Substance P Activates the Wnt Signal Transduction Pathway and Enhances the Differentiation of Mouse Preosteoblastic MC3T3-E1 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
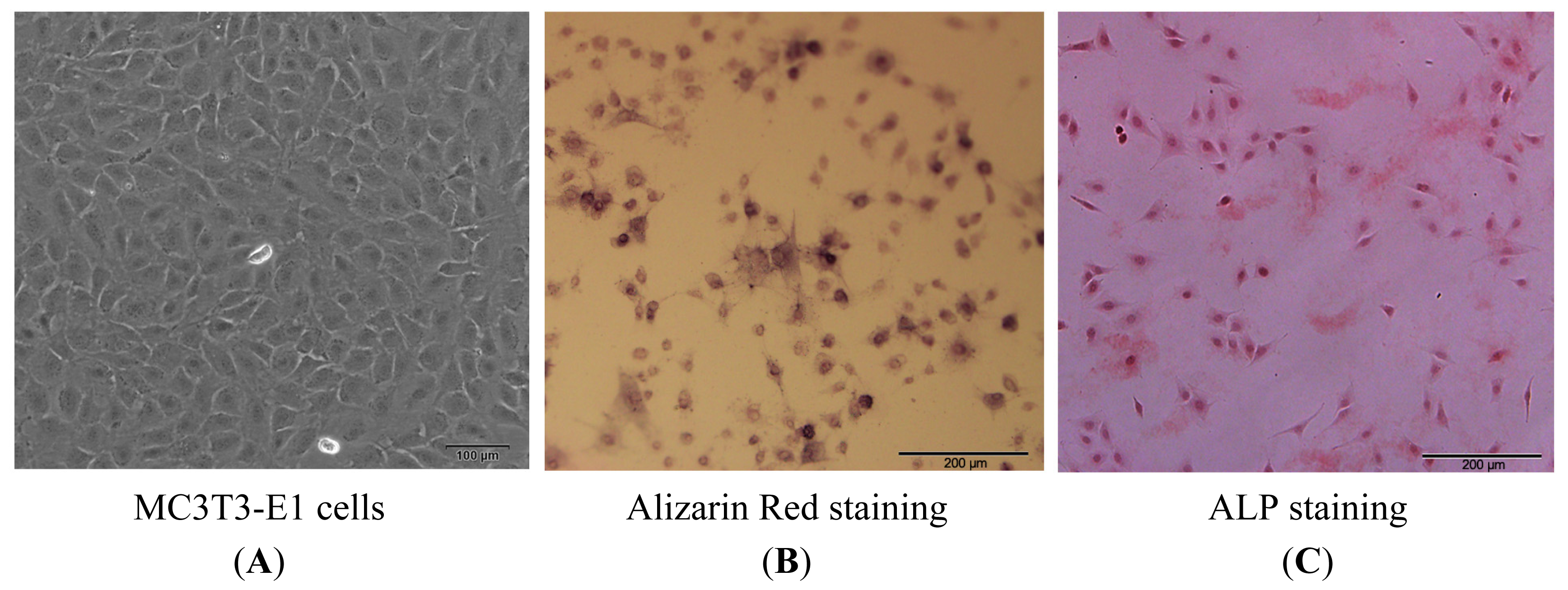
2.1.1. Isolation, Screening, Alkaline Phosphatase (ALP) and Alizarin Red Staining of MC3T3-E1 Cells
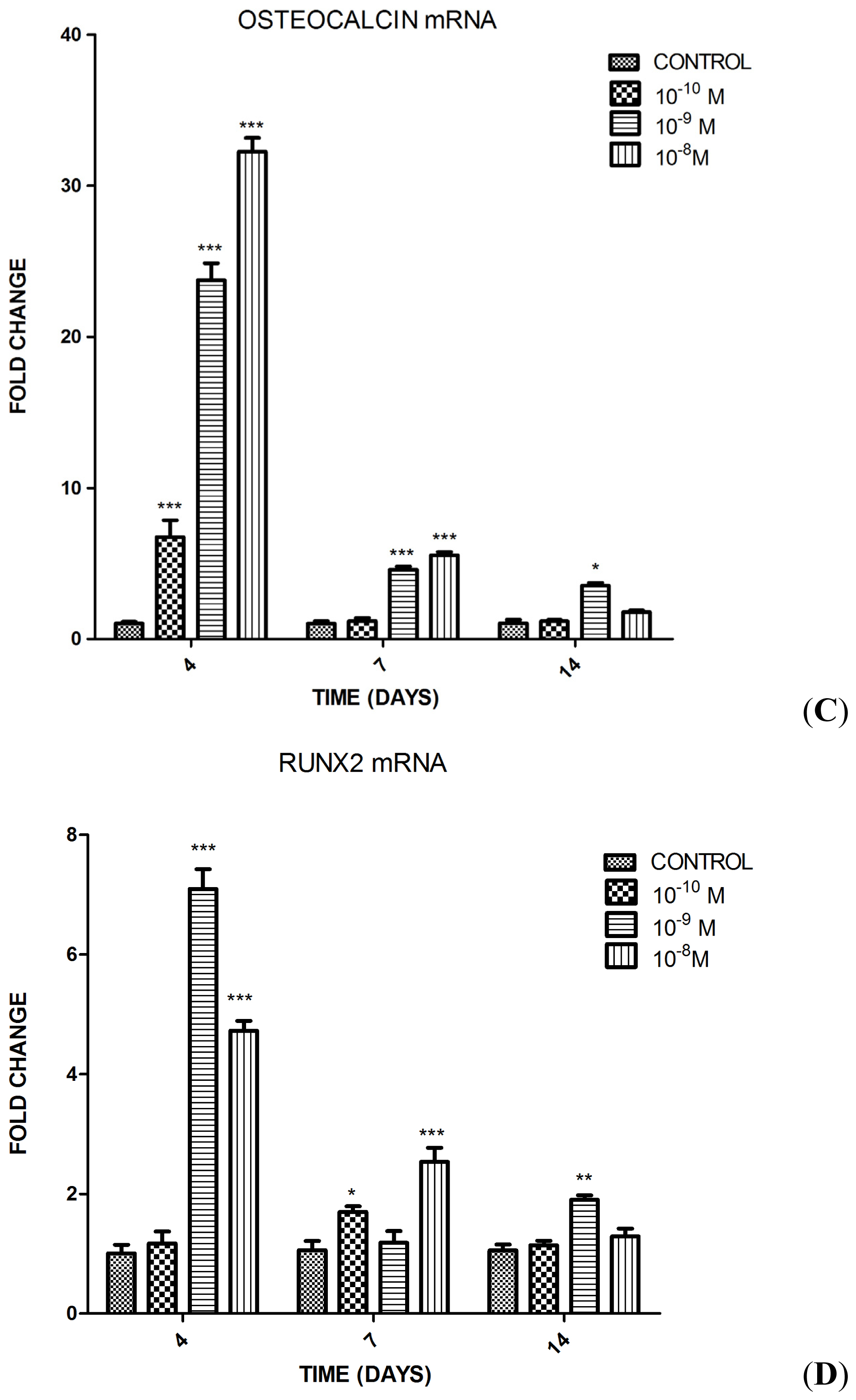
2.1.2. Influence of Substance P (SP) at Different Concentrations on Differentiation of the MC3T3-E1 Cells into Osteoblasts
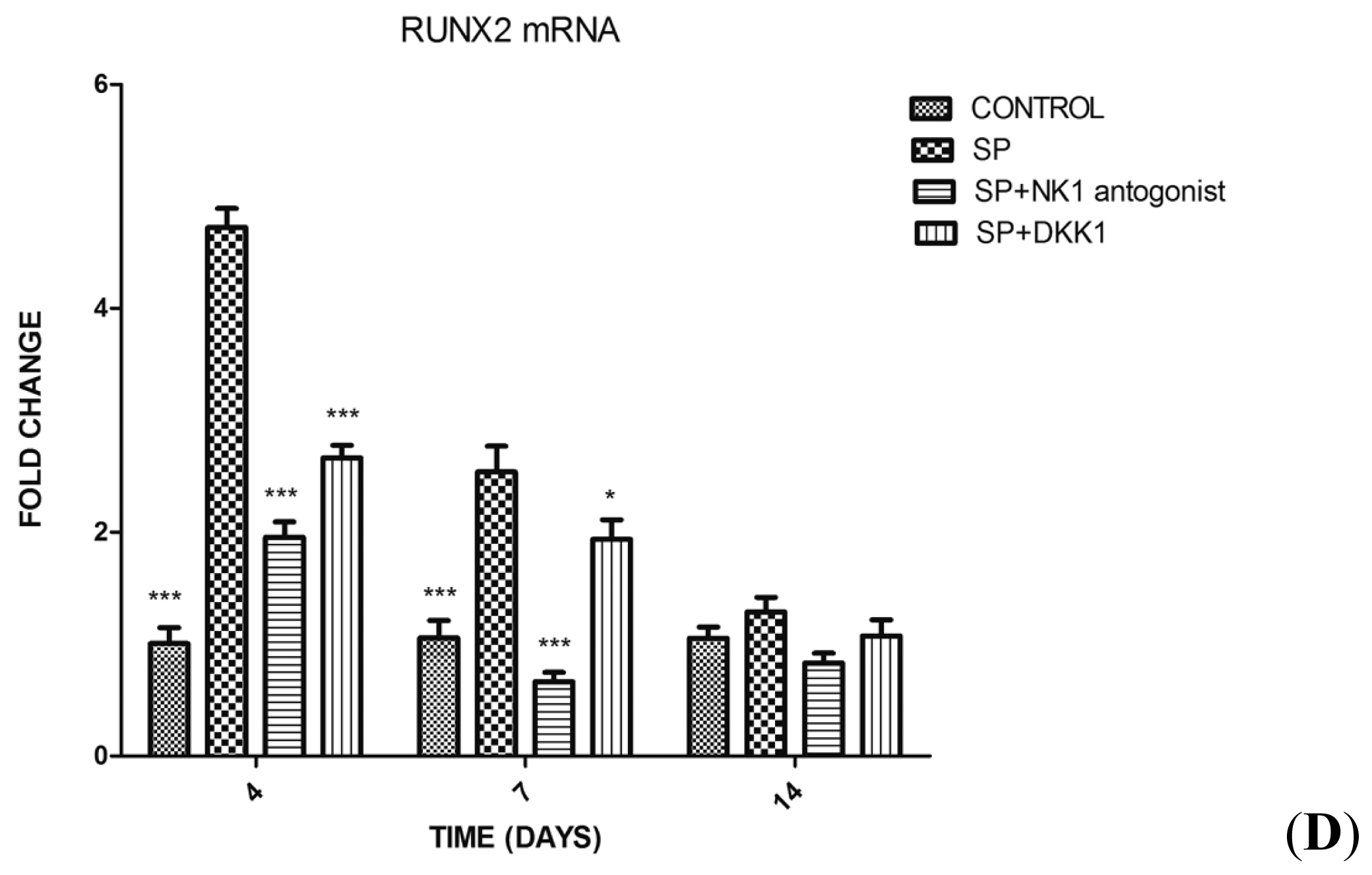
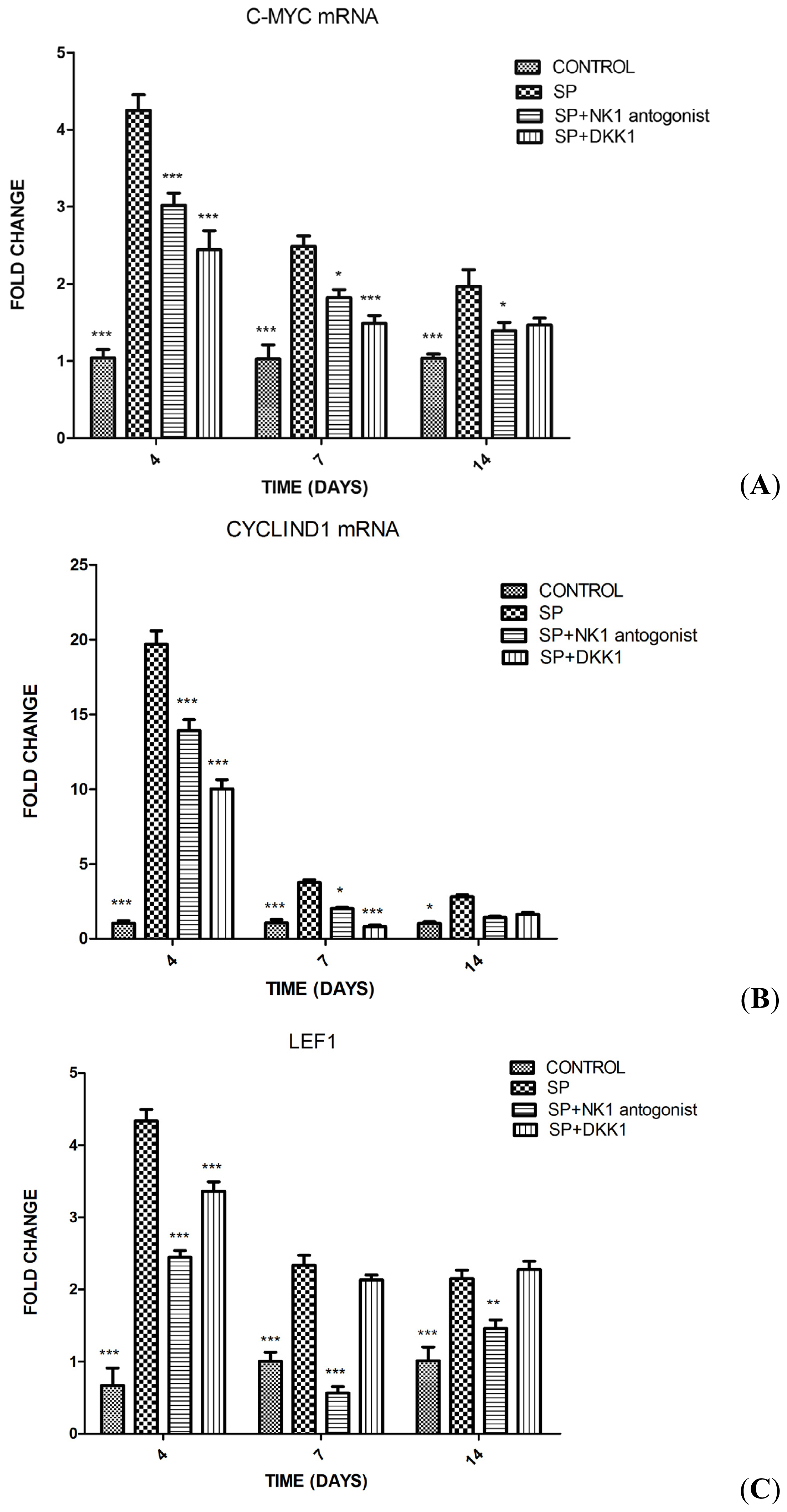
2.1.3. Impact of SP, Neurokinin-1 (NK1) Antagonist and Dickkopf-1 (DKK1) on the Differentiation of MC3T3-E1 Cells into Osteoblasts
2.1.4. Impact of SP, NK1 Antagonist and DKK1 on the Expression of Genes of the Wnt/β-Catenin Signaling Pathway
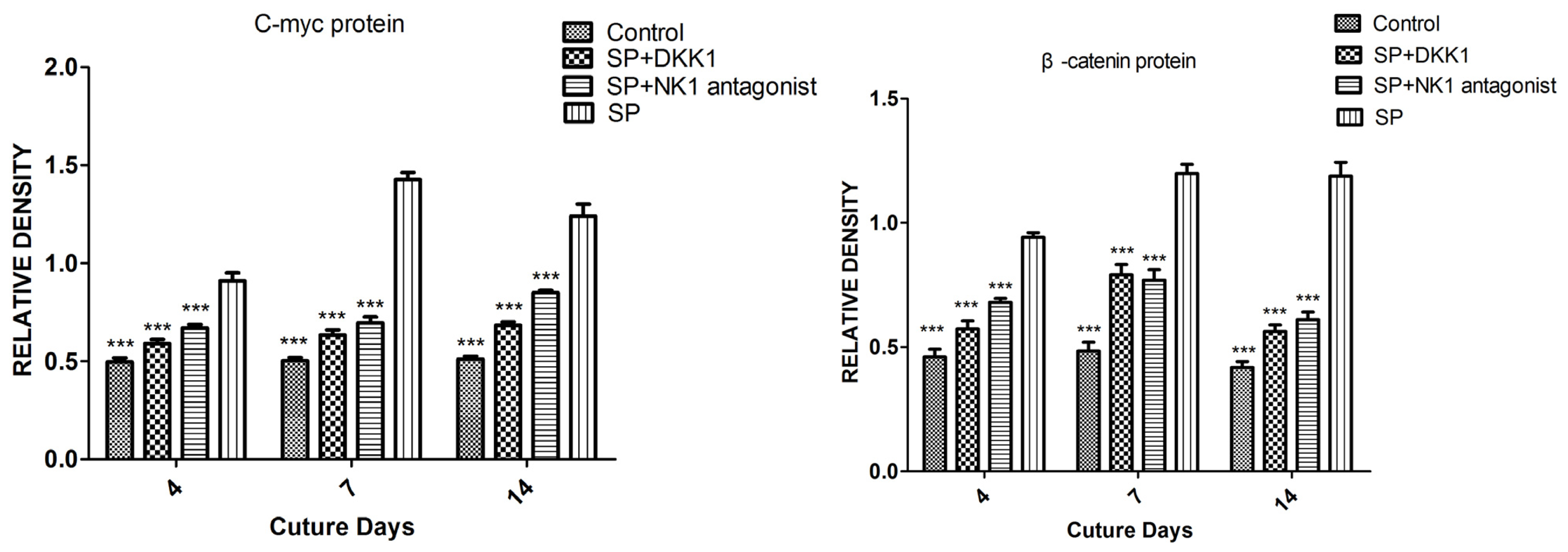
2.1.5. Impact of SP, NK1 Antagonist and DKK1 on the Protein Levels of the Wnt/β-Catenin Signaling Pathway
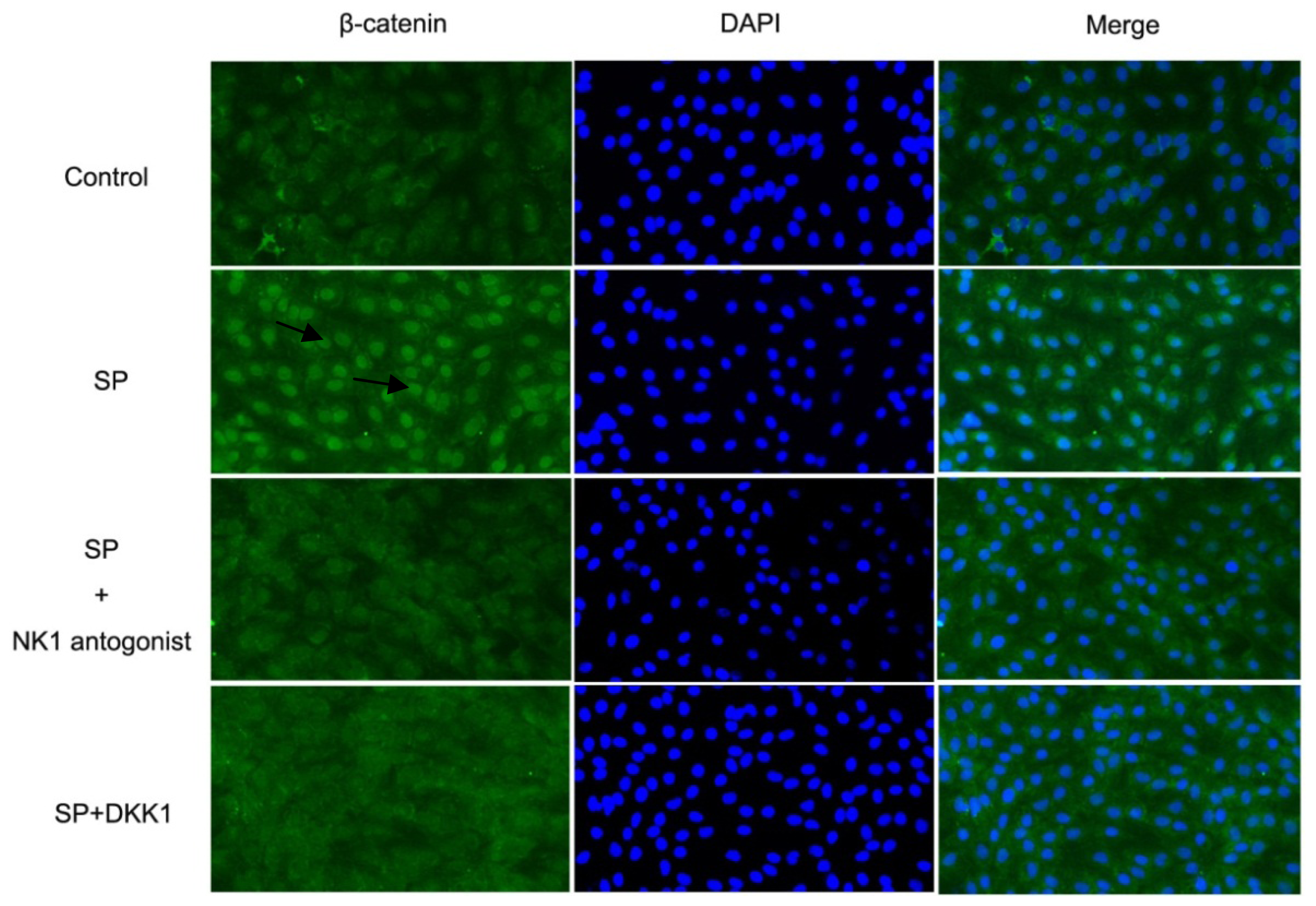
2.1.6. Impact of SP, NK1 Antagonist and DKK1 on β-Catenin Activation in MC3T3-E1 Cells
2.2. Discussion
3. Experimental Section
3.1. Cell Culture
3.2. Alizarin Red Staining and Alkaline Phosphatase Staining
3.3. Quantitative Polymerase Chain Reaction (qPCR)
3.4. Protein Extraction and Western Blot
3.5. Immunofluorescence Staining
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsGang Mei and Dan Jin conceived the project and wrote the first draft. All authors contributed to the analysis and interpretations of date and to the writing of subsequent drafts. All authors have seen and approved the final version of the manuscript. All authors read and approved the final manuscript.
References
- Bjurholm, A.; Kreicbergs, A.; Brodin, E.; Schultzberg, M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides 1988, 9, 165–171. [Google Scholar]
- Lundberg, P.; Lundgren, I.; Mukohyama, H.; Lehenkari, P.P.; Horton, M.A.; Lerner, U.H. Vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase-activating peptide receptor subtypes in mouse calvarial osteoblasts: Presence of VIP-2 receptors and differentiation-induced expression of VIP-1 receptors. Endocrinology 2001, 142, 339–347. [Google Scholar]
- Tuo, Y.; Guo, X.; Zhang, X.; Wang, Z.; Zhou, J.; Xia, L.; Zhang, Y.; Wen, J.; Jin, D. The biological effects and mechanisms of calcitonin gene-related peptide on human endothelial cell. J. Recept. Signal Transduct. Res 2013, 33, 114–123. [Google Scholar]
- An, Y.S.; Lee, E.; Kang, M.H.; Hong, H.S.; Kim, M.R.; Jang, W.S.; Son, Y.; Yi, J.Y. Substance P stimulates the recovery of bone marrow after the irradiation. J. Cell. Physiol 2011, 226, 1204–1213. [Google Scholar]
- Malkesman, O.; Braw, Y.; Weller, A. Assessment of antidepressant and anxiolytic properties of NK1 antagonists and substance P in Wistar Kyoto rats. Physiol. Behav 2007, 90, 619–625. [Google Scholar]
- Delgado, A.V.; McManus, A.T.; Chambers, J.P. Production of tumor necrosis factor-α, interleukin 1-β, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides 2003, 37, 355–361. [Google Scholar]
- Marolda, R.; Ciotti, M.T.; Matrone, C.; Possenti, R.; Calissano, P.; Cavallaro, S.; Severini, C. Substance P activates ADAM9 mRNA expression and induces α-secretase-mediated amyloid precursor protein cleavage. Neuropharmacology 2012, 62, 1954–1963. [Google Scholar]
- Goto, T.; Tanaka, T. Tachykinins and tachykinin receptors in bone. Microsc. Res. Tech 2002, 58, 91–97. [Google Scholar]
- Goto, T.; Nakao, K.; Gunjigake, K.K.; Kido, M.A.; Kobayashi, S.; Tanaka, T. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides 2007, 41, 25–31. [Google Scholar]
- Shih, C.; Bernard, G.W. Neurogenic substance P stimulates osteogenesisin vitro. Peptides 1997, 18, 323–326. [Google Scholar]
- Sohn, S.J. Substance P upregulates osteoclastogenesis by activating nuclear factor κB in osteoclast precursors. Acta Otolaryngol 2005, 125, 130–133. [Google Scholar]
- Wang, L.; Zhao, R.; Shi, X.; Wei, T.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activityin vitro. Bone 2009, 45, 309–320. [Google Scholar]
- Kim, S.Y.; Kim, S.; Yun-Choi, H.S.; Jho, E.H. Wnt5a potentiates U46619-induced platelet aggregation via the PI3K/Akt pathway. Mol. Cells 2011, 32, 333–336. [Google Scholar]
- Zheng, Q.; Chen, P.; Xu, Z.; Li, F.; Yi, X.P. Expression and redistribution of β-catenin in the cardiac myocytes of left ventricle of spontaneously hypertensive rat. J. Mol. Histol 2013, 44, 565–573. [Google Scholar]
- Peterson-Nedry, W.; Erdeniz, N.; Kremer, S.; Yu, J.; Baig-Lewis, S.; Wehrli, M. Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev. Biol. 2008, 320, 226–241. [Google Scholar]
- Li, J.; Li, J.; Chen, B. Oct4 was a novel target of Wnt signaling pathway. Mol. Cell. Biochem 2012, 362, 233–240. [Google Scholar]
- Sun, Y.C. Examination of effects of GSK3β phosphorylation, β-catenin phosphorylation, and β-catenin degradation on kinetics of Wnt signaling pathway using computational method. Theor. Biol. Med. Model 2009. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.G.; Ahn, J.M.; Park, H.J.; Park, S.K.; Yoo, J.S.; Yates, J.R.; Cho, J.Y. Role of PI3K on the regulation of BMP2-induced β-catenin activation in human bone marrow stem cells. Bone 2010, 46, 1522–1532. [Google Scholar]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar]
- Fleury, D.; Vayssiere, B.; Touitou, R.; Gillard, C.; Lebhar, H.; Rawadi, G.; Mollat, P. Expression, purification and functional characterization of Wnt signaling co-receptors LRP5 and LRP6. Protein Expr. Purif 2010, 70, 39–47. [Google Scholar]
- Georgiou, K.R.; King, T.J.; Scherer, M.A.; Zhou, H.; Foster, B.K.; Xian, C.J. Attenuated Wnt/β-catenin signalling mediates methotrexate chemotherapy-induced bone loss and marrow adiposity in rats. Bone 2012, 50, 1223–1233. [Google Scholar]
- Tamura, M.; Sato, M.M.; Nashimoto, M. Regulation of CXCL12 expression by canonical Wnt signaling in bone marrow stromal cells. Int. J. Biochem. Cell Biol 2011, 43, 760–767. [Google Scholar]
- Guo, J.; Liu, M.; Yang, D.; Bouxsein, M.L.; Saito, H.; Galvin, R.J.; Kuhstoss, S.A.; Thomas, C.C.; Schipani, E.; Baron, R.; et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab 2010, 11, 161–171. [Google Scholar]
- Wang, L.; Shi, X.; Zhao, R.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-κB activation, osteoclastogenesis and bone resorption. Bone 2010, 46, 1369–1379. [Google Scholar]
- Zhang, M.; Yan, Y.; Lim, Y.B.; Tang, D.; Xie, R.; Chen, A.; Tai, P.; Harris, S.E.; Xing, L.; Qin, Y.X.; et al. BMP-2 modulates β-catenin signaling through stimulation of Lrp5 expression and inhibition of β-TrCP expression in osteoblasts. J. Cell. Biochem 2009, 108, 896–905. [Google Scholar]
- Lee, E.; Salic, A.; Kruger, R.; Heinrich, R.; Kirschner, M.W. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 2003, 1, e10. [Google Scholar]
- Kestler, H.A.; Kuhl, M. From individual Wnt pathways towards a Wnt signalling network. Philos. Trans. R. Soc 2008, 363, 1333–1347. [Google Scholar]
- Cho, K.H.; Baek, S.; Sung, M.H. Wnt pathway mutations selected by optimal β-catenin signaling for tumorigenesis. FEBS Lett 2006, 580, 3665–3670. [Google Scholar]
- Hoppler, S.; Kavanagh, C.L. Wnt signalling: Variety at the core. J. Cell Sci 2007, 120, 385–393. [Google Scholar]
- Lee, J.E.; Wu, S.F.; Goering, L.M.; Dorsky, R.I. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development 2006, 133, 4451–4461. [Google Scholar]
- Barolo, S. Transgenic Wnt/TCF pathway reporters: All you need is Lef? Oncogene 2006, 25, 7505–7511. [Google Scholar]
- Shimizu, N.; Kawakami, K.; Ishitani, T. Visualization and exploration of Tcf/Lef function using a highly responsive Wnt/β-catenin signaling-reporter transgenic zebrafish. Dev. Biol 2012, 370, 71–85. [Google Scholar]
- Vlad, A.; Rohrs, S.; Klein-Hitpass, L.; Muller, O. The first five years of the Wnt targetome. Cell Signal 2008, 20, 795–802. [Google Scholar]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol 2004, 20, 781–810. [Google Scholar]
- Juan, J.; Muraguchi, T.; Iezza, G.; Sears, R.C.; McMahon, M. Diminished WNT → β-catenin → c-myc signaling is a barrier for malignant progression of BRAFV600E-induced lung tumors. Genes Dev 2014, 28, 561–575. [Google Scholar]
- Sukhotnik, I.; Roitburt, A.; Pollak, Y.; Dorfman, T.; Matter, I.; Mogilner, J.G.; Bejar, J.; Coran, A.G. Wnt/β-catenin signaling cascade down-regulation following massive small bowel resection in a rat. Pediatr. Surg. Int 2014, 30, 173–180. [Google Scholar]
- Ramnath, R.D.; Sun, J.; Adhikari, S.; Bhatia, M. Effect of mitogen-activated protein kinases on chemokine synthesis induced by substance P in mouse pancreatic acinar cells. J. Cell. Mol. Med 2007, 11, 1326–1341. [Google Scholar]
- Schmidt, P.T.; Rasmussen, T.N.; Holst, J.J. Tachykinins may mediate capsaicin-induced, but not vagally induced motility in porcine antrum. Peptides 1997, 18, 1511–1516. [Google Scholar]
- Sharon, N.; Mor, I.; Golan-lev, T.; Fainsod, A.; Benvenisty, N. Molecular and functional characterizations of gastrula organizer cells derived from human embryonic stem cells. Stem Cells 2011, 29, 600–608. [Google Scholar]
- Alcantara, E.H.; Shin, M.Y.; Sohn, H.Y.; Park, Y.M.; Kim, T.; Lim, J.H.; Jeong, H.J.; Kwon, S.T.; Kwun, I.S. Diosgenin stimulates osteogenic activity by increasing bone matrix protein synthesis and bone-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells. J. Nutr. Biochem 2011, 22, 1055–1063. [Google Scholar]
- Dong, J.; He, Y.; Zhang, X.; Wang, L.; Sun, T.; Zhang, M.; Liang, Y.; Qi, M. Calcitonin gene-related peptide regulates the growth of epidermal stem cellsin vitro. Peptides 2010, 31, 1860–1865. [Google Scholar]




| Gene | Source | Sequence | Predicted length (bp) |
|---|---|---|---|
| ALP | NM_007431 | 5′-AACCCAGACACAAGCATTCC-3′ | 200 |
| 5′-GCCTTTGAGGTTTTTGGTCA-3′ | |||
| COL1 | NM_007742 | 5′-GCCAAGAAGACATCCCTGAA-3′ | 107 |
| 5′-GCCATTGTGGCAGATACAGA-3′ | |||
| OCN | NM_007541 | 5′-TGACAAAGCCTTCATGTCCA-3′ | 175 |
| 5′-TTTGTAGGCGGTCTTCAAGC-3′ | |||
| RUNX2 | NM_009820 | 5′-AAGTGCGGTGCAAACTTTCT-3′ | 175 |
| 5′-ACGCCATAGTCCCTCCTTTT-3′ | |||
| C-MYC | NM_001177353 | 5′-TCCTGTACCTCGTCCGATTC-3′ | 195 |
| 5′-GGTTTGCCTCTTCTCCACAG-3′ | |||
| CCND1 | NM_007631 | 5′-GCGTACCCTGACACCAATCT-3′ | 183 |
| 5′-CTCCTCTTCGCACTTCTGCT-3′ | |||
| LEF1 | NM_010703 | 5′-TATGAACAGCGACCCGTACA-3′ | 132 |
| 5′-TCGTCGCTGTAGGTGATGAG-3′ | |||
| TCF7 | NM_009331 | 5′-ATCCTTGATGCTGGGATCTG-3′ | 139 |
| 5′-CTTCTCTGCCTTGGGTTCTG-3′ | |||
| β-CATENIN | NM_007614 | 5′-ATGGCTTGGAATGAGACTGC-3′ | 150 |
| 5′-ATGCTCCATCATAGGGTCCA-3′ | |||
| GAPDH | NM_008084 | 5′-ATTGTCAGCAATGCATCCTG-3′ | 102 |
| 5′-ATGGACTGTGGTCATGAGCC-3′ |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mei, G.; Zou, Z.; Fu, S.; Xia, L.; Zhou, J.; Zhang, Y.; Tuo, Y.; Wang, Z.; Jin, D. Substance P Activates the Wnt Signal Transduction Pathway and Enhances the Differentiation of Mouse Preosteoblastic MC3T3-E1 Cells. Int. J. Mol. Sci. 2014, 15, 6224-6240. https://doi.org/10.3390/ijms15046224
Mei G, Zou Z, Fu S, Xia L, Zhou J, Zhang Y, Tuo Y, Wang Z, Jin D. Substance P Activates the Wnt Signal Transduction Pathway and Enhances the Differentiation of Mouse Preosteoblastic MC3T3-E1 Cells. International Journal of Molecular Sciences. 2014; 15(4):6224-6240. https://doi.org/10.3390/ijms15046224
Chicago/Turabian StyleMei, Gang, Zhenlv Zou, Su Fu, Liheng Xia, Jian Zhou, Yongtao Zhang, Yonghua Tuo, Zhao Wang, and Dan Jin. 2014. "Substance P Activates the Wnt Signal Transduction Pathway and Enhances the Differentiation of Mouse Preosteoblastic MC3T3-E1 Cells" International Journal of Molecular Sciences 15, no. 4: 6224-6240. https://doi.org/10.3390/ijms15046224




