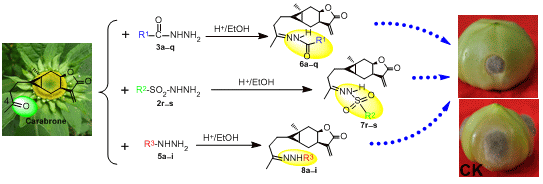
Synthesis, Antifungal Activities and Qualitative Structure Activity Relationship of Carabrone Hydrazone Derivatives as Potential Antifungal Agents
Abstract
:1. Introduction
2. Results and Discussion
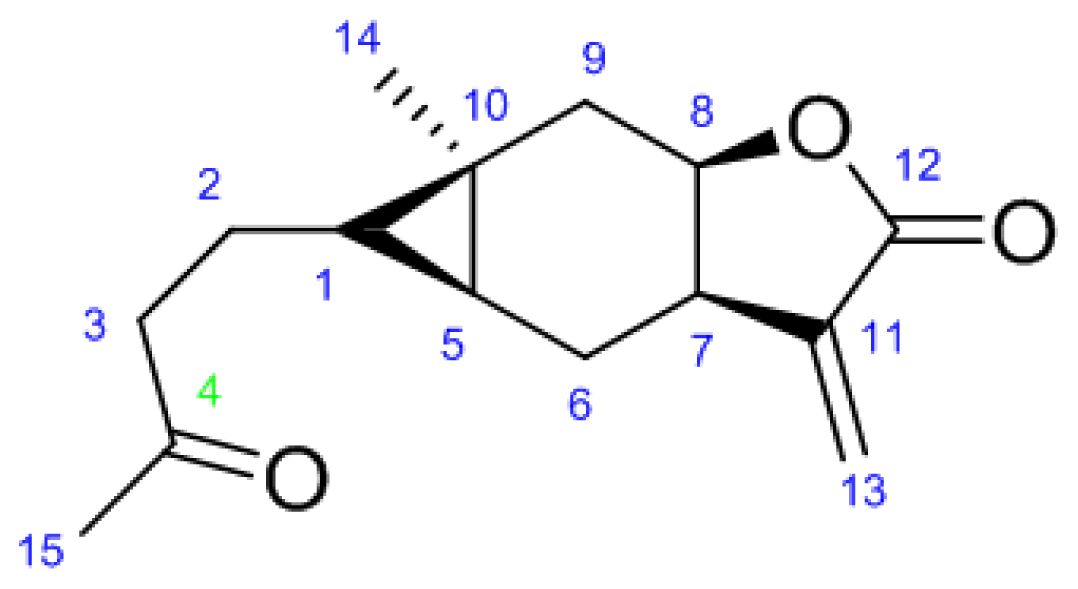
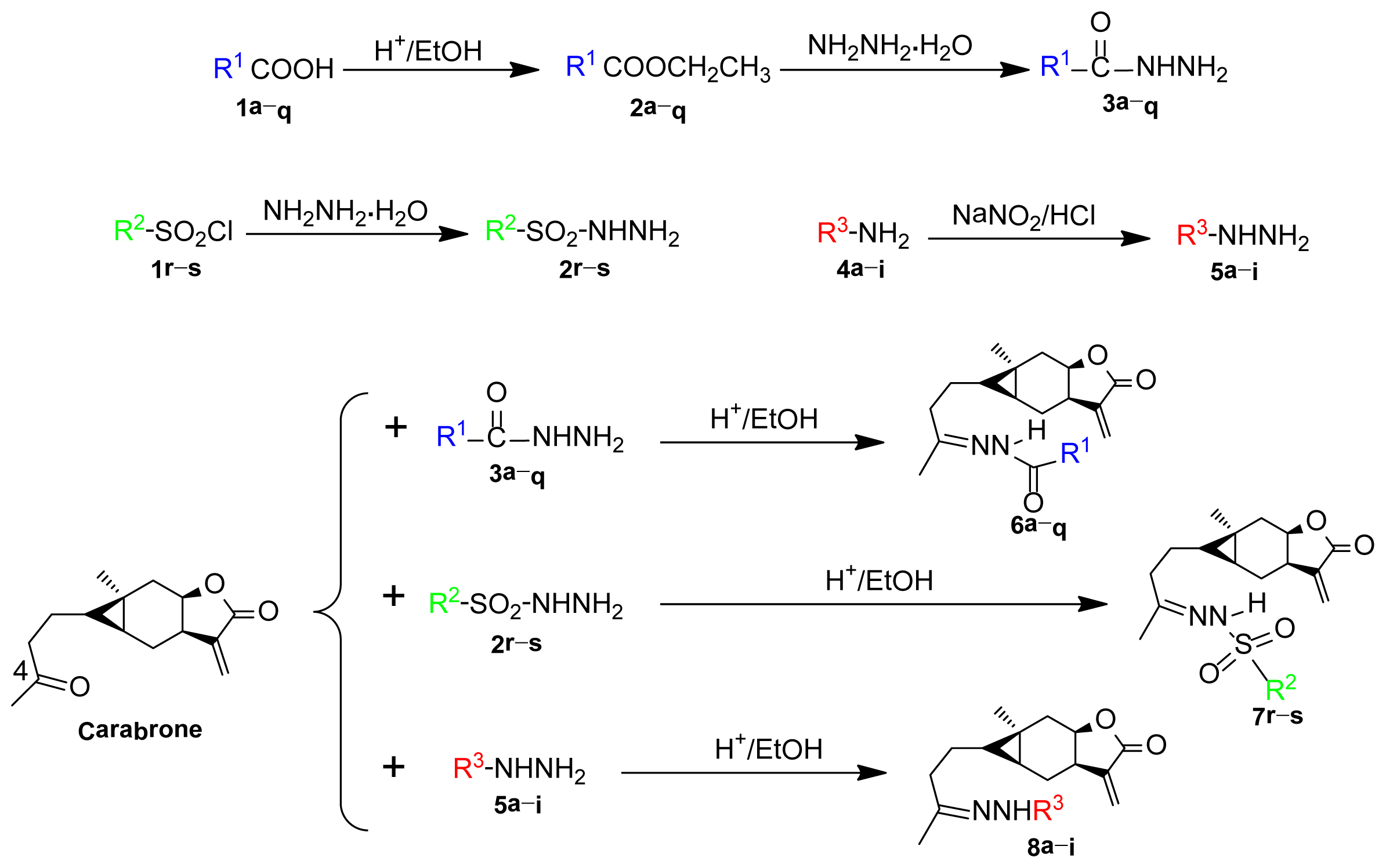
2.1. Synthesis
2.2. Antifungal Activity
2.3. Qualitative Structure–Activity Relationship
3. Experimental Section
3.1. General
3.2. Synthesis of Title Compounds
3.2.1. N′-(4-((4aS,5S,5aR)-5a-Methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)acetohydrazide (6a)
3.2.2. 2-Cyano-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)acetohydrazide (6b)
3.2.3. N′1, N′2-Bis(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclo-propa[f]benzofuran- 5-yl)butan-2-ylidene)oxalohydrazide (6c)
3.2.4. N′-(4-((4aS,5S,5aR)-5a-Methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]-benzofuran- 5-yl)butan-2-ylidene)thiophene-2-carbohydrazide (6d)
3.2.5. 5-Chloro-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)thiophene-2-carbohydrazide (6e)
3.2.6. 5-Bromo-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclo-propa[f]benzofuran- 5-yl)butan-2-ylidene)thiophene-2-carbohydrazide (6f)
3.2.7. N′-(4-((4aS,5S,5aR)-5a-Methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]-benzofuran- 5-yl)butan-2-ylidene)nicotinohydrazide (6g)
3.2.8. 4-Amino-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclo-propa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6h)
3.2.9. 4-Hydroxy-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6i)
3.2.10. 2-Hydroxy-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzo -furan-5-yl)butan-2-ylidene)benzohydrazide (6j)
3.2.11. 2-Chloro-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6k)
3.2.12. 3-Chloro-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6l)
3.2.13. N′-(4-((4aS,5S,5aR)-5a-Methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]-benzofuran- 5-yl)butan-2-ylidene)-4-nitrobenzohydrazide (6m)
3.2.14. 4-Cyano-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6n)
3.2.15. 3-Methyl-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6o)
3.2.16. 4-Methoxy-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6p)
3.2.17. 3-Methoxy-N′-(4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzohydrazide (6q)
3.2.18. N′-((E)-4-((4aS,5S,5aR)-5a-Methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)benzenesulfonohydrazide (7r)
3.2.19. 4-Methyl-N′-((E)-4-((4aS,5S,5aR)-5a-methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran-5-yl)butan-2-ylidene)benzenesulfonohydrazide (7s)
3.2.20. (4aS,5S,5aR)-5-(3-(2-(2-Hydroxyethyl)hydrazono)butyl)-5a-methyl-3-methyl-eneoctahydro- 2H-cyclopropa[f]benzofuran-2-one (8a)
3.2.21. (4aS,5S,5aR)-5a-Methyl-3-methylene-5-(3-(2-phenylhydrazono)butyl)-octa-hydro-2H-cyclopropa[ f]benzofuran-2-one (8b)
3.2.22. (4aS,5S,5aR)-5a-Methyl-3-methylene-5-(3-(2-(2,4,6-trichlorophenyl)-hydrazono)butyl)- octahydro-2H-cyclopropa[f]benzofuran-2-one (8c)
3.2.23. (4aS,5S,5aR)-5a-Methyl-3-methylene-5-(3-(2-(4-nitrophenyl)hydrazono)-butyl)-octahydro-2H-cyclopropa[ f]benzofuran-2-one (8d)
3.2.24. (4aS,5S,5aR)-5a-Methyl-3-methylene-5-(3-(2-(2-nitrophenyl)hydrazono)butyl)-octahydro-2H-cyclopropa[ f]benzofuran-2-one (8e)
3.2.25. (4aS,5S,5aR)-5a-Methyl-3-methylene-5-(3-(2-(4-(trifluoromethyl)phenyl)-hydrazono)butyl)- octahydro-2H-cyclopropa[f]benzofuran-2-one (8f)
3.2.26. (4aS,5S,5aR)-5a-Methyl-3-methylene-5-(3-(2-(2,3,5,6-tetrafluorophenyl)-hydrazono)butyl)- octahydro-2H-cyclopropa[f]benzofuran-2-one (8g)
3.2.27. (4aS,5S,5aR)-5-(3-(2-(5,6-Dimethylthieno[2,3-d]pyrimidin-4-yl)hydrazono)-butyl)-5a-methyl- 3-methyleneoctahydro-2H-cyclopropa[f]benzofuran-2-one (8h)
3.2.28. 4-(2-(4-((4aS,5S,5aR)-5a-Methyl-3-methylene-2-oxooctahydro-2H-cyclopropa[f]benzofuran- 5-yl)butan-2-ylidene)hydrazinyl)benzoic acid (8i)
3.3. Microorganism and Preparation of Spore Suspension
3.4. Spore Germination Assay
3.5. Preparation of Tested Tomato Fruits
3.6. Culture of B. cinerea and Inoculation
3.7. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-15-04257-s001.pdfAcknowledgments
Conflicts of Interest
- Author ContributionsWang Hao designed and conducted the experiment and wrote the paper; Ren Shuang-Xi and Wang De-Long assisted in the completion of synthesis of the title compounds; He Ze-Yu and Yan Xiao-Nan took part in the experiments on activity evaluation; Feng Jun-Tao and Zhang Xing supervised the whole experiment and provided technical guidance. All the authors contributed to the analysis of the data.
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar]
- Savary, S.; Ficke, A.; Aubertot, J.N.; Hollier, C. Crop losses due to diseases and their implications for global food production losses and food security. Food Secur. 2012, 4, 519–537. [Google Scholar]
- Shimizu, M.; Yazawa, S.; Ushijima, Y. A promising strain of endophytic Streptomyces sp for biological control of cucumber anthracnose. J. Gen. Plant. Pathol. 2009, 75, 27–36. [Google Scholar]
- Oercke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar]
- Oercke, E.C.; Dehne, H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar]
- Minato, H.; Nosaka, S.; Horibe, I. Studies on sesquiterpenoids Part VIII The structure of carabrone a new component of Carpesium abrotanoides Linn. J. Chem. Soc. 1964, 5503–5510. [Google Scholar]
- Maruyama, M.; Omura, S. Carpesiolin from Carpesium abrotanoides. Phytochemistry 1977, 16, 782–783. [Google Scholar]
- Yang, C.; Shi, Y.P.; Jia, Z.J. Sesquiterpene lactone glycosides eudesmanolides and other constituents from Carpesium macrocephalum. Planta Med. 2002, 68, 626–630. [Google Scholar]
- Jiang, J.W. Manuscript of Active Ingredients of Vegetable Drug; People’s Health Press: Beijing, China, 1986; pp. 832–833. [Google Scholar]
- Lee, J.; Min, B.; Lee, S.; Na, M.; Kwon, B.; Lee, C.; Bae, K. Cytotoxic sesquiterpene lactones from Carpesium abrotanoides. Planta. Med. 2002, 68, 745–747. [Google Scholar]
- Feng, J.T.; Ma, Z.Q.; Li, J.H.; He, J.; Xu, H.; Zhang, X. Synthesis and antifungal activity of carabrone derivatives. Molecules 2010, 15, 6485–6492. [Google Scholar]
- Feng, J.T.; Wang, H.; Ren, S.X.; He, J.; Liu, Y.; Zhang, X. Synthesis and antifungal activities of carabrol ester derivatives. J. Agric. Food Chem. 2012, 60, 3817–3823. [Google Scholar]
- Fardella, G.; Barbetti, P.; Grandolini, G.; Chiappini, I.; Ambrogi, V.; Scarcia, V.; Candiani, A.F. Phenylthio-derivatives of α-methylene-γ-lactones as pro-drugs of cytotoxic agents. Eur. J. Med. Chem. 1999, 34, 515–523. [Google Scholar]
- Lee, K.H.; Wu, Y.S.; Hall, I.H. Antitumor agents 25 Synthesis and antitumor activity of uracil and thymine a-methylene-y-lactones and related derivatives. J. Med. Chem. 1977, 20, 911–914. [Google Scholar]
- Makovitzki, A.; Viterbo, A.; Brotman, Y.; Chet, I.; Shai, Y. Inhibition of fungal and bacterial plant pathogens in vitro and in planta with ultrashort cationic lipopeptides. Appl. Environ. Microbiol. 2007, 73, 6629–6636. [Google Scholar]
- Ji, Y.L.; Liu, L.; Jia, D.Z. Synthesis and crystal structure of N-(1-phenyl-3-methyl-4-benzylidene- 5-pyrazolone)p-nitrobenzoylhydrazide. Chin. J. Struct. Chem. 2002, 21, 553–556. [Google Scholar]
- Rollas, S.; Kucukguzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar]
- Kumar, K.R.; Prasad, A.R.G.; Srilalitha, V.; Swamy, G.N.; Ravindranath, L.K. Synthesis and electrochemical investigations on certain pyrazolin-5-ones. Sci. Iran. Trans. C 2012, 19, 605–618. [Google Scholar]
- Shen, Y.H.; Yu, J.L. A Synthetic Method of 3-Bromophenyl hydrazine. Chin. Patent CN 101157636, 9 April 2008. [Google Scholar]
- Li, S.K.; Ji, Z.Q.; Zhang, J.W.; Guo, Z.Y.; Wu, W.J. Synthesis of 1-acyl-3- isopropenylbenzimidazolone derivatives and their activity against Botrytis cinerea. J. Agric. Food Chem. 2010, 58, 2668–2672. [Google Scholar]
- Fang, X.L.; Li, Z.Z.; Wang, Y.H.; Zhang, X. In vitro and in vivo antimicrobial activity of Xenorhabdus bovienii YL002 against Phytophthora capsici and Botrytis cinerea. J. Appl. Microbiol. 2011, 111, 145–154. [Google Scholar]

| Compound No. | Yield a (%) | In vitro (IC50 b, μg/mL) | In vivo (IC50 b, μg/mL) | |
|---|---|---|---|---|
| B. cinerea | C. lagenarium | B. cinerea | ||
| 6a | 78 | 27.33 ± 1.29 | 10.18 ± 1.02 | 29.62 ± 3.12 |
| 6b | 82 | 9.77 ± 0.68 | 8.14 ± 0.34 | 7.55 ± 0.68 |
| 6c | 93 | 22.07 ± 0.70 | 10.22 ± 0.62 | 30.80 ± 1.51 |
| 6d | 78 | 7.81 ± 0.37 | 10.83 ± 1.79 | 12.84 ± 0.31 |
| 6e | 87 | 5.57 ± 0.89 | 9.98 ± 0.30 | 9.57 ± 0.99 |
| 6f | 72 | 4.83 ± 0.41 | 10.02 ± 0.72 | 7.03 ± 0.58 |
| 6g | 70 | 8.50 ± 0.69 | 2.06 ± 0.86 | 4.02 ± 0.26 |
| 6h | 78 | 2.67 ± 0.10 | 2.10 ± 0.19 | 4.85 ± 0.52 |
| 6i | 59 | 3.46 ± 0.39 | 1.24 ± 0.47 | 4.73 ± 1.13 |
| 6j | 48 | 13.16 ± 0.23 | 2.01 ± 0.34 | 13.44 ± 1.02 |
| 6k | 77 | 3.35 ± 0.65 | 3.52 ± 0.21 | 9.67 ± 0.95 |
| 6l | 78 | 2.57 ± 0.12 | 1.97 ± 0.78 | 8.76 ± 1.08 |
| 6m | 61 | 3.39 ± 0.56 | 5.49 ± 0.73 | 6.67 ± 0.49 |
| 6n | 88 | 2.47 ± 0.72 | 1.69 ± 0.54 | 4.29 ± 0.51 |
| 6o | 65 | 9.00 ± 1.09 | 0.98 ± 0.19 | 18.08 ± 0.91 |
| 6p | 53 | 10.30 ± 0.86 | 2.56 ± 0.32 | 16.42 ± 1.24 |
| 6q | 47 | 6.37 ± 0.71 | 0.77 ± 0.27 | 12.52 ± 0.96 |
| 7r | 66 | 13.32 ± 0.87 | 6.43 ± 0.95 | 17.46 ± 0.88 |
| 7s | 73 | 12.99 ± 0.62 | 5.33 ± 0.89 | 17.26 ± 1.05 |
| 8a | 58 | 17.37 ± 0.91 | 15.23 ± 1.14 | 16.69 ± 2.37 |
| 8b | 47 | 16.32 ± 0.58 | 7.62 ± 0.19 | 18.85 ± 1.25 |
| 8c | 75 | 1.51 ± 0.73 | 1.53 ± 0.46 | 2.10 ± 0.47 |
| 8d | 65 | 3.79 ± 0.82 | 3.95 ± 0.28 | 4.62 ± 0.29 |
| 8e | 77 | 10.31 ± 1.31 | 4.27 ± 0.35 | 8.93 ± 0.96 |
| 8f | 72 | 1.99 ± 0.34 | 4.05 ± 0.57 | 5.52 ± 0.37 |
| 8g | 63 | 1.27 ± 0.16 | 2.65 ± 0.91 | 2.59 ± 0.63 |
| 8h | 81 | 13.32 ± 0.87 | 1.63 ± 0.45 | 11.46 ± 1.09 |
| 8i | 45 | 14.77 ± 0.59 | 7.06 ± 0.77 | 14.59 ± 0.95 |
| Carabrone | 14.14 ± 0.94 | 8.29 ± 0.51 | 16.74 ± 1.32 | |
| Chlorothalonil c | 0.49 ± 0.28 | 0.52 ± 0.17 | 1.29 ± 0.33 | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, H.; Ren, S.-X.; He, Z.-Y.; Wang, D.-L.; Yan, X.-N.; Feng, J.-T.; Zhang, X. Synthesis, Antifungal Activities and Qualitative Structure Activity Relationship of Carabrone Hydrazone Derivatives as Potential Antifungal Agents. Int. J. Mol. Sci. 2014, 15, 4257-4272. https://doi.org/10.3390/ijms15034257
Wang H, Ren S-X, He Z-Y, Wang D-L, Yan X-N, Feng J-T, Zhang X. Synthesis, Antifungal Activities and Qualitative Structure Activity Relationship of Carabrone Hydrazone Derivatives as Potential Antifungal Agents. International Journal of Molecular Sciences. 2014; 15(3):4257-4272. https://doi.org/10.3390/ijms15034257
Chicago/Turabian StyleWang, Hao, Shuang-Xi Ren, Ze-Yu He, De-Long Wang, Xiao-Nan Yan, Jun-Tao Feng, and Xing Zhang. 2014. "Synthesis, Antifungal Activities and Qualitative Structure Activity Relationship of Carabrone Hydrazone Derivatives as Potential Antifungal Agents" International Journal of Molecular Sciences 15, no. 3: 4257-4272. https://doi.org/10.3390/ijms15034257