The Canonical Notch Signaling Was Involved in the Regulation of Intestinal Epithelial Cells Apoptosis after Intestinal Ischemia/Reperfusion Injury
Abstract
:1. Introduction
2. Results
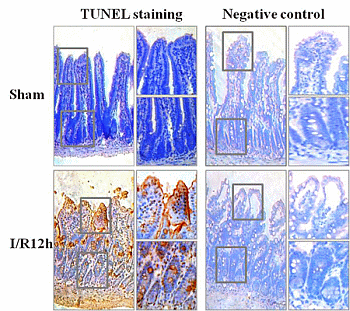
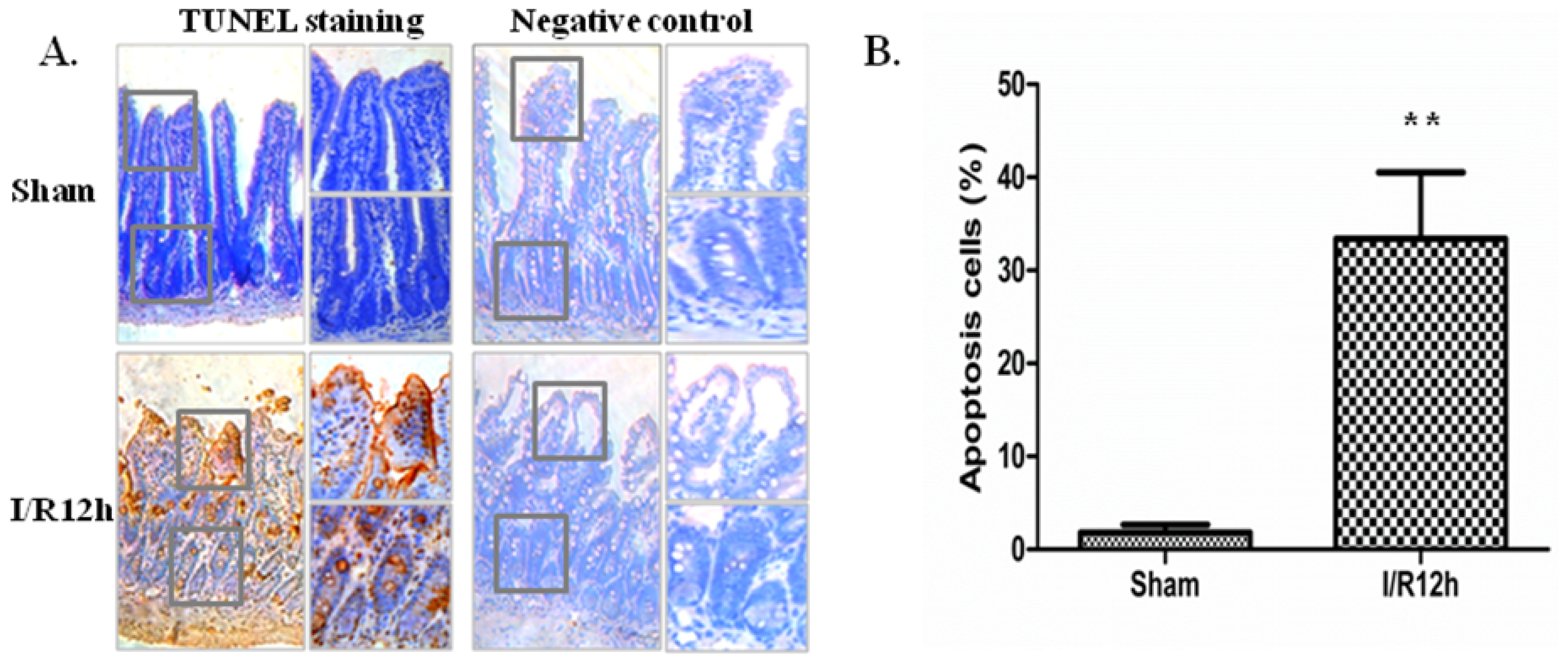
2.1. Intestinal I/R Injury Increased Apoptosis of Intestinal Epithelial Cells
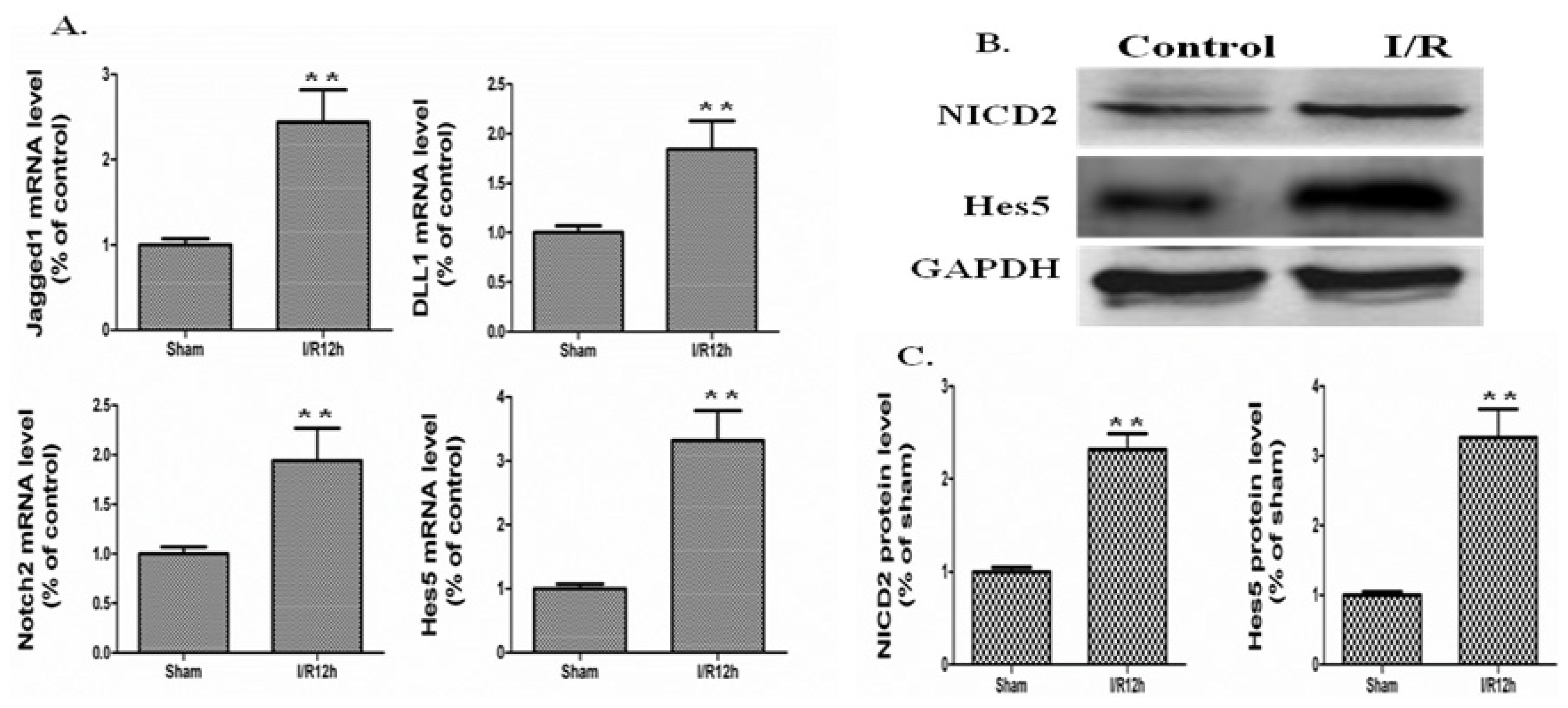
2.2. The Notch Signaling Was Activated in a Mouse Model of Intestinal I/R
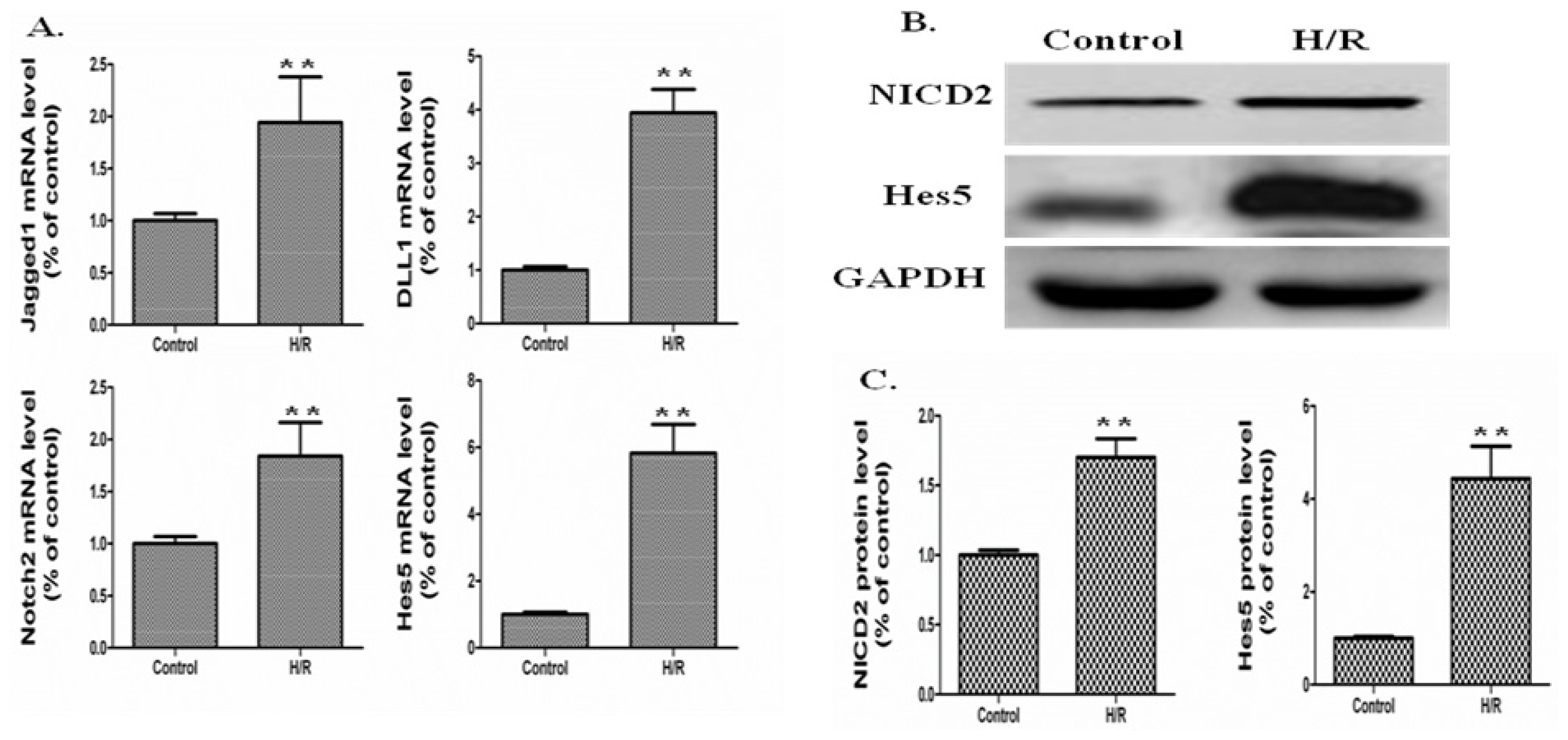
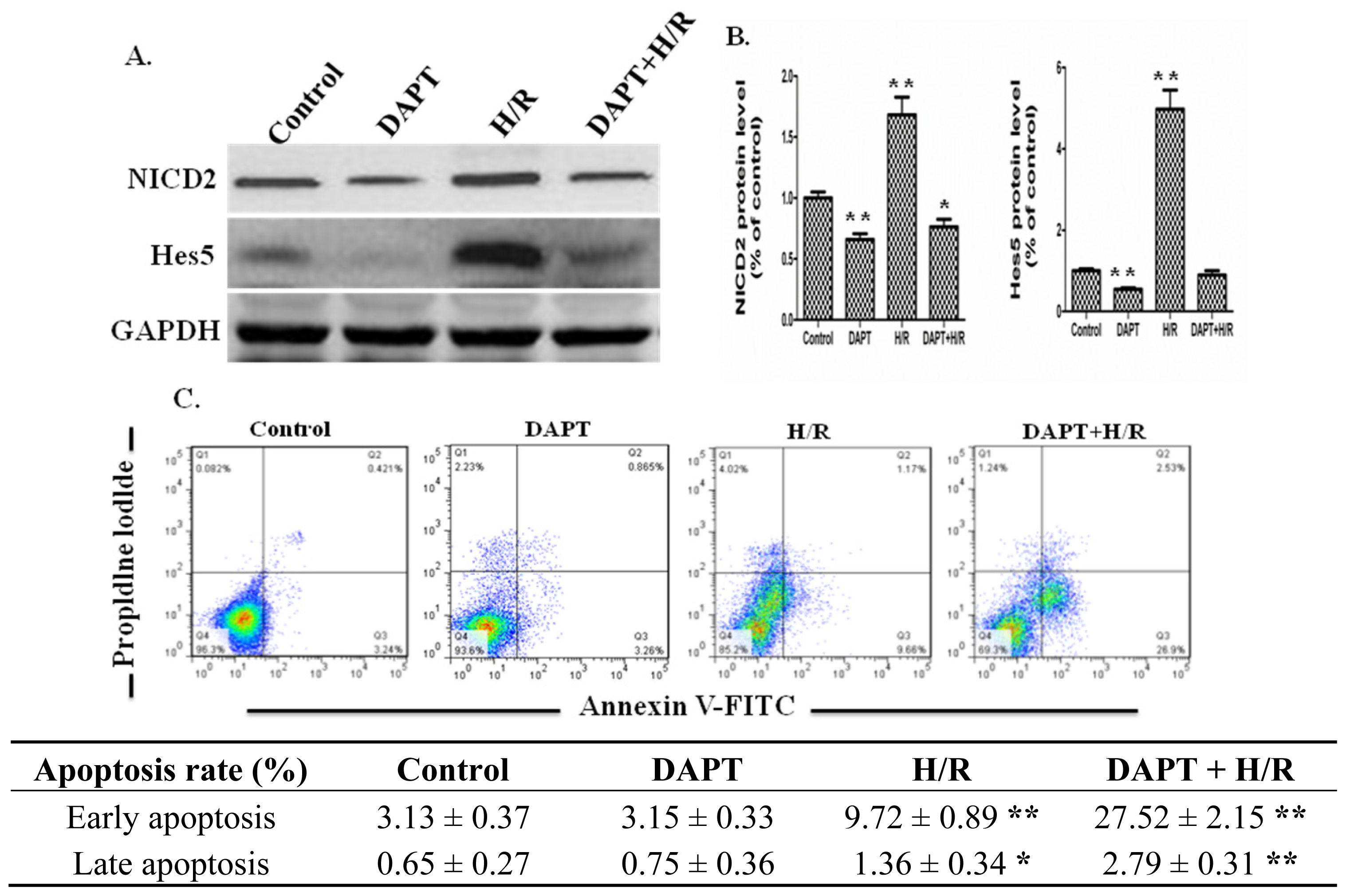
2.3. Effects of DAPT on the Apoptosis of IEC-6 Cells
2.4 Effects of Silencing RNA for Hes5 on the Apoptosis of IEC-6 Cells
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Cell Culture
4.3. Real-Time PCR Analysis
4.4. Western Blot Analysis
4.5. Silencing Hes5 Using siRNA and in Vitro Transfection
4.6. Detection of Epithelial Apoptosis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Deitch, E.A.; Xu, D.; Lu, Q. Gut lymph hypot Hesis of early shock and trauma-induced multiple organ dysfunction: A new look at gut origin of sepsis. J. Org. Dys 2006, 2, 70. [Google Scholar]
- An, S.C.; Hishikawa, Y.; Koji, T. Induction of cell death in rat small intestine by ischemia reperfusion: Differential roles of Fas/Fas ligand and Bcl-2/Bax systems depending upon cell types. Histochem. Cell Biol 2005, 123, 249–261. [Google Scholar]
- Higa, O.H.; Parra, E.R.; Ab’Saber, A.M.; Farhat, C.; Higa, R.; Capelozzi, V.L. Protective effects of ascorbic acid pretreatment in a rat model of intestinal ischemia-reperfusion injury: A histomorphometric study. Clinics 2007, 62, 315–320. [Google Scholar]
- Sukhotnik, I.; Brod, V.; Lurie, M.; Rahat, M.A.; Shnizer, S.; Lahat, N.; Mogilner, J.G.; Bitterman, H. The effect of 100% oxygen on intestinal preservation and recovery following ischemia-reperfusion injury in rats. Crit. Care Med 2009, 37, 1054–1061. [Google Scholar]
- Huang, C.Y.; Hsiao, J.K.; Lu, Y.Z.; Lee, T.C.; Yu, L.C.H. Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1 reduces epithelial barrier damage and bacterial translocation in intestinal ischemia. Lab. Investig 2011, 91, 294–309. [Google Scholar]
- Ban, K.; Peng, Z.; Kozar, R.A. Inhibition of ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS One 2013, 8, e76790. [Google Scholar]
- Feinman, R.; Deitch, E.A.; Watkins, A.C.; Abungu, B.; Colorado, I.; Kannan, K.B.; Sheth, S.U.; Caputo, F.J.; Lu, Q.; Ramanathan, M.; et al. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. Am. J. Physiol.-Gastr. L 2010, 299, G833–G843. [Google Scholar]
- Robinson, G.W. Using notches to track mammary epithelial cell homeostasis. Cell Stem Cell 2008, 3, 359–360. [Google Scholar]
- Ueo, T.; Imayoshi, I.; Kobayashi, T.; Ohtsuka, T.; Seno, H.; Nakase, H.; Chiba, T.; Kageyama, R. The role of Hes genes in intestinal development, homeostasis and tumor formation. Development 2012, 139, 1071–1082. [Google Scholar]
- Baron, M. An overview of the Notch signalling pathway. Semin. Cell Dev. Biol 2003, 14, 113–119. [Google Scholar]
- Bailey, A.M.; Posakony, J.W. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev 1995, 9, 2609–2622. [Google Scholar]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar]
- Yu, H.C.; Qin, H.Y.; He, F.; Wang, L.; Fu, W.; Liu, D.; Guo, F.C.; Liang, L.; Dou, K.F.; Han, H. Canonical Notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology 2011, 54, 979–988. [Google Scholar]
- Zhou, X.L.; Wan, L.; Xu, Q.R.; Zhao, Y.; Liu, J.C. Notch signaling activation contributes to cardioprotection provided by ischemic preconditioning and postconditioning. J. Transl. Med 2013, 11, 251. [Google Scholar]
- Chang, L.D.; Wong, F.; Niessen, K.; Karsan, A. Notch activation promotes endothelial survival through a PI3K-Slug axis. Microvasc. Res 2013, 89, 80–85. [Google Scholar]
- Duechler, M.; Shehata, M.; Schwarzmeier, J.D.; Hoelbl, A.; Hilgarth, M.; Hubmann, R. Induction of apoptosis by proteasome inhibitors in B-CLL cells is associated with downregulation of CD23 and inactivation of Notch 2. Leukemia 2005, 19, 260–267. [Google Scholar]
- Ma, M.M.; Wang, X.J.; Ding, X.B.; Teng, J.F.; Shao, F.M.; Zhang, J.W. Numb/Notch signaling plays an important role in cerebral ischemia-induced apoptosis. Neurochem. Res 2013, 38, 254–261. [Google Scholar]
- Yu, H.C.; Bai, L.; Yue, S.Q.; Wang, D.S.; Wang, L.; Han, H.; Dou, K.F. Notch signal protects non-parenchymal cells from ischemia/reperfusion injury in vitro in vitro by repressing ROS. Ann. Hepatol 2013, 12, 815–821. [Google Scholar]
- Chen, G.Q.; Qiu, Y.; Sun, L.H.; Yu, M.; Wang, W.S.; Xiao, W.D.; Yang, Y.; Liu, Y.; Yang, S.; Teitelbaum, D.H.; et al. The Jagged-2/Notch-1/Hes-1 Pathway Is Involved in Intestinal Epithelium Regeneration after Intestinal Ischemia-Reperfusion Injury. PLoS One 2013, 8, e76274. [Google Scholar]
- Schroder, N.; Gossler, A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr. Patterns 2002, 2, 247–250. [Google Scholar]
- Ban, K.; Kozar, R.A. Glutamine protects against apoptosis via downregulation of Sp3 in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol 2010, 299, G1344–G1353. [Google Scholar]
- Shima, Y.; Tajiri, T.; Taguchi, T.; Suita, S. Increased expression of c-fos and c-jun in the rat small intestinal epithelium after ischemia-reperfusion injury: A possible correlation with the proliferation or apoptosis of intestinal epithelial cells. J. Pediatr. Surg 2006, 41, 830–836. [Google Scholar]
- Itoh, H.; Yagi, M.; Hasebe, K.; Fushida, S.; Tani, T.; Hashimoto, T.; Shimizu, K.; Miwa, K. Regeneration of small intestinal mucosa after acute ischemia-reperfusion injury. Dig. Dis. Sci 2002, 47, 2704–2710. [Google Scholar]
- Zheng, S.Y.; Fu, X.B.; Xu, J.G.; Zhao, J.Y.; Sun, T.Z.; Chen, W. Inhibition of p38 mitogen-activated protein kinase may decrease intestinal epithelial cell apoptosis and improve intestinal epithelial barrier function after ischemia-reperfusion injury. World J. Gastroenterol 2005, 11, 656–660. [Google Scholar]
- Jin, X.L.; Zimmers, T.A.; Zhang, Z.X.; Pierce, R.H.; Koniaris, L.G. Interleukin-6 is an important in vivo in vivo inhibitor of intestinal epithelial cell death in mice. Gut 2010, 59, 186–196. [Google Scholar]
- Kuenzler, K.A.; Pearson, P.Y.; Schwartz, M.Z. Hepatoctye growth factor pretreatment reduces apoptosis and mucosal damage after intestinal ischemia-reperfusion. J. Pediatr. Surg 2002, 37, 1093–1097. [Google Scholar]
- Cai, Y.J.; Wang, W.S.; Liang, H.Y.; Sun, L.H.; Teitelbaum, D.H.; Yang, H. Keratinocyte growth factor improves epithelial structure and function in a mouse model of intestinal ischemia/reperfusion. PLoS One 2012, 7, e44772. [Google Scholar]
- van Es, J.H.; van Gijn, M.E.; Riccio, O.; van den Born, M.; Vooijs, M.; Begthel, H.; Cozijnsen, M.; Robine, S.; Winton, D.J.; Radtke, F.; et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005, 435, 959–963. [Google Scholar]
- Wong, G.T.; Manfra, D.; Poulet, F.M.; Zhang, Q.; Josien, H.; Bara, T.; Engstrom, L.; Pinzon-Ortiz, M.; Fine, J.S. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J. Biol. Chem 2004, 279, 12876–12882. [Google Scholar]
- Milano, J.; McKay, J.; Dagenais, C.; Foster-Brown, L.; Pognan, F.; Gadient, R.; Jacobs, R.T.; Zacco, A.; Greenberg, B.; Ciaccio, P.J. Modulation of Notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci 2004, 82, 341–358. [Google Scholar]
- Taguchi, T.; Shima, Y.; Nakao, M.; Fujii, Y.; Tajiri, T.; Ogita, K.; Suita, S. Activation of immediate early genes in relation to proliferation and apoptosis of enterocytes after ischemia-reperfusion injury of small intestine. Transplant. Proc 2002, 34, 983. [Google Scholar]
- Chen, G.Q.; Sun, L.H.; Yu, M.; Meng, D.; Wang, W.S.; Yang, Y.; Yang, H. The Jagged-1/Notch-1/Hes-1 Pathway Is Involved in Intestinal Adaptation in a Massive Small Bowel Resection Rat Model. Dig. Dis. Sci 2013, 58, 2478–2486. [Google Scholar]
- Sander, G.R.; Powell, B.C. Expression of Notch receptors and ligands in the adult gut. J. Histochem. Cytochem 2004, 52, 509–516. [Google Scholar]
- Qiu, Y.; Yu, M.; Yang, Y.; Sheng, H.; Wang, W.; Sun, L.; Chen, G.; Liu, Y.; Xiao, W.; Yang, H. Disturbance of intraepithelial lymphocytes in a murine model of acute intestinal ischemia/reperfusion. J. Mol. Histol 2013, 45, 217–227. [Google Scholar]

© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, G.; Zhang, Z.; Cheng, Y.; Xiao, W.; Qiu, Y.; Yu, M.; Sun, L.; Wang, W.; Du, G.; Gu, Y.; et al. The Canonical Notch Signaling Was Involved in the Regulation of Intestinal Epithelial Cells Apoptosis after Intestinal Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2014, 15, 7883-7896. https://doi.org/10.3390/ijms15057883
Chen G, Zhang Z, Cheng Y, Xiao W, Qiu Y, Yu M, Sun L, Wang W, Du G, Gu Y, et al. The Canonical Notch Signaling Was Involved in the Regulation of Intestinal Epithelial Cells Apoptosis after Intestinal Ischemia/Reperfusion Injury. International Journal of Molecular Sciences. 2014; 15(5):7883-7896. https://doi.org/10.3390/ijms15057883
Chicago/Turabian StyleChen, Guoqing, Zhicao Zhang, Yingdong Cheng, Weidong Xiao, Yuan Qiu, Min Yu, Lihua Sun, Wensheng Wang, Guangsheng Du, Yingchao Gu, and et al. 2014. "The Canonical Notch Signaling Was Involved in the Regulation of Intestinal Epithelial Cells Apoptosis after Intestinal Ischemia/Reperfusion Injury" International Journal of Molecular Sciences 15, no. 5: 7883-7896. https://doi.org/10.3390/ijms15057883