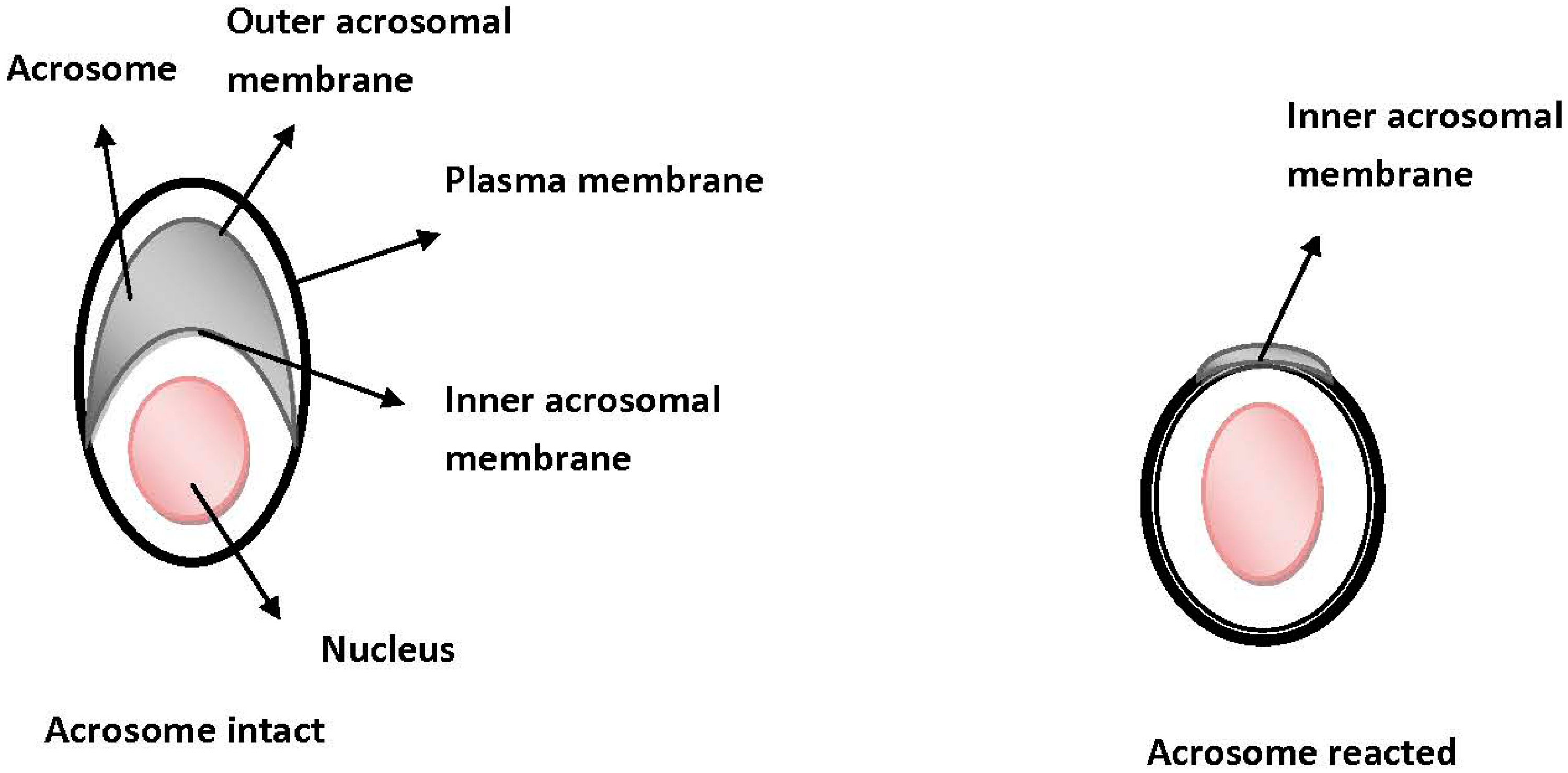
2.3. Sperm and Oocyte Plasma Membrane Interactions
3. Fertilization in the IVF Era-Conclusion Remarks
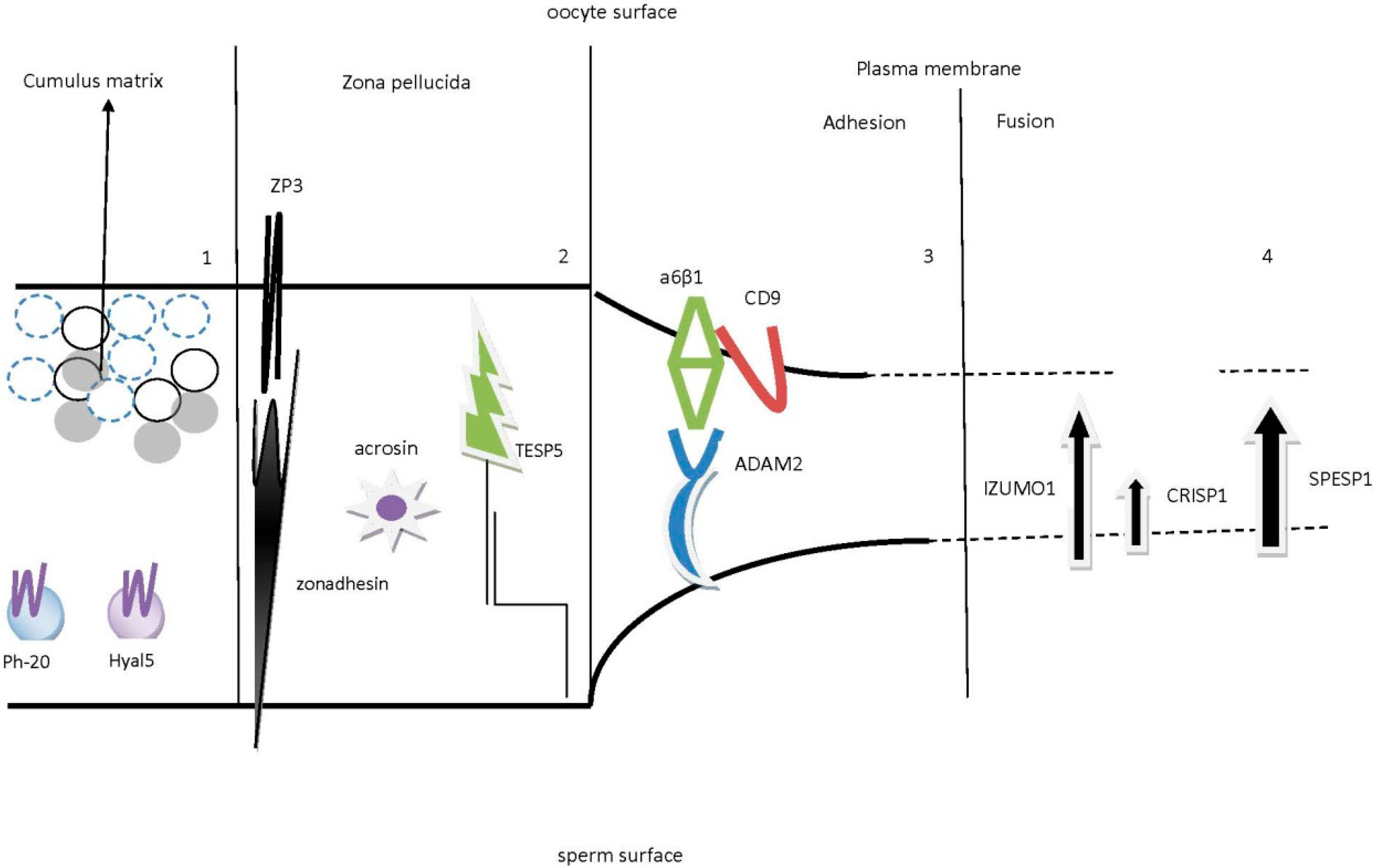
Author Contributions
Conflicts of Interest
References
- Hagaman, J.R.; Moyer, J.S.; Bachman, E.S.; Sibony, M.; Magyar, P.L.; Welch, J.E.; Smithies, O.; Krege, J.H.; O’Brien, D.A. Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. USA 1998, 95, 2552–2557. [Google Scholar] [CrossRef]
- Cho, C.; Bunch, D.O.; Faure, J.E.; Goulding, E.H.; Eddy, E.M.; Primakoff, P.; Myles, D.G. Fertilization defects in sperm from mice lacking fertilin α. Science 1998, 281, 1857–1859. [Google Scholar] [CrossRef]
- Ikawa, M.; Nakanishi, T.; Yamada, S.; Wada, I.; Kominami, K.; Tanaka, H.; Nozaki, M.; Nishimune, Y.; Okabe, M. Calmegin is required for fertilin α/β heterodimerization and sperm fertility. Dev. Biol. 2001, 240, 254–261. [Google Scholar] [CrossRef]
- Nishimura, H.; Kim, E.; Nakanishi, T.; Baba, T. Possible function of the ADAM1a/ADAM2 Fertilin complex in the appearance of ADAM3 on the sperm surface. J. Biol. Chem. 2004, 279, 34957–34962. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Muro, Y.; Isotani, A.; Tokuhiro, K.; Takumi, K.; Adham, I.; Ikawa, M.; Okabe, M. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol. Reprod. 2009, 81, 142–146. [Google Scholar] [CrossRef]
- Ikawa, M.; Wada, I.; Kominami, K.; Watanabe, D.; Toshimori, K.; Nishimune, Y.; Okabe, M. The putative chaperone calmegin is required for sperm fertility. Nature 1997, 387, 607–611. [Google Scholar] [CrossRef]
- Tollner, T.L.; Yudin, A.I.; Treece, C.A.; Overstreet, J.W.; Cheer, G.N. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum. Reprod. 2008, 23, 2523–2534. [Google Scholar] [CrossRef]
- Lefebvre, J.; Fan, J.; Chevalier, S.; Sullivan, R.; Carmona, E.; Manjunath, P. Genomic structure and tissue-specific expression of human and mouse genes encoding homologues of the major bovine seminal plasma proteins. Mol. Hum. Reprod. 2007, 13, 45–53. [Google Scholar]
- Ignotz, G.G.; Cho, M.Y.; Suarez, S.S. Annexins are candidate oviductal receptors for bovine sperm surface proteins and thus may serve to hold bovine sperm in the oviductal reservoir. Biol. Reprod. 2007, 77, 906–913. [Google Scholar] [CrossRef]
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef]
- Dobrinski, I.; Smith, T.T.; Suarez, S.S.; Ball, B.A. Membrane contact with oviductal epithelium modulates the intracellular calcium concentration of equine spermatozoa in vitro. Biol. Reprod. 1997, 56, 861–869. [Google Scholar] [CrossRef]
- Ignotz, G.G.; Lo, M.C.; Perez, C.L.; Gwathmey, T.M.; Suarez, S.S. Characterization of a fucose-binding protein from bull sperm and seminal plasma that may be responsible for formation of the oviductal sperm reservoir. Biol. Reprod. 2001, 64, 1806–1811. [Google Scholar] [CrossRef]
- Demott, R.P.; Suarez, S.S. Hyperactivated sperm progress in the mouse oviduct. Biol. Reprod. 1992, 46, 779–785. [Google Scholar] [CrossRef]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichoc, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef]
- Avenarius, M.R.; Hildebrand, M.S.; Zhang, Y.; Meyer, N.C.; Smith, L.L.; Kahrizi, K.; Najmabadi, H.; Smith, R.J. Human male infertility caused by mutations in the CATSPER1 channel protein. Am. J. Hum. Genet. 2009, 84, 505–510. [Google Scholar] [CrossRef]
- Hardy, C.M.; Clarke, H.G.; Nixon, B.; Grigg, J.A.; Hinds, L.A.; Holland, M.K. Examination of the immunocontraceptive potential of recombinant rabbit fertilin subunits in rabbit. Biol. Reprod. 1997, 57, 879–886. [Google Scholar] [CrossRef]
- Yuan, R.; Primakoff, P.; Myles, D.G. A role for the disintegrin domain of cyritestin, a sperm surface protein belonging to the ADAM family, in mouse sperm-egg plasma membrane adhesion and fusion. J. Cell. Biol. 1997, 273, 7345–7350. [Google Scholar]
- McLaughlin, E.A.; Frayne, J.; Barker, H.L.; Jury, J.A.; Jones, R.; Ford, W.C.; Hall, L. Cloning and sequence analysis of rat fertilin α and β—Developmental expression, processing and immunolocalization. Mol. Hum. Reprod. 1997, 7, 313–317. [Google Scholar]
- Nishimura, H.; Kim, E.; Fujimori, T.; Kashiwabara, S.; Kuroiwa, A.; Matsuda, Y.; Baba, T. The ADAM1a and ADAM1b genes, instead of the ADAM1 (fertilin α) gene, are localized on mouse chromosome 5. Gene 2002, 291, 67–76. [Google Scholar] [CrossRef]
- Primakoff, P.; Hyatt, H.; Tredick-Kline, J. Identification and purification of a sperm surface protein with a potential role in sperm—Egg membrane fusion. J. Cell. Biol. 1987, 104, 141–149. [Google Scholar] [CrossRef]
- Cho, C.; Ge, H.; Branciforte, D.; Promakoff, P.; Myles, D.G. Analysis of mouse fertilin in wild-type and fertilin β(−/−) sperm: Evidence for C-terminal modification, α/β dimerization, and lack of essential role of fertilin α in sperm–egg fusion. Dev. Biol. 2000, 222, 289–295. [Google Scholar] [CrossRef]
- Primakoff, P. Sperm proteins being studied for use in a contraceptive vaccine. Am. J. Reprod. Immunol. 1994, 31, 208–210. [Google Scholar] [CrossRef]
- Nishimura, H.; Cho, C.; Branciforte, D.R.; Myles, D.G.; Primakoff, P. Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin β. Dev. Biol. 2001, 233, 204–213. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Yamagata, K.; Ikawa, M.; Moss, S.B.; Okabe, M. Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (clgn)-deficient mice. Biol. Reprod. 2006, 75, 760–766. [Google Scholar] [CrossRef]
- Cohen-Dayag, A.; Tur-Kaspa, I.; Dor, J.; Mashiach, S.; Einsenbach, M. Sperm capacitation in humans is transient and correlates with chemotactic responsiveness to follicular factors. Proc. Natl. Acad. Sci. USA 1995, 92, 11039–11043. [Google Scholar] [CrossRef]
- Sun, F.; Bahat, A.; Gakamsky, A.; Girsh, E.; Katz, N.; Giojalas, L.C.; Tur-Kaspa, I.; Eisenbach, M. Human sperm chemotaxis: Both the oocyte and its surrounding cumulus cells secrete sperm chemoattractants. Hum. Reprod. 2005, 20, 761–767. [Google Scholar] [CrossRef]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2005, 299, 2054–2058. [Google Scholar]
- Fülöp, C.; Szántó, S.; Mukhopadhyay, D.; Bárdos, T.; Kamath, R.V.; Rugg, M.S.; Day, A.J.; Salustri, A.; Hascall, V.C.; Glant, T.T.; et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 2003, 130, 2253–2261. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Asari, A.; Rugg, M.S.; Day, A.J.; Fülöp, C. Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-α-trypsin inhibitor to hyaluronan: Implications for the assembly of the cumulus extracellular matrix. J. Biol. Chem. 2004, 279, 11119–11128. [Google Scholar]
- Florman, H.M.; Ducibella, T. Fertilization in mammals. In Knobil and Neill’s Physiology of Reproduction; Neil, J.D., Ed.; Eslevier: New York, NY, USA, 2006; pp. 55–112. [Google Scholar]
- Primakoff, P.; Hyatt, H.; Myles, D.G. A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucida. J. Cell. Biol. 1985, 101, 2239–2244. [Google Scholar] [CrossRef]
- Baba, D.; Kashiwabara, S.; Honda, A.; Yamagata, K.; Wu, Q.; Ikawa, M.; Okabe, M.; Baba, T. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J. Biol. Chem. 2002, 277, 30310–30314. [Google Scholar]
- Kim, E.; Baba, D.; Kimura, M.; Yamashita, M.; Kashiwabara, S.; Baba, T. Identification of a hyaluronidase, Hyal5, involved in penetration of mouse sperm through cumulus mass. Proc. Natl. Acad. Sci. USA 2005, 102, 18028–18033. [Google Scholar] [CrossRef]
- Cherr, G.N.; Meyers, S.A.; Yudin, A.I.; VandeVoort, C.A.; Myles, D.G.; Primakoff, P.; Overstreet, J.W. The PH-20 protein in cynomolgus macaque spermatozoa: Identification of two different forms exhibiting hyaluronidase activity. Dev. Biol. 1996, 175, 142–153. [Google Scholar] [CrossRef]
- Liu, C.; Litscher, E.S.; Mortillo, S.; Sakai, Y.; Kinloch, R.A.; Stewart, C.L.; Wassarman, P.M. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc. Natl. Acad. Sci. USA 1996, 93, 5431–5436. [Google Scholar]
- Rankin, T.; Familari, M.; Lee, E.; Ginsberg, A.; Dwyer, N.; Blanchette-Mackie, J.; Drago, J.; Westphal, H.; Dean, J. Mice homozygous for an insertional mutation in the ZP3 gene lack a zona pellucida and are infertile. Development 1996, 122, 2903–2910. [Google Scholar]
- Wassarman, P.M.; Liu, C.; Chen, J.; Qi, H.; Litscher, E.S. Ovarian development in mice bearing homozygous or heterozygous null mutations in zona pellucida glycoprotein gene mZP3. Histol. Histopathol. 1998, 13, 293–300. [Google Scholar]
- Wassarman, P.M.; Lewandoski, M.; Campbell, K.; Joyner, A.L.; Rubenstein, J.L.; Martinez, S.; Martin, G.R. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development 1997, 125, 2923–2934. [Google Scholar]
- Jovine, L.; Qi, H.; Williams, Z.; Litscher, E.S.; Wassarman, P.M. Features that affect secretion and assembly of zona pellucida glycoproteins during mammalian oogenesis. Soc. Reprod. Fertil. Suppl. 2007, 63, 187–201. [Google Scholar]
- Florman, H.M.; Wassarman, P.M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell 1985, 41, 313–324. [Google Scholar] [CrossRef]
- Litscher, E.S.; Wassarman, P.M. Characterization of mouse ZP3-derived glycopeptide, gp55, that exhibits sperm receptor and acrosome reaction-inducing activity in vitro. Biochemistry 1996, 35, 3980–3985. [Google Scholar] [CrossRef]
- Florman, H.M.; Bechtol, K.B.; Wassarman, P.M. Enzymatic dissection of the functions of the mouse egg’s receptor for sperm. Dev. Biol. 1984, 106, 243–255. [Google Scholar] [CrossRef]
- Yanagimachi, R. Fertility of mammalian spermatozoa: Its development and relativity. Zygote 1994, 2, 371–372. [Google Scholar]
- Mortillo, S.; Wassarman, P.M. Differential binding of gold-labeled zona pellucida glycoproteins mZP2 and mZP3 to mouse sperm membrane compartments. Development 1991, 113, 141–149. [Google Scholar]
- Cohen, N.; Wassarman, P.M. Association of egg zona pellucida glycoprotein mZP3 with sperm protein sp56 during fertilization in mice. Int. J. Dev. Biol. 2001, 45, 569–576. [Google Scholar]
- Hardy, D.M.; Garbers, D.L. A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J. Biol. Chem. 1995, 270, 26025–26028. [Google Scholar] [CrossRef]
- Bi, M.; Hickox, J.R.; Winfrey, V.P.; Olson, G.E.; Hardy, D.M. Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem. J. 2003, 375, 477–488. [Google Scholar] [CrossRef]
- Olson, G.E.; Winfrey, V.P.; Hardy, D.M.; NaqDas, S.K. Zonadhesin assembly into the hamster sperm acrosomal matrix occurs by distinct targeting strategies during spermiogenesis and maturation in the epididymis. Biol. Reprod. 2004, 71, 1128–1134. [Google Scholar] [CrossRef]
- Tardif, S.; Wilson, M.D.; Wagner, R.; Hunt, P.; Gertsenstein, M.; Nagy, A.; Lobe, C.; Koop, B.F.; Hardy, D.M. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J. Biol. Chem. 2010, 285, 24863–24870. [Google Scholar] [CrossRef]
- Tardif, S. Green spermatozoa illuminate a 30-year-old model: Sperm—Egg adhesion involves intra-acrosomal proteins. Asian J. Androl. 2011, 13, 665–666. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar]
- Gasper, J.; Swanson, W.J. Molecular population genetics of the gene encoding the human fertilization protein zonadhesin reveals rapid adaptive evolution. Am. J. Hum. Genet. 2006, 79, 820–830. [Google Scholar] [CrossRef]
- Muro, Y.; Buffone, M.G.; Okabe, M.; Gerton, G.L. Function of the acrosomal matrix: Zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biol. Reprod. 2012, 86, 1–6. [Google Scholar]
- Wassarman, P.M.; Litscher, E.S. The multifunctional zona pellucida and mammalian fertilization. J. Reprod. Immunol. 2009, 83, 45–49. [Google Scholar] [CrossRef]
- Buffone, M.G.; Zhuang, T.; Ord, T.S.; Hui, L.; Moss, S.B.; Gerton, G.L. Recombinant mouse sperm ZP3-binding protein (ZP3R/sp56) forms a high order oligomer that binds eggs and inhibits mouse fertilization in vitro. J. Biol. Chem. 2008, 283, 12438–12445. [Google Scholar]
- Baba, T.; Kashiwabara, S.; Watanabe, K.; Itoh, H.; Michikawa, Y.; Kimura, K.; Takada, M.; Fukamizu, A.; Arai, Y. Activation and maturation mechanisms of boar acrosin zymogen based on the deduced primary structure. J. Biol. Chem. 1989, 264, 11920–11927. [Google Scholar]
- Honda, A.; Yamagata, K.; Sugiura, S.; Watanabe, K.; Baba, T. A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 2002, 277, 16976–16984. [Google Scholar]
- Sutovsky, P.; Manandhar, G.; McCauley, T.C.; Caamaño, J.N.; Sutovsky, M.; Thompson, W.E.; Day, B.N. Proteasomal interference prevents zona pellucida penetration and fertilization in mammals. Biol. Reprod. 2004, 71, 1625–1637. [Google Scholar] [CrossRef]
- Baba, T.; Azuma, S.; Kashiwabara, S.; Toyoda, Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 1994, 269, 31845–31849. [Google Scholar]
- Kawano, N.; Kang, W.; Yamashita, M.; Koga, Y.; Yamazaki, T.; Hata, T.; Miyado, K.; Baba, T. Mice lacking two sperm serine proteases, ACR and PRSS21, are subfertile, but the mutant sperm are infertile in vitro. Biol. Reprod. 2010, 83, 359–369. [Google Scholar] [CrossRef]
- Yamashita, M.; Honda, A.; Ogura, A.; Kashiwabara, S.; Fukami, K.; Baba, T. Reduced fertility of mouse epididymal sperm lacking Prss21/Tesp5 is rescued by sperm exposure to uterine microenvironment. Cell Mol. Biol. 2008, 10, 1001–1013. [Google Scholar]
- Yamagata, K.; Murayama, K.; Okabe, M.; Toshimori, K.; Nakanishi, T.; Kashiwabara, S.; Baba, T. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. J. Biol. Chem. 1998, 273, 10470–10474. [Google Scholar]
- Baibakov, B.; Gauthier, L.; Talbot, P.; Rankin, T.L.; Dean, J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development 2007, 134, 933–943. [Google Scholar] [CrossRef]
- Fábryová, K.; Simon, M. Function of the cell surface molecules (CD molecules) in the reproduction processes. Gen. Physiol. Biophys. 2009, 28, 1–7. [Google Scholar] [CrossRef]
- Rubinstein, E.; le Naour, F.; Lagaudrière-Gesbert, C.; Billard, M.; Conjeaud, H.; Boucheix, C. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur. J. Immunol. 1996, 26, 2657–2665. [Google Scholar] [CrossRef]
- Boucheix, C.; Benoit, P.; Frachet, P.; Billard, M.; Worthington, R.E.; Gagnon, J.; Uzan, G. Molecular cloning of the CD9 antigen. A new family of cell surface proteins. J. Biol. Chem. 1991, 266, 117–122. [Google Scholar]
- Chen, M.S.; Tung, K.S.; Coonrod, S.A.; Takahashi, Y.; Bigler, D.; Chang, A.; Yamashita, Y.; Kincade, P.W.; Herr, J.C.; White, J.M. Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin α6β1: Implications for murine fertilization. Proc. Natl. Acad. Sci. USA 1999, 96, 11830–11835. [Google Scholar] [CrossRef]
- Longo, F.J.; Chen, D.Y. Development of surface polarity in mouse eggs. Scan Eletron. Microsc. 1984, 2, 703–716. [Google Scholar]
- Kaji, K.; Oda, S.; Shikano, T.; Ohnuki, T.; Uematsu, Y.; Sakagami, J.; Tada, N.; Miyazaki, S.; Kudo, A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 2000, 24, 279–282. [Google Scholar] [CrossRef]
- Runge, K.E.; Evans, J.E.; He, Z.Y.; Gupta, S.; McDonald, K.L.; Stahlberg, H.; Primakoff, P.; Myles, D.G. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev. Biol. 2007, 304, 317–325. [Google Scholar] [CrossRef]
- Le Naour, F.; Rubinstein, E.; Jasmin, C.; Prenant, M.; Boucheix, C. Severely reduced female fertility in CD9-deficient mice. Science 2000, 287, 319–321. [Google Scholar] [CrossRef]
- Miyado, K.; Yamada, G.; Yamada, S.; Hasuwa, H.; Nakamura, Y.; Ryu, F.; Suzuki, K.; Kosai, K.; Inoue, K.; Ogura, A.; et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 2000, 287, 321–324. [Google Scholar] [CrossRef]
- Zhu, G.Z.; Miller, B.J.; Boucheix, C.; Rubinstein, E.; Liu, C.C.; Hynes, R.O.; Myles, D.G.; Primakoff, P. Residues SFQ (173–175) in the large extracellular loop of CD9 are required for gamete fusion. Development 2002, 129, 1995–2002. [Google Scholar]
- Seigneuret, M.; Delaquillaumie, A.; Laqaudriere-Gesbert, C.; Conjeaud, H. Structure of the tetraspanin main extracellular domain. A partially conserved fold with a structurally variable domain insertion. J. Biol. Chem. 2001, 276, 40055–40064. [Google Scholar]
- Rubinstein, E.; Ziyyat, A.; Prenant, M.; Wrobel, E.; Wolf, J.P.; Levy, S.; le Naour, F.; Boucheix, C. Reduced fertility of female mice lacking CD81. Dev. Biol. 2006, 290, 351–358. [Google Scholar] [CrossRef]
- Evans, J.P. Sperm—Egg Interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef]
- Anderson, D.J.; Michaelson, J.S.; Johnson, P.M. Trophoblast/leukocyte-common antigen is expressed by human testicular germ cells and appears on the surface of acrosome-reacted sperm. Biol. Reprod. 1989, 41, 285–293. [Google Scholar] [CrossRef]
- Antalíková, J.; Simon, M.; Jankovicová, J.; Horovská, L. Identification of MCP/CD46 analogue on bovine erythrocytes using the new monoclonal antibody IVA-520. Vet. Immunol. Immunopathol. 2007, 115, 155–159. [Google Scholar] [CrossRef]
- Anderson, D.J.; Abbott, A.F.; Jack, R.M. The role of complement component C3b and its receptors in sperm–oocyte interaction. Proc. Natl. Acad. Sci. USA 1993, 90, 10051–10055. [Google Scholar] [CrossRef]
- Kitamura, M.; Matsumiya, K.; Yamanaka, M.; Takahara, S.; Hara, T.; Matsumoto, M.; Namiki, M.; Okuyama, A.; Seya, T. Possible association of infertility with sperm-specific abnormality of CD46. J. Reprod. Immunol. 1997, 33, 83–38. [Google Scholar] [CrossRef]
- Ziyyat, A.; Rubinstein, E.; Monier-Gavelle, F.; Barraud, V.; Kulski, O.; Prenant, M.; Boucheix, C.; Bomsel, M.; Wolf, J.P. CD9 controls the formation of clusters that contain tetraspanins and the integrin α6β1, which are involved in human and mouse gamete fusion. J. Cell. Sci. 2006, 119, 416–424. [Google Scholar] [CrossRef]
- Barclay, A.N.; Brown, M.H. Heterogeneity of interactions mediated by membrane glycoproteins of lymphocytes. Biochem. Soc. Trans. 1997, 25, 224–228. [Google Scholar]
- Miller, B.J.; Georges-Labouesse, E.; Primakoff, P.; Myles, D.G. Normal fertilization occurs with eggs lacking the integrin α6β1 and is CD9-dependent. J. Cell. Biol. 2000, 149, 1289–1296. [Google Scholar] [CrossRef]
- Jégou, A.; Ziyyat, A.; Barraud-Lange, V.; Perez, E.; Wolf, J.P.; Pincet, F.; Gourier, C. CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 10946–10951. [Google Scholar]
- Zhu, X.; Evans, J.P. Analysis of the roles of RGD-binding integrins, α(4)/α(9) integrins, α(6) integrins, and CD9 in the interaction of the fertilin β (ADAM2) disintegrin domain with the mouse egg membrane. Biol. Reprod. 2002, 66, 1193–1202. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes—Vesicular carriers for intercellular communication. Curr. Opin. Cell. Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Barraud-Lange, V.; Naud-Barriant, N.; Bomsel, M.; Wolf, J.P.; Ziyyat, A. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J. 2007, 21, 3446–3449. [Google Scholar] [CrossRef]
- Miyado, K.; Yoshida, K.; Yamagata, K.; Sakakibara, K.; Okabe, M.; Wang, X.; Miyamoto, K.; Akutsu, H.; Kondo, T.; Takahashi, Y.; et al. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12921–12926. [Google Scholar] [CrossRef]
- Alfieri, J.A.; Martin, A.D.; Takeda, J.; Kondoh, G.; Myles, D.G.; Primakoff, P. Infertility in female mice with an oocyte-specific knockout of GPI-anchored proteins. J. Cell. Sci. 2003, 116, 2149–2155. [Google Scholar] [CrossRef]
- Claas, C.; Stipp, C.S.; Hemler, M.E. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J. Biol. Chem. 2001, 276, 7974–7984. [Google Scholar] [CrossRef]
- Espenel, C.; Margeat, E.; Dosset, P.; Arduise, C.; le Grimellec, C.; Royer, C.A.; Boucheix, C.; Rubinstein, E.; Milhiet, P.E. Single-molecule analysis of CD9 dynamics and partitioning reveals multiple modes of interaction in the tetraspanin web. J. Cell. Biol. 2008, 182, 765–776. [Google Scholar] [CrossRef]
- Kasahara, M.; Gutknecht, J.; Brew, K.; Spurr, N.; Goodfellow, P.N. Cloning and mapping of a testis-specific gene with sequence similarity to a sperm-coating glycoprotein gene. Genomics 1989, 5, 527–534. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Loveland, K.L.; Herszfeld, D.; McFarlane, J.R.; Hearn, M.T.; de Kretser, D.M. Identification of a rat testis-specific gene encoding a potential rat outer dense fibre protein. Mol. Reprod. Dev. 1998, 50, 313–322. [Google Scholar] [CrossRef]
- Mizuki, N.; Kasahara, M. Mouse submandibular glands express an androgen-regulated transcript encoding an acidic epididymal glycoprotein-like molecule. Mol. Cell. Endocrinol. 1992, 89, 25–32. [Google Scholar] [CrossRef]
- Schambony, A.; Gentzel, M.; Wolfes, H.; Raida, M.; Neumann, U.; Töpfer-Petersen, E. Equine CRISP-3: Primary structure and expression in the male genital tract. Biochim. Biophys. Acta 1998, 1387, 206–216. [Google Scholar] [CrossRef]
- Cuasnicú, P.S.; González Echeverría, F.; Piazza, A.D.; Cameo, M.S.; Blaquier, J.A. Antibodies against epididymal glycoproteins block fertilizing ability in rat. J. Reprod. Fertil. 1984, 72, 467–471. [Google Scholar] [CrossRef]
- Ellerman, D.A.; Brantua, V.S.; Martinez, S.P.; Cohen, D.J.; Conesa, D.; Cuasnicu, P.S. Potential contraceptive use of epididymal proteins: Immunization of male rats with epididymal protein DE inhibits sperm fusion ability. Biol. Reprod. 1998, 59, 1029–1036. [Google Scholar] [CrossRef]
- Rochwerger, L.; Cuasnicu, P.S. Redistribution of a rat sperm epididymal glycoprotein after in vitro and in vivo capacitation. Mol. Reprod. Dev. 1992, 31, 34–41. [Google Scholar] [CrossRef]
- Rochwerger, L.; Cohen, D.J.; Cuasnicú, P.S. Mammalian sperm–egg fusion: The rat egg has complementary sites for a sperm protein that mediates gamete fusion. Dev. Biol. 1992, 153, 83–90. [Google Scholar] [CrossRef]
- Cohen, D.J.; Ellerman, D.A.; Busso, D.; Morgenfeld, M.M.; Piazza, A.D.; Hayashi, M.; Young, E.T.; Kasahara, M.; Cuasnicu, P.S. Evidence that human epididymal protein ARP plays a role in gamete fusion through complementary sites on the surface of the human egg. Biol. Reprod. 2001, 65, 1000–1005. [Google Scholar] [CrossRef]
- Okabe, M.; Yagasaki, M.; Oda, H.; Matzno, S.; Kohama, Y.; Mimura, T. Effect of a monoclonal anti-mouse sperm antibody (OBF13) on the interaction of mouse sperm with zona-free mouse and hamster eggs. J. Reprod. Immunol. 1988, 13, 211–219. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 10. 434, 234–238. [Google Scholar] [CrossRef]
- Ellerman, D.A.; Pei, J.; Gupta, S.; Snell, W.J.; Myles, D.; Primakoff, P. Izumo is part of a multiprotein family whose members form large complexes on mammalian sperm. Mol. Reprod. Dev. 2009, 76, 1188–1199. [Google Scholar] [CrossRef]
- Brümmendorf, T.; Lemmon, V. Immunoglobulin superfamily receptors: cis-Interactions, intracellular adapters and alternative splicing regulate adhesion. Curr. Opin. Cell. Biol. 2001, 13, 611–618. [Google Scholar] [CrossRef]
- Inoue, N.; Kasahara, T.; Ikawa, M.; Okabe, M. Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. PLoS One 2010, 22, e10301. [Google Scholar]
- Wolkowicz, M.J.; Shetty, J.; Westbrook, A.; Klotz, K.; Jayes, F.; Mandal, A.; Flickinger, C.J.; Herr, J.C. Equatorial segment protein defines a discrete acrosomal subcompartment persisting throughout acrosomal biogenesis. Biol. Reprod. 2003, 69, 7357–7345. [Google Scholar]
- Wolkowicz, M.J.; Digilio, L.; Klotz, K.; Shetty, J.; Flickinger, C.J.; Herr, J.C. Equatorial segment protein (ESP) is a human alloantigen involved in sperm–egg binding and fusion. J. Androl. 2008, 29, 272–282. [Google Scholar] [CrossRef]
- Lv, Z.M.; Wang, M.; Xu, C. Antifertility characteristics of the N-terminal region of mouse equatorial segment protein. Anat. Rec. 2010, 293, 171–181. [Google Scholar] [CrossRef]
- Fujihara, Y.; Murakami, M.; Inoue, N.; Satouh, Y.; Kaseda, K.; Ikawa, M.; Okabe, M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J. Cell. Sci. 2010, 123, 1531–1536. [Google Scholar] [CrossRef]
- Evans, J.P. The molecular basis of sperm–oocyte membrane interactions during mammalian fertilization. Hum. Reprod. Updat. 2002, 8, 297–311. [Google Scholar] [CrossRef]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 24. 508, 483–487. [Google Scholar] [CrossRef]
- Wassarman, P.M. Sperm protein finds its mate. Nature 2014, 508, 466–467. [Google Scholar] [CrossRef]
- Bjerregaard, B.; Lemmen, J.G.; Petersen, M.R.; Ostrup, E.; Iversen, L.H.; Almstrup, K.; Larsson, L.I.; Ziebe, S. Syncytin-1 and its receptor is present in human gametes. J. Assist. Reprod. Genet. 2014, 5, 533–539. [Google Scholar]
- Albertini, D.F. Confronting the fissions and fusions of human fertilization. J. Assist. Reprod. Genet. 2014, 31, 507–508. [Google Scholar] [CrossRef]
- Foresta, C.; Ferlin, A.; Bertoldo, A.; Patassini, C.; Zuccarello, D.; Garolla, A. Human papilloma virus in the sperm cryobank: An emerging problem? Int. J. Androl. 2011, 34, 242–246. [Google Scholar] [CrossRef]
- Foresta, C.; Garolla, A.; Zuccarello, D.; Pizzol, D.; Moretti, A.; Barzon, L.; Palù, G. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil. Steril. 2010, 93, 802–806. [Google Scholar] [CrossRef]
- Foresta, C.; Patassini, C.; Bertoldo, A.; Menegazzo, M.; Francavilla, F.; Barzon, L.; Ferlin, A. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS One 2011, 6, e15036. [Google Scholar]
- Foresta, C.; Pizzol, D.; Bertoldo, A.; Menegazzo, M.; Barzon, L.; Garolla, A. Semen washing procedures do not eliminate human papilloma virus sperm infection in infertile patients. Fertil. Steril. 2011, 96, 1077–1082. [Google Scholar] [CrossRef]
- Garolla, A.; Lenzi, A.; Palù, G.; Pizzol, D.; Bertoldo, A.; de Toni, L.; Foresta, C. Human papillomavirus sperm infection and assisted reproduction: A dangerous hazard with a possible safe solution. Hum. Reprod. 2012, 27, 967–973. [Google Scholar] [CrossRef]
- Garolla, A.; Pizzol, D.; Bertoldo, A.; de Toni, L.; Barzon, L.; Foresta, C. Association, prevalence, and clearance of human papillomavirus and antisperm antibodies in infected semen samples from infertile patients. Fertil. Steril. 2013, 99, 125–131. [Google Scholar] [CrossRef]
- Gizzo, S.; Ferrari, B.; Noventa, M.; Ferrari, E.; Patrelli, T.S.; Gangemi, M.; Nardelli, G.B. Male and couple fertility impairment due to HPV–DNA sperm infection: Update on molecular mechanism and clinical impact-systematic review. Biomed. Res. Int. 2014, 2014, 230263. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anifandis, G.; Messini, C.; Dafopoulos, K.; Sotiriou, S.; Messinis, I. Molecular and Cellular Mechanisms of Sperm-Oocyte Interactions Opinions Relative to in Vitro Fertilization (IVF). Int. J. Mol. Sci. 2014, 15, 12972-12997. https://doi.org/10.3390/ijms150712972
Anifandis G, Messini C, Dafopoulos K, Sotiriou S, Messinis I. Molecular and Cellular Mechanisms of Sperm-Oocyte Interactions Opinions Relative to in Vitro Fertilization (IVF). International Journal of Molecular Sciences. 2014; 15(7):12972-12997. https://doi.org/10.3390/ijms150712972
Chicago/Turabian StyleAnifandis, George, Christina Messini, Konstantinos Dafopoulos, Sotiris Sotiriou, and Ioannis Messinis. 2014. "Molecular and Cellular Mechanisms of Sperm-Oocyte Interactions Opinions Relative to in Vitro Fertilization (IVF)" International Journal of Molecular Sciences 15, no. 7: 12972-12997. https://doi.org/10.3390/ijms150712972



