Immuno-Expression of Endoglin and Smooth Muscle Actin in the Vessels of Brain Metastases. Is There a Rational for Anti-Angiogenic Therapy?
Abstract
:1. Introduction
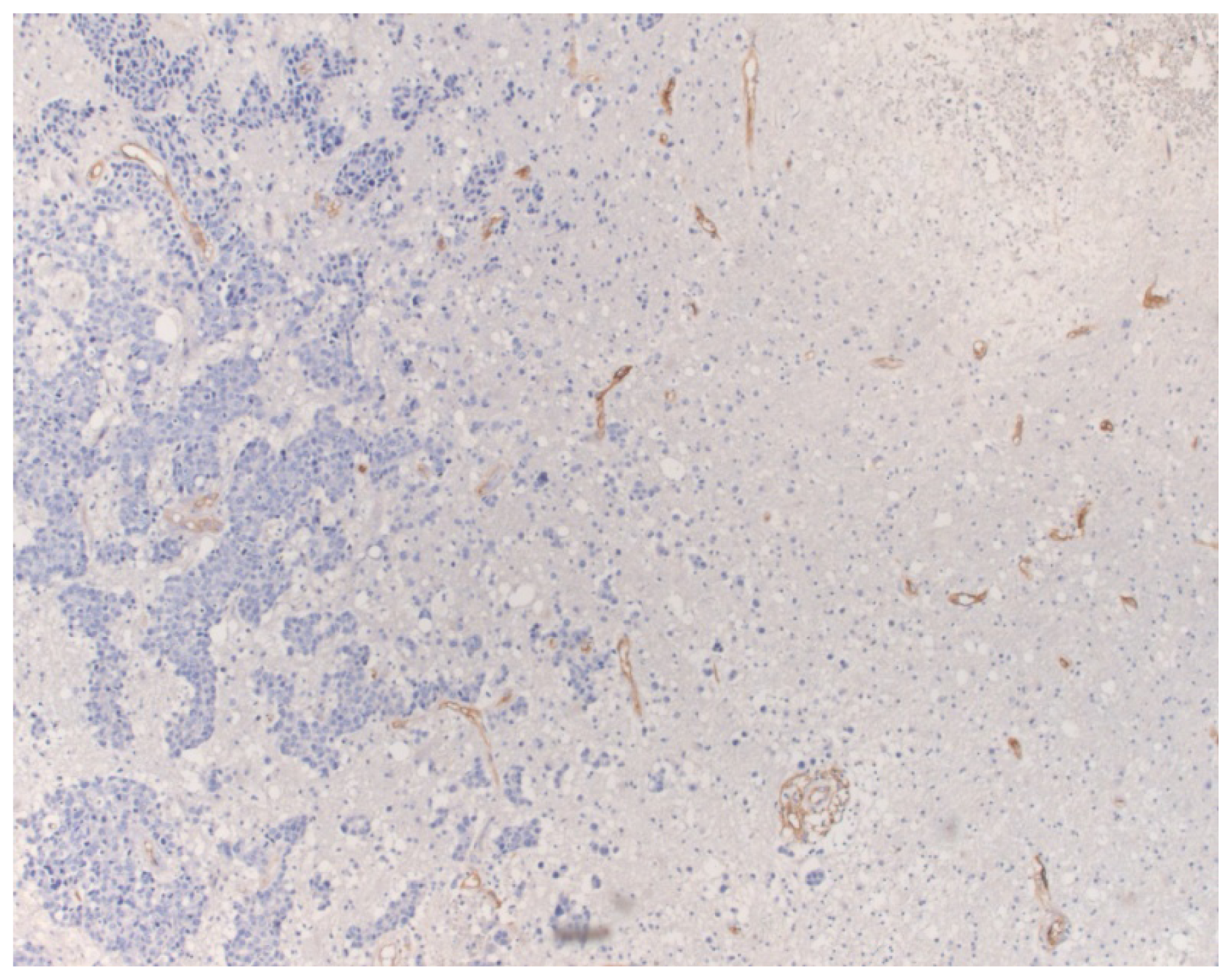
2. Results
3. Discussion
4. Materials and Methods
Immunohistochemistry, Quantification and Statistics
5. Conclusions
Conflicts of Interest
- Author ContributionsValeria Barresi: conception and design of the study, analysis and interpretation of data and drafting of the manuscript.Giovanni Branca: collection of the specimens and participation in drafting the manuscript.Maria Caffo: collection of the specimens and of the clinical data, literature review and participation in drafting the manuscript.Rosario Caltabiano: collection of the specimens, interpretation of data and participation in drafting the manuscript.Antonio Ieni: collection of the clinical data and participation in drafting the manuscript.Enrica Vitarelli: immunohistochemistry, interpretation of the immunohistochemical findings and participation in drafting the manuscript.Salvatore Lanzafame: analysis and interpretation of data and revision of the manuscript.Giovanni Tuccari: analysis and interpretation of data and revision of the manuscript.
References
- Liu, Y.; Carson-Walter, E.B.; Cooper, A.; Winans, B.N.; Johnson, M.D.; Walter, K.A. Vascular gene expression patterns are conserved in primitive and metastatic brain tumors. J. Neurooncol 2010, 99, 13–24. [Google Scholar]
- Hall, W.A.; Djalilian, H.R.; Nussbaum, E.S.; Cho, K.H. Longterm survival with metastatic cancer to the brain. Med. Oncol 2000, 7, 279–286. [Google Scholar]
- Kolomainen, D.F.; Larkin, J.M.; Badran, M.; A’Hern, R.P.; King, D.M.; Fisher, C.; Bridges, J.E.; Blake, P.R.; Barton, D.P.; Shepherd, J.H.; et al. Epithelial ovarian cancer metastasizing to the brain: A late manifestation of the disease with an increasing incidence. J. Clin. Oncol 2002, 20, 982–986. [Google Scholar]
- Kyritsis, A.P.; Markoula, S.; Levin, V.A. A systematic approach to the management of patients with brain metastases of known or unknown primitive site. Cancer Chemother. Pharmacol 2012, 69, 1–13. [Google Scholar]
- Fidler, I.J. The role of the organ microenvironment in brain metastasis. Semin. Cancer Biol 2011, 21, 107–112. [Google Scholar]
- Jahroudi, N.; Greenberger, J.S. The role of endothelial cells in tumor invasion and metastasis. J. Neurooncol 1995, 23, 99–108. [Google Scholar]
- Nicolson, G.L.; Menter, D.G.; Herrmann, J.L.; Yun, Z.; Cavanaugh, P.; Marchetti, D. Brain metastasis: Role of trophic, autocrine, and paracrine factors in tumor invasion and colonization of the central nervous system. Curr. Top. Microbiol. Immunol 1996, 213, 89–115. [Google Scholar]
- Yano, S.; Shinohara, H.; Herbst, R.S.; Kuniyasu, H.; Bucana, C.D.; Ellis, L.; Davis, D.W.; McConkey, D.J.; Fidler, I.J. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res 2000, 60, 4959–4967. [Google Scholar]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999, 13, 9–22. [Google Scholar]
- Kim, L.S.; Huang, S.; Lu, W.; Lev, D.C.; Price, J.E. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin. Exp. Metastasis 2004, 21, 107–118. [Google Scholar]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar]
- Labidi, S.I.; Bachelot, T.; Ray-Coquard, I.; Mosbah, K.; Mosbah, K.; Treilleux, I.; Fayette, J.; Favier, B.; Galy, G.; Blay, J.Y.; et al. Bevacizumab and paclitaxel for breast cancer patients with central nervous system metastases: A case series. Clin. Breast Cancer 2009, 9, 118–121. [Google Scholar]
- De Braganca, K.C.; Janjigian, Y.Y.; Azzoli, G.C.; Kris, M.G.; Pietanza, M.C.; Nolan, C.P.; Omuro, A.M.; Holodny, A.I.; Lassman, A.B. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J. Neurooncol 2010, 100, 443–447. [Google Scholar]
- Besse, B.; Lasserre, S.F.; Compton, P.; Huang, J.; Augustus, S.; Rohr, U.P. Bevacizumab safety in patients with central nervous system metastases. Clin. Cancer Res 2010, 16, 69–78. [Google Scholar]
- Danciu, O.C.; Rayani, S.; Michals, E.A.; Villano, J.L. Prolonged activity of bevacizumab in adenocarcinoma of the lung with multiple brain metastases. Med. Oncol 2012, 29, 2619–2622. [Google Scholar]
- Yamamoto, D.; Iwase, S.; Tsubota, Y.; Sueoka, N.; Yamamoto, C.; Kitamura, K.; Odagiri, H.; Nagumo, Y. Bevacizumab in the treatment of five patients with breast cancer and brain metastases: Japan Breast Cancer Research Network-07 trial. Onco Targets Ther 2012, 5, 185–189. [Google Scholar]
- Cook, K.M.; Figg, W.D. Angiogenesis inhibitors, current strategies and future prospects. CA Cancer J. Clin 2010, 60, 222–243. [Google Scholar]
- Choueiri, T.K.; Duh, M.S.; Clement, J.; Brick, A.J.; Rogers, M.J.; Kwabi, C.; Shah, K.; Percy, A.G.; Antràs, L.; Jayawant, S.S.; et al. Angiogenesis inhibitor therapies for metastatic renal cell carcinoma, effectiveness; safety and treatment patterns in clinical practice-based on medical chart review. BJU Int 2010, 105, 1247–1254. [Google Scholar]
- Donnem, T.; Hu, J.; Ferguson, M.; Adighibe, O.; Snell, C.; Harris, A.L.; Gatter, K.C.; Pezzella, F. Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Med 2013, 2, 427–436. [Google Scholar]
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med 1995, 1, 1024–1028. [Google Scholar]
- Benjamin, L.E.; Hemo, I.; Keshet, E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998, 125, 1591–1598. [Google Scholar]
- Helfrich, I.; Scheffrahn, I.; Bartling, S.; Weis, J.; von Felbert, V.; Middleton, M.; Kato, M.; Ergün, S.; Augustin, H.G.; Schadendorf, D. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med 2010, 207, 491–503. [Google Scholar]
- Barresi, V.; Cerasoli, S.; Vitarelli, E.; Tuccari, G. Density of microvessels positive for CD105 (endoglin) is related to prognosis in meningiomas. Acta Neuropathol 2007, 114, 147–156. [Google Scholar]
- Cheifetz, S.; Bellón, T.; Calés, C.; Vera, S.; Bernabeu, C.; Massagué, J.; Letarte, M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem 1992, 267, 19027–19030. [Google Scholar]
- Folkman, J. Tumor angiogenesis, therapeutic implications. N. Engl. J. Med 1971, 285, 1182–1186. [Google Scholar]
- Madhusudan, S.; Harris, A.L. Drug inhibition of angiogenesis. Curr. Opin. Pharmacol 2002, 2, 403–414. [Google Scholar]
- El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis inhibitors in cancer therapy, mechanistic perspective on classification and treatment rationales. Br. J. Pharmacol 2013, 170, 712–729. [Google Scholar]
- Escudier, B.; Pluzanska, A.; Koralewski, P.; Ravaud, A.; Bracarda, S.; Szczylik, C.; Chevreau, C.; Filipek, M.; Melichar, B.; Bajetta, E.; et al. AVOREN Trial investigators Bevacizumab plus interferon α-2a for treatment of metastatic renal cell carcinoma, a randomised; double-blind phase III trial. Lancet 2007, 370, 2103–2111. [Google Scholar]
- Ince, W.L.; Jubb, A.M.; Holden, S.N.; Holmgren, E.B.; Tobin, P.; Sridhar, M.; Hurwitz, H.I.; Kabbinavar, F.; Novotny, W.F.; Hillan, K.J.; et al. Association of k-ras; b-raf; and p53 status with the treatment effect of bevacizumab. J. Natl. Cancer Inst 2005, 97, 981–989. [Google Scholar]
- Jubb, A.M.; Hurwitz, H.I.; Bai, W.; Holmgren, E.B.; Tobin, P.; Guerrero, A.S.; Kabbinavar, F.; Holden, S.N.; Novotny, W.F.; Frantz, G.D.; et al. Impact of vascular endothelial growth factor-A expression; thrombospondin-2 expression; and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J. Clin. Oncol 2006, 24, 217–227. [Google Scholar]
- Jubb, A.M.; Cesario, A.; Ferguson, M.; Congedo, M.T.; Gatter, K.C.; Lococo, F.; Mulè, A.; Pezzella, F. Vascular phenotypes in primitive non-small cell lung carcinomas and matched brain metastases. Br. J. Cancer 2011, 104, 1877–1881. [Google Scholar]
- Salgado, K.B.; Toscani, N.V.; Silva, L.L.; Hilbig, A.; Barbosa-Coutinho, L.M. Immunoexpression of endoglin in brain metastasis secondary to malignant melanoma, evaluation of angiogenesis and comparison with brain metastasis secondary to breast and lung carcinomas. Clin. Exp. Metastasis 2007, 24, 403–410. [Google Scholar]
- Burrows, F.J.; Derbyshire, E.J.; Tazzari, P.L.; Amlot, P.; Gazdar, A.F.; King, S.W.; Letarte, M.; Vitetta, E.S.; Thorpe, P.E. Up-regulation of endoglin on vascular endothelial cells in human solid tumors, implications for diagnosis and therapy. Clin. Cancer Res 1995, 1, 1623–1634. [Google Scholar]
- Auguste, P.; Lemiere, S.; Larrieu-Lahargue, F.; Bikfalvi, A. Molecular mechanisms of tumor vascularization. Crit. Rev. Oncol. Hematol 2005, 54, 53–61. [Google Scholar]
- Maniotis, A.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.G.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J.C. Vascular channel formation by human melanoma cells in vivo and in vitro, vasculogenic mimicry. Am. J. Pathol 1999, 155, 739–752. [Google Scholar]
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegang, S.J. Vessel cooption; regression; and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284, 1994–1998. [Google Scholar]
- Wesseling, P.; Ruiter, D.J.; Burger, P.C. Angiogenesis in brain tumors; pathobiological and clinical aspects. J. Neurooncol 1997, 32, 253–265. [Google Scholar]
- Leenders, W.; Kusters, B.; de Waal, R. Vessel co-option, how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium 2002, 9, 83–87. [Google Scholar]
- Kusters, B.; Leenders, W.P.; Wesseling, P.; Smits, D.; Verrijp, K.; Ruiter, D.J.; Peters, J.P.; van der Kogel, A.J.; de Waal, R.M. Vascular endothelial growth factor-A165 induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res 2002, 62, 341–345. [Google Scholar]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med 2010, 16, 116–122. [Google Scholar]
- Yadzani, S.; Miki, Y.; Tamaki, K.; Ono, K.; Iwabuchi, E.; Abe, K.; Suzuki, T.; Sato, Y.; Kondo, T.; Sasano, H. Proliferation and maturation of intratumoral blood vessels in non-small cell lung cancer. Hum. Pathol 2013, 44, 1586–1596. [Google Scholar]
- Rivera, L.B.; Brekken, R.A. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-β1 activity. J. Cell Biol 2011, 193, 1305–1319. [Google Scholar]
- Barresi, V.; Alafaci, C.; Salpietro, F.; Tuccari, G. Sstr2A immunohistochemical expression in human meningiomas: Is there a correlation with the histological grade; proliferation or microvessel density? Oncol. Rep 2008, 20, 485–492. [Google Scholar]
- Barresi, V.; Vitarelli, E.; Tuccari, G.; Barresi, G. Correlative study of microvessel density and 5-lipoxygenase expression in human sporadic colorectal cancer. Arch. Pathol. Lab. Med 2008, 132, 1807–1812. [Google Scholar]
- Barresi, V.; Cerasoli, S.; Tuccari, G. Correlative evidence that tumor cell-derived caveolin-1 mediates angiogenesis in meningiomas. Neuropathology 2008, 28, 472–478. [Google Scholar]
- Barresi, V.; Vitarelli, E.; Cerasoli, S. Semaphorin3A immunohistochemical expression in human meningiomas, correlation with the microvessel density. Virchows Arch 2009, 454, 563–571. [Google Scholar]
- Barresi, V.; Tuccari, G.; Barresi, G. Adiponectin immunohistochemical expression in colorectal cancer and its correlation with histological grade and tumor microvessel density. Pathology 2009, 41, 533–538. [Google Scholar]
- Barresi, V.; di Gregorio, C.; Regiani-Bonetti, L.; Ponz-De Leon, M.; Barresi, G.; Vitarelli, E. Stage I colorectal carcinoma, VEGF immunohistochemical expression; microvessel density; and their correlation with clinical outcome. Virchows Arch 2010, 457, 11–19. [Google Scholar]
- Barresi, V.; Tuccari, G. Increased ratio of vascular endothelial growth factor to semaphorin3A is a negative prognostic factor in human meningiomas. Neuropathology 2010, 30, 537–546. [Google Scholar]
- Barresi, V. Angiogenesis in meningiomas. Brain Tumor. Pathol 2011, 28, 99–106. [Google Scholar]
- Barresi, V.; Branca, G.; Granata, F.; Alafaci, C.; Caffo, M.; Tuccari, G. Embolized meningiomas, risk of overgrading and neo-angiogenesis. J. Neurooncol 2013, 113, 207–219. [Google Scholar]
- Rosen, L.S.; Hurwitz, H.I.; Wong, M.K.; Goldman, J.; Mendelson, D.S.; Figg, W.D.; Spencer, S.; Adams, B.J.; Alvarez, D.; Seon, B.K.; et al. A phase I first-in-human study of TRC105 Anti-Endoglin Antibody in patients with advanced cancer. Clin. Cancer Res 2012, 18, 4820–4829. [Google Scholar]
- Paauwe, M.; ten Dijke, P.; Hawinkels, L.J. Endoglin for tumor imaging and targeted cancer therapy. Expert Opin. Ther. Targets 2013, 17, 421–435. [Google Scholar]


| Case | Age | Sex | Site | MVD (v/mm2) | Counts range (v/HPF) | Mean diameter of endoglin-stained vessels (μm) | Maturation index | Primary tumor | Status | FU |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | M | parietal | 10 | 3 | 39.12 | 1 | CRC | Dead | 7 |
| 2 | 65 | M | parietal | 18.8 | 2–8 | 25.09 | 1 | CRC | Dead | 56 |
| 3 | 58 | M | frontal | 93.3 | 25–31 | 44.17 | 1 | CRC | Dead | 5 |
| 4 | 71 | M | frontal | 46.6 | 14–16 | 38.7 | 1 | CRC | Alive | 18 |
| 5 | 62 | F | frontal | 42.2 | 9–15 | 22.99 | 1 | breast ductal adenocarcinoma | Dead | 43 |
| 6 | 55 | F | temporal | 80 | 24 | 29.13 | 0.97 | breast ductal adenocarcinoma | Dead | 17 |
| 7 | 71 | F | parietal | 20 | 3–9 | 39.29 | 1 | breast ductal adenocarcinoma | Dead | 43 |
| 8 | 62 | F | cerebellar | 36 | 9–12 | 32.14 | 1 | breast ductal adenocarcinoma | Dead | 6 |
| 9 | 40 | F | temporal | 17.7 | 4–7 | 22.17 | 1 | breast ductal adenocarcinoma | Alive | 98 |
| 10 | 47 | F | temporal | 12.2 | 1–6 | 25.78 | 1 | breast ductal adenocarcinoma | Dead | 4 |
| 11 | 73 | F | parietal | 40 | 12 | 33.97 | 1 | breast ductal adenocarcinoma | n.a. | |
| 12 | 44 | F | parietal | 90 | 24–25 | 22.68 | 0.94 | breast ductal adenocarcinoma | Dead | 6 |
| 13 | 69 | F | cerebellar | 9.3 | 2–3 | 22.52 | 1 | breast ductal adenocarcinoma | Dead | 3 |
| 14 | 75 | F | parietal | 1.11 | 0–1 | 74.85 | 1 | breast ductal adenocarcinoma | Alive | 27 |
| 15 | 71 | F | parietal | 70.66 | 20–22 | 24.81 | 0.82 | breast ductal adenocarcinoma | Dead | 26 |
| 16 | 46 | F | cerebellar | 58 | 15–18 | 26.14 | 0.84 | breast ductal adenocarcinoma | Dead | 3 |
| 17 | 60 | F | frontal | 35.5 | 9–12 | 31.18 | 1 | breast ductal adenocarcinoma | Dead | 1 |
| 18 | 59 | M | cerebellar | 1.11 | 0–1 | 27.16 | 1 | CCRCC | Alive | 8 |
| 19 | 61 | M | cerebellar | 40 | 11–12 | 30.93 | 1 | CCRCC | n.a. | |
| 20 | 71 | F | cerebellar | 23.3 | 5–8 | 35.19 | 1 | CCRCC | n.a. | |
| 21 | 57 | F | temporal | 20 | 4–8 | 12.53 | 1 | CCRCC | Dead | 33 |
| 22 | 78 | M | parietal | 54 | 15–17 | 42.11 | 1 | thymic carcinoma | n.a. | |
| 23 | 73 | F | temporal | 16.6 | 4–5 | 47.38 | 1 | melanoma | Dead | 24 |
| 24 | 44 | M | occipital | 57.3 | 9–19 | 46.22 | 1 | melanoma | n.a. | |
| 25 | 68 | F | frontal | 44 | 12–14 | 48.32 | 1 | melanoma | Alive | 9 |
| 26 | 69 | M | frontal | 10 | 2–4 | 42.22 | 1 | melanoma | Alive | 6 |
| 27 | 58 | M | frontal | 2.22 | 0–2 | 45.28 | 1 | melanoma | Alive | 1 |
| 28 | 46 | M | frontal | 36.66 | 9–13 | 43.18 | 1 | melanoma | Alive | 3 |
| 29 | 67 | F | cerebellar | 24.44 | 4–12 | 38.17 | 1 | melanoma | Dead | 6 |
| 30 | 76 | M | cerebellar | 15.55 | 3–6 | 32.16 | 1 | melanoma | Dead | 22 |
| 31 | 66 | M | frontal | 27.7 | 7–9 | 40.11 | 1 | MPNST | Alive | 11 |
| 32 | 49 | F | frontal | 40 | 6–18 | 58.17 | 1 | serous papillary ovarian carcinoma | n.a. | |
| 33 | 80 | F | temporo-parietal | 93.3 | 23–31 | 35.67 | 1 | serous papillary ovarian carcinoma | Alive | 13 |
| 34 | 61 | F | temporo-parietal | 11.3 | 2–5 | 29.6 | 1 | serous papillary ovarian carcinoma | Alive | 23 |
| 35 | 58 | F | parieto-occipital | 12 | 2–7 | 31.34 | 1 | serous papillary ovarian carcinoma | Alive | 59 |
| 36 | 55 | F | frontal | 43.3 | 9–15 | 38.14 | 1 | serous papillary ovarian carcinoma | Alive | 21 |
| 37 | 74 | M | frontal | 97.7 | 28–30 | 38.78 | 0.89 | small cell lung carcinoma | Dead | 8 |
| 38 | 71 | F | cerebellar | 38 | 8–14 | 39.01 | 1 | lung adenocarcinoma | n.a. | |
| 39 | 67 | M | cerebellar | 34.6 | 8–13 | 40.35 | 0.73 | lung adenocarcinoma | Dead | 1 |
| 40 | 58 | M | frontal | 100 | 29–31 | 39.15 | 0.96 | lung adenocarcinoma | n.a. | |
| 41 | 59 | M | frontal | 45.3 | 10–17 | 31.25 | 1 | lung adenocarcinoma | Dead | 25 |
| 42 | 69 | F | temporal | 41.3 | 12–13 | 48.56 | 1 | lung adenocarcinoma | n.a. | |
| 43 | 77 | M | cerebellar | 88.6 | 19–25 | 48.92 | 0.73 | lung adenocarcinoma | Alive | 35 |
| 44 | 60 | M | frontal | 28 | 6–10 | 24.45 | 1 | lung adenocarcinoma | Alive | 20 |
| 45 | 55 | F | cerebellar | 64.6 | 16–21 | 37.06 | 0.56 | lung adenocarcinoma | Dead | 2 |
| 46 | 74 | M | frontal | 33.3 | 3–15 | 38.12 | 1 | lung adenocarcinoma | Alive | 38 |
| 47 | 50 | F | frontal | 56.6 | 15–19 | 43.13 | 1 | lung adenocarcinoma | Alive | 34 |
| 48 | 72 | M | cerebellar | 140 | 40–44 | 41.78 | 0.59 | lung adenocarcinoma | Alive | 33 |
| 49 | 65 | M | temporal | 98.8 | 27–32 | 38.78 | 0.82 | lung adenocarcinoma | Alive | 24 |
| 50 | 79 | F | frontal | 41.11 | 11–13 | 34.67 | 1 | lung adenocarcinoma | Alive | 7 |
| 51 | 46 | M | frontal | 77.7 | 19–27 | 30.78 | 0.6 | lung adenocarcinoma | Alive | 68 |
| 52 | 67 | M | temporo-parietal | 72.2 | 20–22 | 32.16 | 0.86 | lung adenocarcinoma | Dead | 23 |
| 53 | 73 | M | temporal | 34.6 | 10–11 | 41.12 | 1 | lung adenocarcinoma | Dead | 35 |
| 54 | 57 | F | parietal | 46.6 | 13–14 | 29.6 | 0.72 | lung adenocarcinoma | n.a. | |
| 55 | 61 | M | frontal | 63.3 | 15–23 | 32.78 | 1 | lung adenocarcinoma | Dead | 4 |
| 56 | 58 | M | parietal | 91.1 | 27–28 | 45.12 | 0.8 | lung adenocarcinoma | Dead | 1 |
| 57 | 69 | M | frontal | 83.3 | 24–26 | 39.37 | 0.8 | lung adenocarcinoma | Alive | 4 |
| 58 | 71 | M | temporal | 6 | 0–3 | 26.08 | 1 | lung adenocarcinoma | Dead | 19 |
| 59 | 67 | F | frontal | 12 | 3–5 | 38.76 | 1 | lung adenocarcinoma | n.a. | |
| 60 | 56 | M | frontal | 100 | 29–32 | 21.91 | 0.83 | lung adenocarcinoma | Dead | 13 |
| 61 | 49 | F | parietal | 26 | 6–10 | 58.23 | 1 | lung adenocarcinoma | Alive | 16 |
| 62 | 60 | M | temporal | 57.3 | 19–21 | 29.58 | 1 | lung adenocarcinoma | Dead | 12 |
| 63 | 66 | M | frontal | 32.6 | 8–10 | 38.5 | 1 | lung adenocarcinoma | n.a. | |
| 64 | 59 | M | frontal | 72 | 17–24 | 59.23 | 1 | lung adenocarcinoma | n.a. | |
| 65 | 49 | M | temporal | 12 | 2–5 | 42.22 | 1 | lung squamous cell carcinoma | Dead | 25 |
| 66 | 50 | M | frontal | 20.6 | 4–8 | 41.11 | 1 | lung squamous cell carcinoma | Alive | 16 |
| 67 | 56 | M | cerebellar | 53.3 | 4–6 | 44.35 | 1 | lung squamous cell carcinoma | Alive | 17 |
| 68 | 59 | M | frontal | 13.3 | 2–5 | 32.67 | 1 | lung squamous cell carcinoma | n.a. | |
| 69 | 58 | M | temporal | 25.5 | 6–8 | 40.13 | 1 | lung squamous cell carcinoma | Dead | 13 |
| 70 | 57 | M | frontal | 22.2 | 5–7 | 38.14 | 1 | lung squamous cell carcinoma | Dead | 4 |
| 71 | 74 | M | frontal | 83.3 | 23–27 | 35.67 | 0.92 | lung large cell carcinoma | Dead | 7 |
| 72 | 68 | M | cerebellar | 123.3 | 34–38 | 33.84 | 0.52 | lung large cell carcinoma | Dead | 1 |
| 73 | 69 | M | temporal | 110.6 | 35–38 | 29.16 | 0.8 | lung large cell carcinoma | Dead | 1 |
| 74 | 72 | M | cerebellar | 82 | 23–27 | 37.94 | 0.96 | lung large cell carcinoma | n.a. | |
| 75 | 76 | M | frontal | 66.6 | 19–21 | 36.64 | 0.78 | lung large cell carcinoma | n.a. | |
| 76 | 68 | F | parietal | 9.3 | 0–4 | 37.56 | 1 | uterine clear cell adenocarcinoma | n.a. | |
| 77 | 70 | F | frontal | 113.3 | 30–37 | 52.34 | 0.7 | uterine endometrioid carcinoma | n.a. | |
| 78 | 75 | F | frontal | 4 | 0–2 | 35.33 | 1 | uterine serous carcinoma | n.a. |
| Primary tumor | Mean MVD (v/mm2) | Mean maturation index |
|---|---|---|
| lung carcinoma | 58.85 ± 33.84 | 0.89 ± 0.14 |
| large cell | 93.16 ± 23.12 | 0.79 ± 0.17 |
| adenocarcinoma | 58.7 ± 31.49 | 0.88 ± 0.14 |
| squamous cell carcinoma | 24.48 ± 15.05 | 1 |
| breast ductal adenocarcinoma | 39.43 ± 28.17 | 0.96 ± 0.06 |
| CRC | 42.17 ± 37.48 | 1 |
| NOS | 69.95 ± 33.02 | 1 |
| mucinous | 14.4 ± 6.2 | 1 |
| CCRCC | 20.82 ± 16.41 | 1 |
| melanoma | 25.84 ± 15.1 | 1 |
| ovarian serous papillary carcinoma | 39.98 ± 33.38 | 1 |
| uterine carcinoma | 42.2 ± 61.63 | 0.9 ± 0.17 |
| serous | 4 | |
| endometrioid | 113.3 | |
| clear cell | 9.3 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barresi, V.; Branca, G.; Caffo, M.; Caltabiano, R.; Ieni, A.; Vitarelli, E.; Lanzafame, S.; Tuccari, G. Immuno-Expression of Endoglin and Smooth Muscle Actin in the Vessels of Brain Metastases. Is There a Rational for Anti-Angiogenic Therapy? Int. J. Mol. Sci. 2014, 15, 5663-5679. https://doi.org/10.3390/ijms15045663
Barresi V, Branca G, Caffo M, Caltabiano R, Ieni A, Vitarelli E, Lanzafame S, Tuccari G. Immuno-Expression of Endoglin and Smooth Muscle Actin in the Vessels of Brain Metastases. Is There a Rational for Anti-Angiogenic Therapy? International Journal of Molecular Sciences. 2014; 15(4):5663-5679. https://doi.org/10.3390/ijms15045663
Chicago/Turabian StyleBarresi, Valeria, Giovanni Branca, Maria Caffo, Rosario Caltabiano, Antonio Ieni, Enrica Vitarelli, Salvatore Lanzafame, and Giovanni Tuccari. 2014. "Immuno-Expression of Endoglin and Smooth Muscle Actin in the Vessels of Brain Metastases. Is There a Rational for Anti-Angiogenic Therapy?" International Journal of Molecular Sciences 15, no. 4: 5663-5679. https://doi.org/10.3390/ijms15045663





