Maternal High Folic Acid Supplement Promotes Glucose Intolerance and Insulin Resistance in Male Mouse Offspring Fed a High-Fat Diet
Abstract
:1. Introduction
2. Results
2.1. Maternal Status and Folate Concentrations
2.2. Body Weight, Body Composition and Dietary Intake
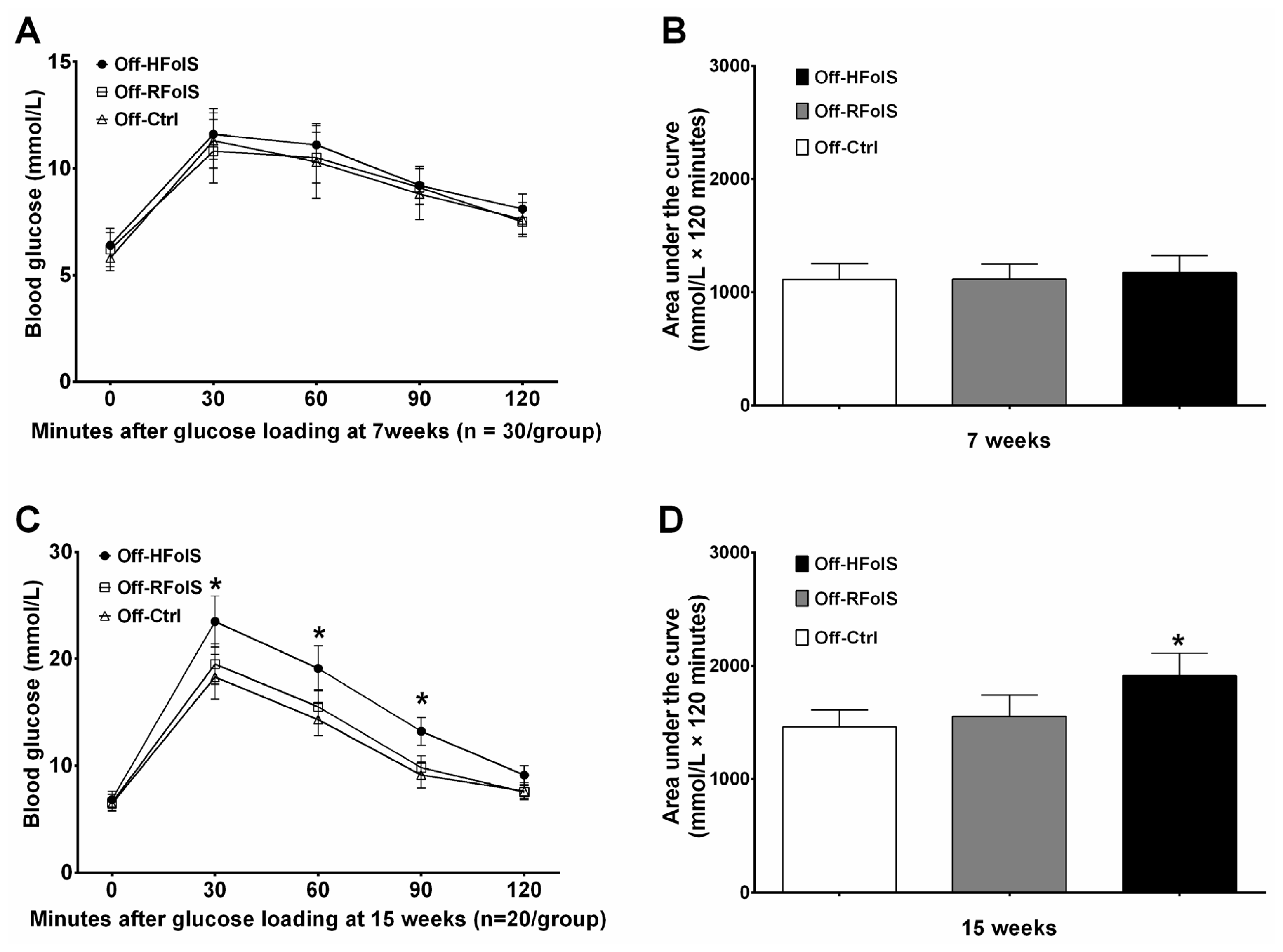
2.3. Glucose Response, Insulin Resistance and Lipid Variables
2.4. Inflammatory Factors and Adipokines
2.5. Global Methylation
3. Discussion
3.1. FolS, Growth and Development
3.2. FolS, Insulin Resistance, Inflammatory Cytokines and Adipokines
3.3. FolS and DNA Methylation
3.4. Clinical Relevance
3.5. Limitations and Strengths
4. Experimental Section
4.1. Selection of Folic Acid Dose
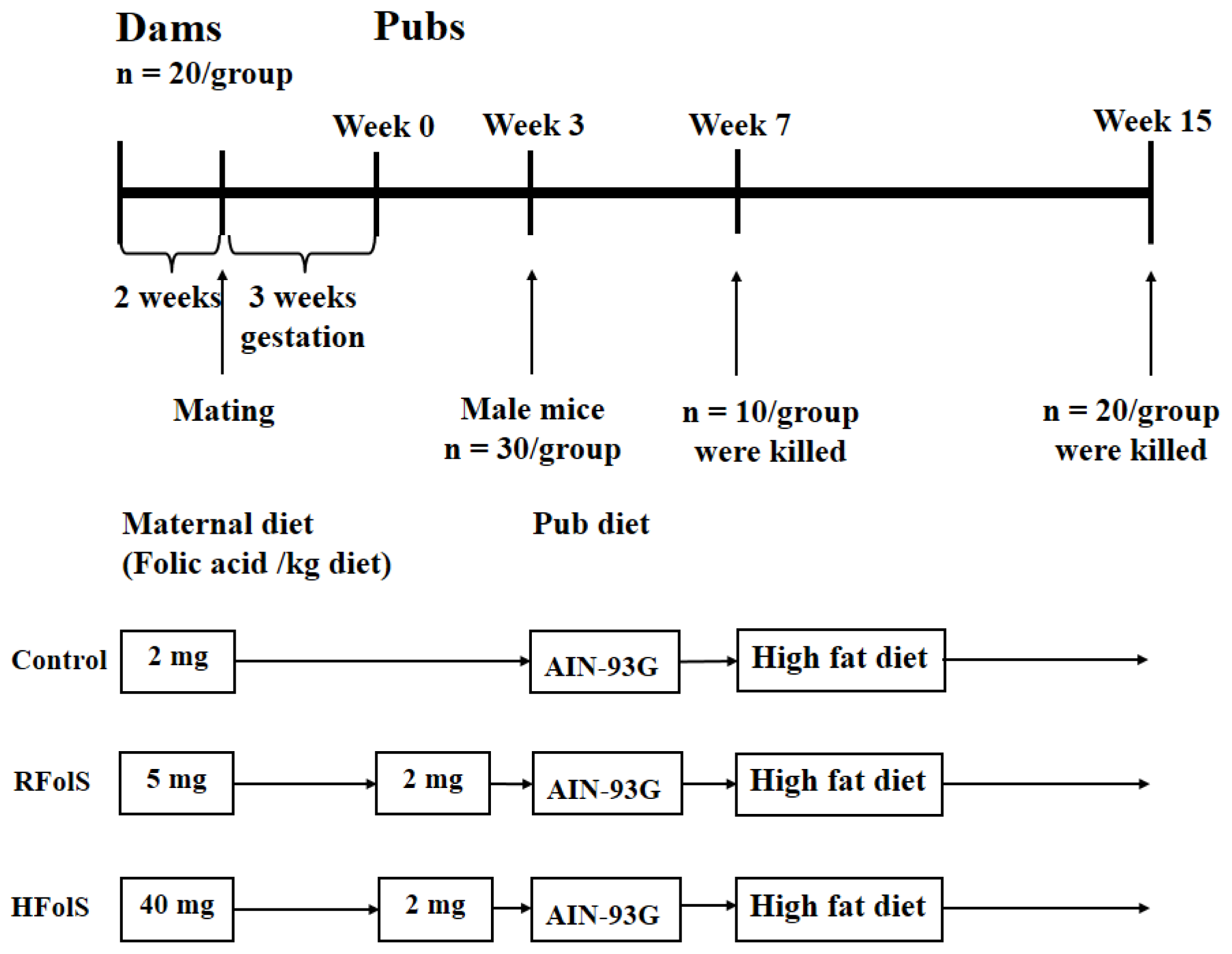
4.2. Animals and Design
4.3. Observational Parameters and Glucose Tolerance Test
4.4. Sampling and Biomedical Measurements
4.5. Quantitative Real-Time PCR
4.6. Global Methylation
4.7. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-15-06298-s001.pdfAcknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| FolS | folic acid supplement |
| HFD | high-fat diet |
| HFolS | high folic acid supplement |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| IL-6 | interleukin-6 |
| Off-Ctrl | offspring of the control dams |
| Off-HFolS | offspring of the high folic acid supplement dams |
| Off-RFolS | offspring of the recommended folic acid supplement dams |
| RFolS | recommended folic acid supplement |
| TNF-α | tumour necrosis factor-α |
- Author ContributionsChanghao Sun, Ying Li and Yifan Huang designed the research; Yifan Huang and Xiaowei Sun conducted the research; Yifan Huang, Yonghan He, Xiaowei Sun and Yujie He analyzed and interpreted the data; Yifan Huang wrote the original manuscript; Yifan Huang and Yonghan He modified and revised the manuscript. Yifan Huang and Changhao Sun had primary responsibility for final content. All authors read and approved the final manuscript.
References
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract 2011, 94, 311–321. [Google Scholar]
- Winzell, M.S.; Ahren, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53, S215–S219. [Google Scholar]
- Ojha, S.; Saroha, V.; Symonds, M.E.; Budge, H. Excess nutrient supply in early life and its later metabolic consequences. Clin. Exp. Pharmacol. Physiol 2013, 40, 817–823. [Google Scholar]
- Jones, R.H.; Ozanne, S.E. Intra-uterine origins of type 2 diabetes. Arch. Physiol. Biochem 2007, 113, 25–29. [Google Scholar]
- Haggarty, P.; Hoad, G.; Campbell, D.M.; Horgan, G.W.; Piyathilake, C.; McNeill, G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr 2013, 97, 94–99. [Google Scholar]
- Fekete, K.; Berti, C.; Trovato, M.; Lohner, S.; Dullemeijer, C.; Souverein, O.W.; Cetin, I.; Decsi, T. Effect of folate intake on health outcomes in pregnancy: A systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr. J 2012, 11, 75. [Google Scholar]
- Burdge, G.C.; Lillycrop, K.A. Folic acid supplementation in pregnancy: Are there devils in the detail? Br. J. Nutr 2012, 108, 1924–1930. [Google Scholar]
- Pickell, L.; Brown, K.; Li, D.; Wang, X.L.; Deng, L.; Wu, Q.; Selhub, J.; Luo, L.; Jerome-Majewska, L.; Rozen, R. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res. Part A 2011, 91, 8–19. [Google Scholar]
- Steegers-Theunissen, R.P.; Obermann-Borst, S.A.; Kremer, D.; Lindemans, J.; Siebel, C.; Steegers, E.A.; Slagboom, P.E.; Heijmans, B.T. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One 2009, 4, e7845. [Google Scholar]
- Hoyo, C.; Murtha, A.P.; Schildkraut, J.M.; Jirtle, R.L.; Demark-Wahnefried, W.; Forman, M.R.; Iversen, E.S.; Kurtzberg, J.; Overcash, F.; Huang, Z.; et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011, 6, 928–936. [Google Scholar]
- Dunstan, J.A.; West, C.; McCarthy, S.; Metcalfe, J.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; D’Vaz, N.; Prescott, S.L. The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy 2012, 67, 50–57. [Google Scholar]
- Ly, A.; Lee, H.; Chen, J.; Sie, K.K.; Renlund, R.; Medline, A.; Sohn, K.J.; Croxford, R.; Thompson, L.U.; Kim, Y.I. Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer Res 2011, 71, 988–997. [Google Scholar]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. Biol 1998, 12, 949–957. [Google Scholar]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar]
- Reza Lopez, S.A.; Poon, A.N.; Szeto, I.M.; Ma, D.W.; Anderson, G.H. High multivitamin intake during pregnancy and postweaning obesogenic diets interact to affect the relationship between expression of PPAR genes and glucose regulation in the offspring. J. Nutr. Biochem 2013, 24, 877–881. [Google Scholar]
- Szeto, I.M.; Das, P.J.; Aziz, A.; Anderson, G.H. Multivitamin supplementation of Wistar rats during pregnancy accelerates the development of obesity in offspring fed an obesogenic diet. Int. J. Obes 2009, 33, 364–372. [Google Scholar]
- Szeto, I.M.; Aziz, A.; Das, P.J.; Taha, A.Y.; Okubo, N.; Reza-Lopez, S.; Giacca, A.; Anderson, G.H. High multivitamin intake by Wistar rats during pregnancy results in increased food intake and components of the metabolic syndrome in male offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol 2008, 295, R575–R582. [Google Scholar]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr. Bull 2008, 29, S101–S111, discussion S112–S115. [Google Scholar]
- Burdge, G.C.; Lillycrop, K.A.; Jackson, A.A.; Gluckman, P.D.; Hanson, M.A. The nature of the growth pattern and of the metabolic response to fasting in the rat are dependent upon the dietary protein and folic acid intake of their pregnant dams and post-weaning fat consumption. Br. J. Nutr 2008, 99, 540–549. [Google Scholar]
- Huot, P.S.; Dodington, D.W.; Mollard, R.C.; Reza-Lopez, S.A.; Sanchez-Hernandez, D.; Cho, C.E.; Kuk, J.; Ward, W.E.; Anderson, G.H. High folic acid Intake during pregnancy lowers body weight and REduces femoral area and strength in female rat offspring. J. Osteoporos 2013, 2013, 154109. [Google Scholar]
- Reaven, G.M. Insulin resistance: The link between obesity and cardiovascular disease. Med. Clin. N. Am 2011, 95, 875–892. [Google Scholar]
- Cho, C.E.; Sanchez-Hernandez, D.; Reza-Lopez, S.A.; Huot, P.S.; Kim, Y.I.; Anderson, G.H. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics 2013, 8, 710–719. [Google Scholar]
- Krishnaveni, G.V.; Veena, S.R.; Karat, S.C.; Yajnik, C.S.; Fall, C.H. Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia 2014, 57, 110–121. [Google Scholar]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev./Aust. Assoc. Clin. Biochem 2005, 26, 19–39. [Google Scholar]
- Arner, P. Insulin resistance in type 2 diabetes—Role of the adipokines. Curr. Mol. Med 2005, 5, 333–339. [Google Scholar]
- Luo, Z.C.; Nuyt, A.M.; Delvin, E.; Fraser, W.D.; Julien, P.; Audibert, F.; Girard, I.; Shatenstein, B.; Deal, C.; Grenier, E.; et al. Maternal and fetal leptin, adiponectin levels and associations with fetal insulin sensitivity. Obesity 2013, 21, 210–216. [Google Scholar]
- de Leal, V.O.; Mafra, D. Adipokines in obesity. Clin. Chim. Acta 2013, 419, 87–94. [Google Scholar]
- Pittas, A.G.; Joseph, N.A.; Greenberg, A.S. Adipocytokines and insulin resistance. J. Clin. Endocrinol. Metab 2004, 89, 447–452. [Google Scholar]
- Wijesekara, N.; Krishnamurthy, M.; Bhattacharjee, A.; Suhail, A.; Sweeney, G.; Wheeler, M.B. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J. Biol. Chem 2010, 285, 33623–33631. [Google Scholar]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig 2006, 116, 1784–1792. [Google Scholar]
- Ryan, A.S.; Macko, R.F.; Peters, M.N.; Ivey, F.M.; Prior, S.J.; Joseph, L.J.; Hafer-Macko, C.E. Plasma adiponectin levels are associated with insulin sensitivity in stroke survivors. J. Stroke Cerebrovasc. Dis 2009, 18, 214–220. [Google Scholar]
- Abdelgadir, M.; Karlsson, A.F.; Berglund, L.; Berne, C. Low serum adiponectin concentrations are associated with insulin sensitivity independent of obesity in Sudanese subjects with type 2 diabetes mellitus. Diabetol. Metab. Syndr 2013, 5, 15. [Google Scholar]
- Yano, W.; Kubota, N.; Itoh, S.; Kubota, T.; Awazawa, M.; Moroi, M.; Sugi, K.; Takamoto, I.; Ogata, H.; Tokuyama, K.; et al. Molecular mechanism of moderate insulin resistance in adiponectin-knockout mice. Endocr. J 2008, 55, 515–522. [Google Scholar]
- Robertson, K.D.; Jones, P.A. DNA methylation: Past, present and future directions. Carcinogenesis 2000, 21, 461–467. [Google Scholar]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr 2012, 3, 21–38. [Google Scholar]
- Yates, A.A.; Schlicker, S.A.; Suitor, C.W. Dietary Reference Intake: The new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J. Am. Diet. Assoc 1998, 98, 699–706. [Google Scholar]
- US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina bIfida and Other Neural Tube Defects; US Department of Health and Human Services, Public Health Service, Centers for Disease Control: Atlanta, GA, USA, 1992.
- Butterworth, C.E., Jr.; Tamura, T. Folic acid safety and toxicity: A brief review. Am. J. Clin. Nutr 1989, 50, 353–358. [Google Scholar]
- Reeves, P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr 1997, 127, 838S–841S. [Google Scholar]
- Kim, Y.I. Folic acid supplementation and cancer risk: Point. Cancer Epidemiol. Biomark. Prev 2008, 17, 2220–2225. [Google Scholar]
- Li, S.; Li, Y.; Ning, H.; Na, L.; Niu, Y.; Wang, M.; Feng, R.; Liu, L.; Guo, F.; Hou, S.; et al. Calcium supplementation increases circulating cholesterol by reducing its catabolism via GPER and TRPC1-dependent pathway in estrogen deficient women. Int. J. Cardiol 2013, 168, 2548–2560. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol 2010, 11, 74. [Google Scholar]


| Variable | Off-Ctrl | Off-RFolS | Off-HFolS | p |
|---|---|---|---|---|
| Litter size | 6.2 ± 0.9 | 5.8 ± 1.2 | 6.1 ± 1.0 | 0.454 |
| 7 weeks | ||||
| Numbers | 10 | 10 | 10 | |
| Intra-abdominal fat pad, g | 0.31 ± 0.03 | 0.34 ± 0.03 | 0.32 ± 0.04 | 0.147 |
| Subcutaneous fat pad, g | 0.13 ± 0.02 | 0.13 ± 0.01 | 0.14 ± 0.02 | 0.344 |
| Adiposity index, % | 1.99 ± 0.21 | 2.17 ± 0.20 | 2.13 ± 0.19 | 0.127 |
| Fasting glucose, mmol/L | 5.94 ± 0.83 | 6.15 ± 0.61 | 6.39 ± 0.81 | 0.424 |
| Insulin, mIU/L | 7.31 ± 1.53 | 7.57 ± 1.13 | 7.67 ± 1.27 | 0.822 |
| HOMA-IR | 1.93 ± 0.50 | 2.08 ± 0.42 | 2.20 ± 0.54 | 0.475 |
| Total cholesterol, mmol/L | 2.67 ± 0.52 | 2.59 ± 0.77 | 3.09 ± 0.85 | 0.273 |
| HDLC, mmol/L | 1.73 ± 0.21 | 1.67 ± 0.21 | 1.60 ± 0.14 | 0.323 |
| LDLC, mmol/L | 0.60 ± 0.10 | 0.61 ± 0.05 | 0.63 ± 0.10 | 0.735 |
| TNF-α, pg/mL | 18.42 ± 1.44 | 18.55 ± 0.97 | 19.84 ± 1.73 | 0.063 |
| IL-6, pg/mL | 15.41 ± 1.12 | 16.13 ± 0.86 | 15.92 ± 1.04 | 0.279 |
| Leptin, pg/mL | 461.73 ± 38.15 | 487.47 ± 69.33 | 512.82 ± 78.71 | 0.226 |
| Adiponectin, mg/L | 8.93 ± 0.97 | 8.37 ± 0.84 | 7.72 ± 1.12 * | 0.035 |
| 15 weeks | ||||
| Numbers | 20 | 20 | 20 | |
| Intra-abdominal fat pad, g | 0.78 ± 0.08 | 0.82 ± 0.07 | 0.87 ± 0.08 * | 0.002 |
| Subcutaneous fat pad, g | 0.47 ± 0.05 | 0.44 ± 0.03 | 0.59 ± 0.06 * | <0.001 |
| Adiposity index, % | 3.41 ± 0.33 | 3.45 ± 0.38 | 3.68 ± 0.41 | 0.057 |
| Fasting glucose, mmol/L | 6.33 ± 0.87 | 6.47 ± 0.66 | 6.82 ± 0.68 | 0.109 |
| Insulin, mIU/L | 7.66 ± 1.21 | 7.71 ± 1.30 | 24.49 ± 4.47 * | <0.001 |
| HOMA-IR | 2.16 ± 0.43 | 2.22 ± 0.42 | 7.39 ± 1.41 * | <0.001 |
| Total cholesterol, mmol/L | 3.98 ± 0.41 | 3.97 ± 0.30 | 4.12 ± 0.43 | 0.392 |
| HDLC, mmol/L | 1.61 ± 0.18 | 1.62 ± 0.22 | 1.63 ± 0.19 | 0.950 |
| LDLC, mmol/L | 0.58 ± 0.10 | 0.58 ± 0.07 | 0.61 ± 0.08 | 0.435 |
| TNF-α, pg/mL | 22.82 ± 3.12 | 22.91 ± 2.84 | 24.58 ± 2.41 | 0.091 |
| IL-6, pg/mL | 20.35 ± 1.84 | 21.31 ± 1.47 | 21.54 ± 2.01 | 0.092 |
| Leptin, pg/mL | 867.40 ± 102.62 | 843.92 ± 88.75 | 916.26 ± 92.38 | 0.056 |
| Adiponectin, mg/L | 6.68 ± 0.76 | 6.20 ± 0.73 | 5.21 ± 0.52 * | <0.001 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, Y.; He, Y.; Sun, X.; He, Y.; Li, Y.; Sun, C. Maternal High Folic Acid Supplement Promotes Glucose Intolerance and Insulin Resistance in Male Mouse Offspring Fed a High-Fat Diet. Int. J. Mol. Sci. 2014, 15, 6298-6313. https://doi.org/10.3390/ijms15046298
Huang Y, He Y, Sun X, He Y, Li Y, Sun C. Maternal High Folic Acid Supplement Promotes Glucose Intolerance and Insulin Resistance in Male Mouse Offspring Fed a High-Fat Diet. International Journal of Molecular Sciences. 2014; 15(4):6298-6313. https://doi.org/10.3390/ijms15046298
Chicago/Turabian StyleHuang, Yifan, Yonghan He, Xiaowei Sun, Yujie He, Ying Li, and Changhao Sun. 2014. "Maternal High Folic Acid Supplement Promotes Glucose Intolerance and Insulin Resistance in Male Mouse Offspring Fed a High-Fat Diet" International Journal of Molecular Sciences 15, no. 4: 6298-6313. https://doi.org/10.3390/ijms15046298





