Protein Tyrosine Kinase 7 (PTK7) as a Predictor of Lymph Node Metastases and a Novel Prognostic Biomarker in Patients with Prostate Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Expression of Protein Tyrosine Kinase 7 (PTK7) mRNA in Benign Prostatic Hyperplasia and Prostate Cancer Tissues by Quantitative Real Time Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.1.2. Expression of PTK7 Protein in Benign Prostatic Hyperplasia and Prostate Cancer Tissues by Western Blot Analysis
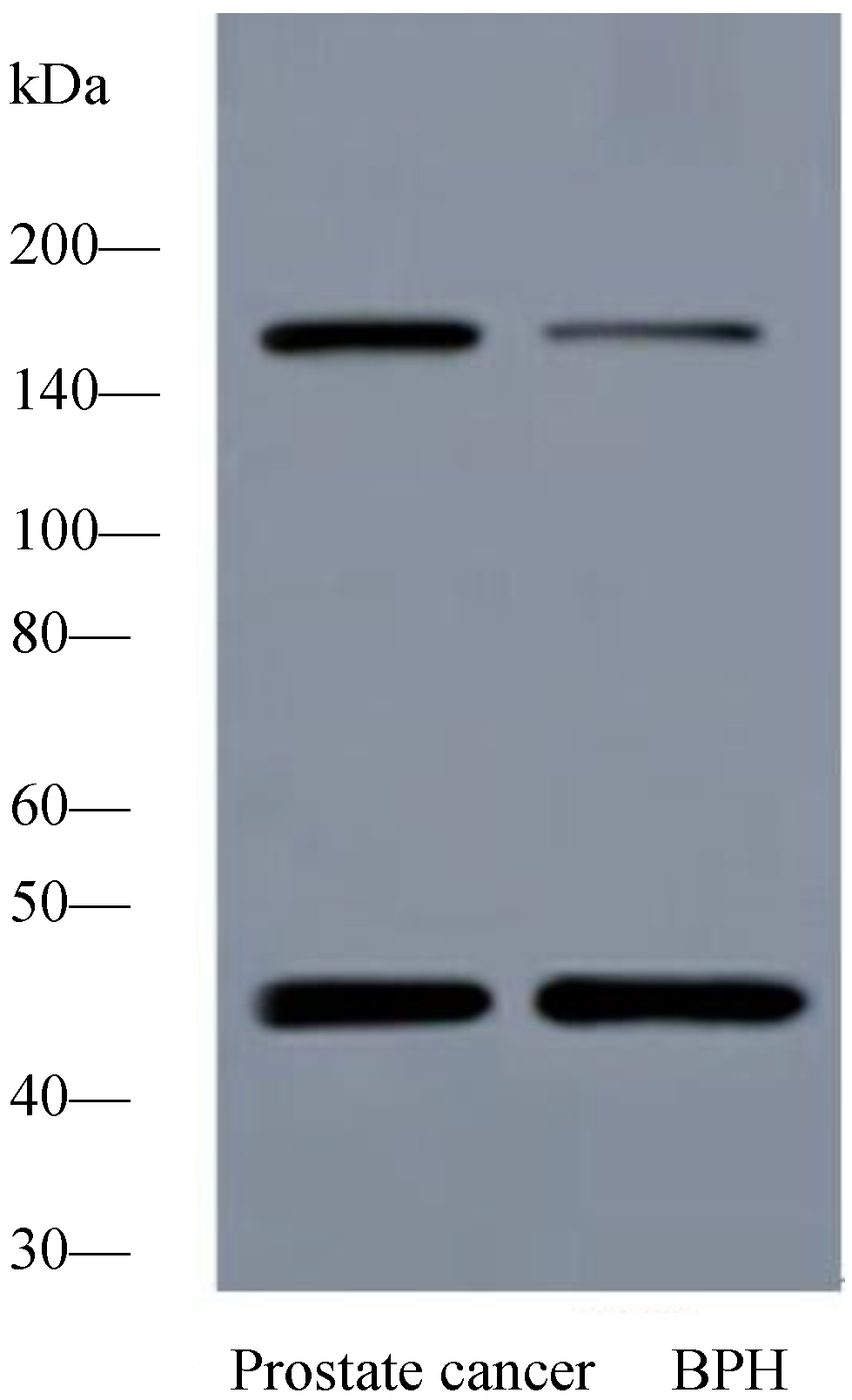
2.1.3. Expression of PTK7 Protein in Benign Prostatic Hyperplasia and Prostate Cancer Tissues by Immunothistochemical Staining

2.1.4. Association between PTK7 Expression and Clinicopathologic Characteristics in Prostate Cancer Patients
| Variable | Group | PTK7 Expression | p Value | ||
|---|---|---|---|---|---|
| n | High | Low | |||
| Age | <70 | 97 | 60 (61.9%) | 37 (38.1%) | 0.782 |
| ≥70 | 83 | 53 (63.9%) | 30 (36.1%) | ||
| Lymph Node Metastasis | Presence | 17 | 15 (88.2%) | 2 (11.8%) | 0.023 |
| Absence | 163 | 98 (60.1%) | 65 (39.9%) | ||
| Surgical Margin Status | Presence | 14 | 9 (64.3%) | 5 (35.7%) | 0.903 |
| Absence | 166 | 104 (71.7%) | 62 (28.3%) | ||
| Seminal Vesicle Invasion | Presence | 35 | 30 (64.3%) | 5 (35.7%) | 0.002 |
| Absence | 145 | 83 (57.2%) | 62 (42.8%) | ||
| Prostate Cancer Stage | T1 | 103 | 54 (52.4%) | 49 (47.6%) | 0.001 |
| T2/T3 | 77 | 59 (76.6%) | 18 (23.4%) | ||
| Preoperative PSA | <4 | 5 | 1 (20%) | 4 (80%) | <0.001 |
| 4–10 | 64 | 23 (35.9%) | 41 (64.1%) | ||
| >10 | 111 | 89 (80.2%) | 22 (19.8%) | ||
| Gleason Score | <7 | 99 | 51 (51.5%) | 48 (48.5%) | 0.002 |
| 7 | 34 | 24 (70.6%) | 10 (29.4%) | ||
| >7 | 47 | 38 (80.9%) | 9 (19.1%) | ||
| Angiolymphatic Invasion | Presence | 35 | 28 (80.0%) | 7(20.0%) | 0.019 |
| Absence | 145 | 85 (58.6%) | 60 (41.4%) | ||
| Biochemical Recurrence | Presence | 52 | 41 (78.8%) | 11 (21.2%) | 0.004 |
| Absence | 128 | 72 (56.3%) | 56 (43.7%) | ||
2.1.5. Predictive Significance of PTK7 Expression in Prostate Cancer Patients with Lymph Node Metastases
| Variable | B | S.E. | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Prostate Cancer Stage | 0.199 | 0.311 | 1.220 | 0.663–2.234 | 0.523 |
| Gleason Score | 0.068 | 0.216 | 1.070 | 0.701–1.633 | 0.753 |
| Preoperative Prostate-Specific Antigen | 0.737 | 0.320 | 2.089 | 1.116–3.911 | 0.021 |
| Age | 0.224 | 0.215 | 1.251 | 0.820–1.908 | 0.299 |
| Surgical Margin Status | −0.265 | 0.336 | 0.767 | 0.397–1.483 | 0.430 |
| Presence of Seminal Vesicle Invasion | 0.166 | 0.216 | 1.181 | 0.773–1.805 | 0.442 |
| PTK7 Overexpression | 0.781 | 0.248 | 2.183 | 1.343–3.549 | 0.002 |
| Presence of Angiolymphatic Invasion | 0.547 | 0.218 | 1.728 | 1.128–2.648 | 0.012 |
| Variable | B | S.E. | OR | 95% CI | p Value |
|---|---|---|---|---|---|
| Preoperative Prostate-Specific Antigen | 0.375 | 0.347 | 1.454 | 0.736–2.873 | 0.281 |
| PTK7 Overexpression | 0.620 | 0.267 | 1.859 | 1.101–3.138 | 0.020 |
| Presence of Angiolymphatic Invasion | 0.458 | 0.222 | 1.581 | 1.023–2.442 | 0.039 |
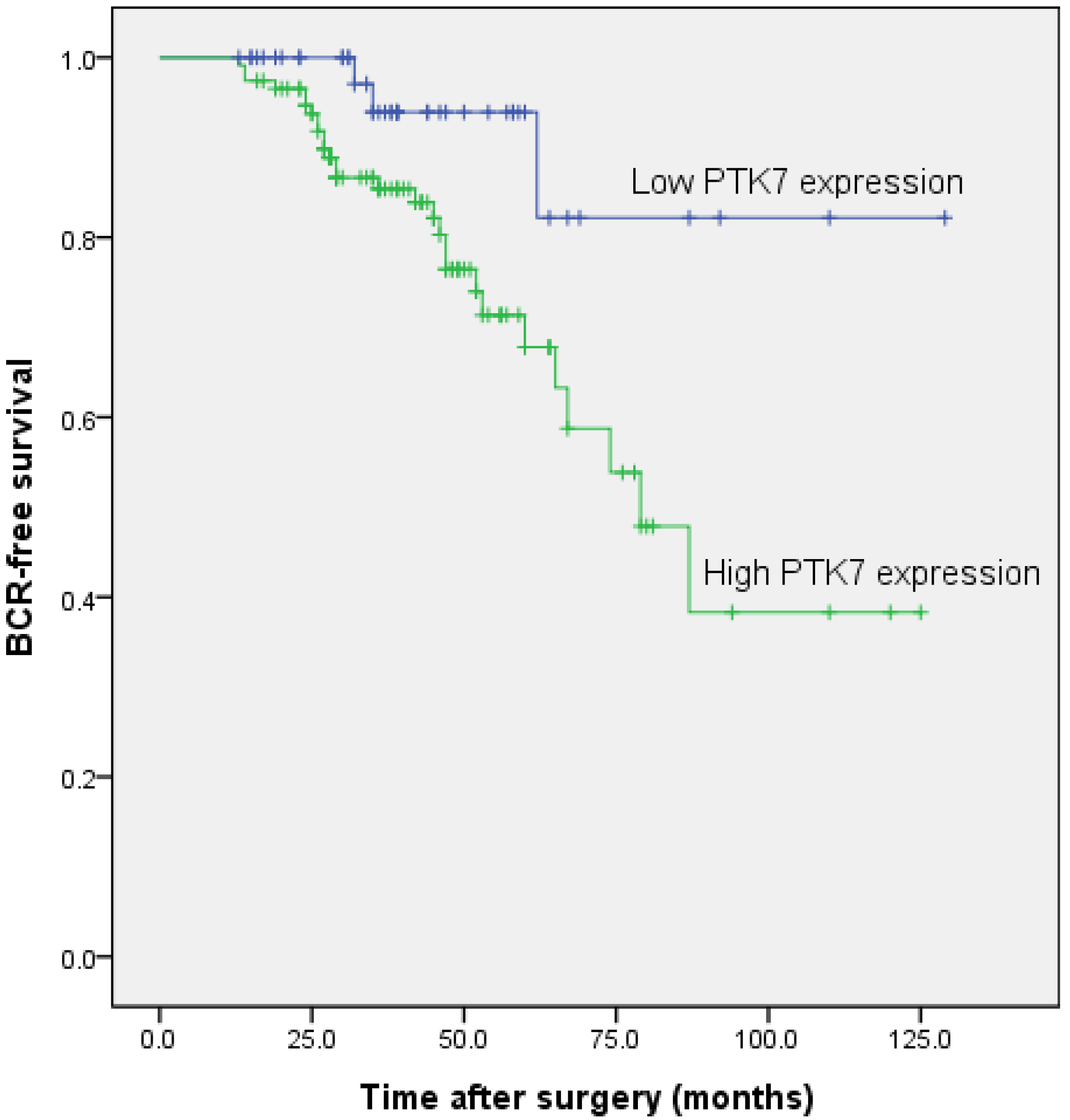
2.1.6. Association between PTK7 Expression and Biochemical Recurrence-Free Survival in Patients with Prostate Cancer
| Covariant | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Exp (B) | 95% CI | p Value | Exp (B) | 95% CI | p Value | |
| PTK7 | 2.477 | 1.432–4.286 | 0.001 | 2.267 | 1.303–3.944 | 0.004 |
| Gleason Score | 1.703 | 1.280–2.265 | <0.001 | 1.667 | 1.253–2.217 | <0.001 |
| Seminal Vesicle Invasion | 1.505 | 1.132–2.003 | 0.005 | 1.371 | 1.029–1.827 | 0.031 |
| Preoperative PSA | 1.241 | 0.705–2.188 | 0.454 | |||
| Age | 1.068 | 0.804–1.419 | 0.650 | |||
| Angiolymphatic Invasion | 1.084 | 0.814–1.443 | 0.580 | |||
| Surgical Margin Status | 1.017 | 0.709–1.459 | 0.925 | |||
| Prostate Cancer Stage | 1.090 | 0.921–1.291 | 0.316 | |||
| Lymph Node Metastasis | 1.140 | 0.850–1.528 | 0.381 | |||
2.1.7. Association between PTK7 Expression and Overall Survival in Patients with Prostate Cancer
| Covariant | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Exp (B) | 95% CI | p Value | Exp (B) | 95% CI | p Value | |
| PTK7 | 5.121 | 2.600–10.083 | <0.001 | 5.650 | 2.843–11.229 | <0.001 |
| Gleason Score | 2.526 | 1.788–3.568 | <0.001 | 2.031 | 1.317–3.132 | 0.001 |
| Preoperative PSA | 2.034 | 1.338–23.092 | 0.001 | 1.819 | 1.278–2.588 | 0.001 |
| Prostate Cancer Stage | 4.131 | 2.888–5.911 | <0.001 | 4.306 | 2.949–6.289 | <0.001 |
| Age | 1.282 | 0.917–1.792 | 0.146 | |||
| Angiolymphatic Invasion | 1.373 | 0.813–2.319 | 0.235 | |||
| Surgical Margin Status | 1.101 | 0.703–1.724 | 0.674 | |||
| Lymph Node Metastasis | 1.044 | 0.746–1.462 | 0.800 | |||
| Seminal Vesicle Invasion | 1.358 | 0.956–1.928 | 0.087 | |||
2.2. Discussion
3. Experimental Section
3.1. Clinical Specimens
3.2. qRT-PCR
3.3. Western Blotting
3.4. Immunohistochemistry
3.5. Semiquantitative Analysis of PTK7 Staining
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA 2012, 62, 10–29. [Google Scholar]
- Pound, C.R.; Partin, A.W.; Eisenberger, M.A.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef]
- Roder, M.A.; Brasso, K.; Christensen, I.J.; Johansen, J.; Langkilde, N.C.; Hvarness, H.; Carlsson, S.; Jakobsen, H.; Borre, M.; Iversen, P. Survival after radical prostatectomy for clinically localised prostate cancer: A population-based study. BJU Int. 2014, 113, 541–547. [Google Scholar]
- Bill-Axelson, A.; Holmberg, L.; Ruutu, M.; Garmo, H.; Stark, J.R.; Busch, C.; Nordling, S.; Haggman, M.; Andersson, S.O.; Bratell, S. Radical prostatectomy versus watchful waiting in early prostate cancer. N. Engl .J. Med. 2011, 364, 1708–1717. [Google Scholar]
- Diamandis, E.P. Prostate-specific antigen: Its usefulness in clinical medicine. Trends Endocrinol. Metab. 1998, 9, 310–316. [Google Scholar] [CrossRef]
- Saha, S.; Bardelli, A.; Buckhaults, P.; Velculescu, V.E.; Rago, C.; St Croix, B.; Romans, K.E.; Choti, M.A.; Lengauer, C.; Kinzler, K.W. A phosphatase associated with metastasis of colorectal cancer. Science 2001, 294, 1343–1346. [Google Scholar] [CrossRef]
- Briganti, A.; Blute, M.L.; Eastham, J.H.; Graefen, M.; Heidenreich, A.; Karnes, J.R.; Montorsi, F.; Studer, U.E. Pelvic lymph node dissection in prostate cancer. Eur. Urol. 2009, 55, 1251–1265. [Google Scholar] [CrossRef]
- Briganti, A.; Karnes, R.J.; da Pozzo, L.F.; Cozzarini, C.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Tutolo, M.; Salonia, A. Combination of adjuvant hormonal and radiation therapy significantly prolongs survival of patients with pT2-4 pN+ prostate cancer: Results of a matched analysis. Eur. Urol. 2011, 59, 832–840. [Google Scholar] [CrossRef]
- Messing, E.M.; Manola, J.; Yao, J.; Kiernan, M.; Crawford, D.; Wilding, G.; di Sant Agnese, P.A.; Trump, D. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006, 7, 472–479. [Google Scholar] [CrossRef]
- Mossie, K.; Jallal, B.; Alves, F.; Sures, I.; Plowman, G.D.; Ullrich, A. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene 1995, 11, 2179–2184. [Google Scholar]
- Jung, J.W.; Ji, A.R.; Lee, J.; Kim, U.J.; Lee, S.T. Organization of the human PTK7 gene encoding a receptor protein tyrosine kinase-like molecule and alternative splicing of its mRNA. Biochim. Biophys. Acta 2002, 1579, 153–163. [Google Scholar]
- Peradziryi, H.; Kaplan, N.A.; Podleschny, M.; Liu, X.; Wehner, P.; Borchers, A.; Tolwinski, N.S. PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. EMBO J. 2011, 30, 3729–3740. [Google Scholar]
- Puppo, F.; Thomé, V.; Lhoumeau, A.C.; Cibois, M.; Gangar, A.; Lembo, F.; Belotti, E.; Marchetto, S.; Lécine, P.; Prébet, T. Protein-tyrosine kinase 7 has a conserved role in Wnt/β-catenin canonical signalling. Eur. Mol. Biol. Organ. Rep. 2011, 12, 43–49. [Google Scholar]
- Lhoumeau, A.C.; Puppo, F.; Prébet, T.; Kodjabachian, L.; Borg, J.P. PTK7: A cell polarity receptor with multiple facets. Cell Cycle 2011, 10, 1233–1236. [Google Scholar]
- Shnitsar, I.; Borchers, A. PTK7 recruits dsh to regulate neural crest migration. Development 2008, 135, 4015–4024. [Google Scholar] [CrossRef]
- Toyofuku, T.; Zhang, H.; Kumanogoh, A.; Takegahara, N.; Yabuki, M.; Harada, K.; Hori, M.; Kikutani, H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 2004, 6, 1204–1211. [Google Scholar]
- Wehner, P.; Shnitsar, I.; Urlaub, H.; Borchers, A. RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development 2011, 138, 1321–1327. [Google Scholar] [CrossRef]
- Whitford, K.L.; Ghosh, A. Plexin signaling via off-track and rho family GTPases. Neuron 2011, 32, 1–3. [Google Scholar] [CrossRef]
- Golubkov, V.S.; Strongin, A.Y. Insights into ectodomain shedding and processing of protein tyrosine pseudokinase 7 (PTK7). J. Biol. Chem. 2012, 287, 42009–42018. [Google Scholar] [CrossRef]
- Shin, W.S.; Maeng, Y.S.; Jung, J.W.; Min, J.K.; Kwon, Y.G.; Lee, S.T. Soluble PTK7 inhibits tube formation, migration, and invasion of endothelial cells and angiogenesis. Biochem. Biophys. Res. Commun. 2008, 371, 793–798. [Google Scholar]
- Gorringe, K.L.; Boussioutas, A.; Bowtell, D.D. Novel regions of chromosomal amplification at 6p21, 5p13, and 12q14 in gastric cancer identified by array comparative genomic hybridization. Genes Chromosom. Cancer 2005, 42, 247–259. [Google Scholar] [CrossRef]
- Endoh, H.; Tomida, S.; Yatabe, Y.; Konishi, H.; Osada, H.; Tajima, K.; Kuwano, H.; Takahashi, T.; Mitsudomi, T. Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J. Clin. Oncol. 2004, 22, 811–819. [Google Scholar] [CrossRef]
- Müller-Tidow, C.; Schwäble, J.; Steffen, B.; Tidow, N.; Brandt, B.; Becker, K.; Schulze-Bahr, E.; Halfter, H.; Vogt, U.; Metzger, R. High-throughput analysis of genome-wide receptor tyrosine kinase expression in human cancers identifies potential novel drug targets. Clin. Cancer Res. 2004, 10, 1241–1249. [Google Scholar]
- Su, H.; Hu, N.; Yang, H.H.; Wang, C.; Takikita, M.; Wang, Q.H.; Giffen, C.; Clifford, R.; Hewitt, S.M.; Shou, J.Z. Global gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypes. Clin. Cancer Res. 2011, 17, 2955–2966. [Google Scholar]
- Gobble, R.M.; Qin, L.X.; Brill, E.R.; Angeles, C.V.; Ugras, S.; O’Connor, R.B.; Moraco, N.H.; Decarolis, P.L.; Antonescu, C.; Singer, S. Expression profiling of liposarcoma yields a multigene predictor of patient outcome and identifies genes that contribute to liposarcomagenesis. Cancer Res. 2011, 71, 2697–2705. [Google Scholar]
- Prebet, T.; Lhoumeau, A.C.; Arnoulet, C.; Aulas, A.; Marchetto, S.; Audebert, S.; Puppo, F.; Chabannon, C.; Sainty, D.; Santoni, M.J. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood 2010, 116, 2315–2323. [Google Scholar] [CrossRef]
- Meng, L.; Sefah, K.; O’Donoghue, M.B.; Zhu, G.; Shangguan, D.; Noorali, A.; Chen, Y.; Zhou, L.; Tan, W. Silencing of PTK7 in colon cancer cells: Caspase-10-dependent apoptosis via mitochondrial pathway. PLoS One 2010, 5, e14018. [Google Scholar]
- Liu, Y.; Chen, J.; Sethi, A.; Li, Q.K.; Chen, L.; Collins, B.; Gillet, L.C.; Wollscheid, B.; Zhang, H.; Aebersold, R. Glycoproteomic analysis of prostate cancer tissues by SWATH mass spectrometry discovers N-acylethanolamine acid amidase and protein tyrosine kinase 7 as signatures for tumor aggressiveness. Mol. Cell Proteomics 2014, 13, 1–45. [Google Scholar]
- Zhang, H.; Qi, C.; Li, L.; Luo, F.; Xu, Y. Clinical significance of NUCB2 mRNA expression in prostate cancer. J. Exp. Clin. Cancer Res. 2013, 32, 56. [Google Scholar] [CrossRef]
- Miyake, H.; Sakai, I.; Harada, K.; Hara, I.; Eto, H. Long-term results of adjuvant hormonal therapy plus radiotherapy following radical prostatectomy for patients with pT3N0 or pT3N1 prostate cancer. Int. J. Urol. 2004, 11, 397–401. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Q.; Liu, R.; Qi, S.; Liang, P.; Qi, C.; Wang, A.; Sheng, B.; Li, L.; Xu, Y. Overexpression of LAPTM4B-35: a novel marker of poor prognosis of prostate cancer. PLoS One 2014, 9, e91069. [Google Scholar]
- Zhang, H.; Cheng, S.; Wang, A.; Ma, H.; Yao, B.; Qi, C.; Liu, R.; Qi, S.; Xu, Y. Expression of RABEX-5 and its clinical significance in prostate cancer. J. Exp. Clin. Cancer Res. 2014, 33, 31. [Google Scholar]
- Zhang, H.; Qi, C.; Wang, A.; Yao, B.; Li, L.; Wang, Y.; Xu, Y. Prognostication of prostate cancerbased on NUCB2 protein assessment: NUCB2 in prostate cancer. J. Exp. Clin. Cancer Res. 2013, 32, 77. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.; Wang, A.; Qi, S.; Cheng, S.; Yao, B.; Xu, Y. Protein Tyrosine Kinase 7 (PTK7) as a Predictor of Lymph Node Metastases and a Novel Prognostic Biomarker in Patients with Prostate Cancer. Int. J. Mol. Sci. 2014, 15, 11665-11677. https://doi.org/10.3390/ijms150711665
Zhang H, Wang A, Qi S, Cheng S, Yao B, Xu Y. Protein Tyrosine Kinase 7 (PTK7) as a Predictor of Lymph Node Metastases and a Novel Prognostic Biomarker in Patients with Prostate Cancer. International Journal of Molecular Sciences. 2014; 15(7):11665-11677. https://doi.org/10.3390/ijms150711665
Chicago/Turabian StyleZhang, Hongtuan, Andi Wang, Shiyong Qi, Shang Cheng, Bing Yao, and Yong Xu. 2014. "Protein Tyrosine Kinase 7 (PTK7) as a Predictor of Lymph Node Metastases and a Novel Prognostic Biomarker in Patients with Prostate Cancer" International Journal of Molecular Sciences 15, no. 7: 11665-11677. https://doi.org/10.3390/ijms150711665




