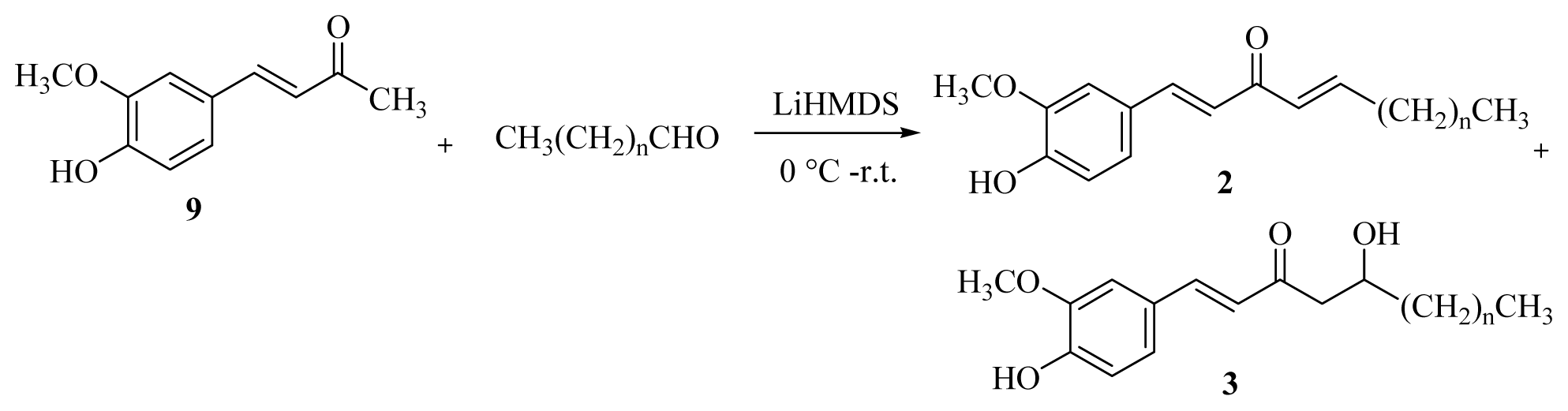
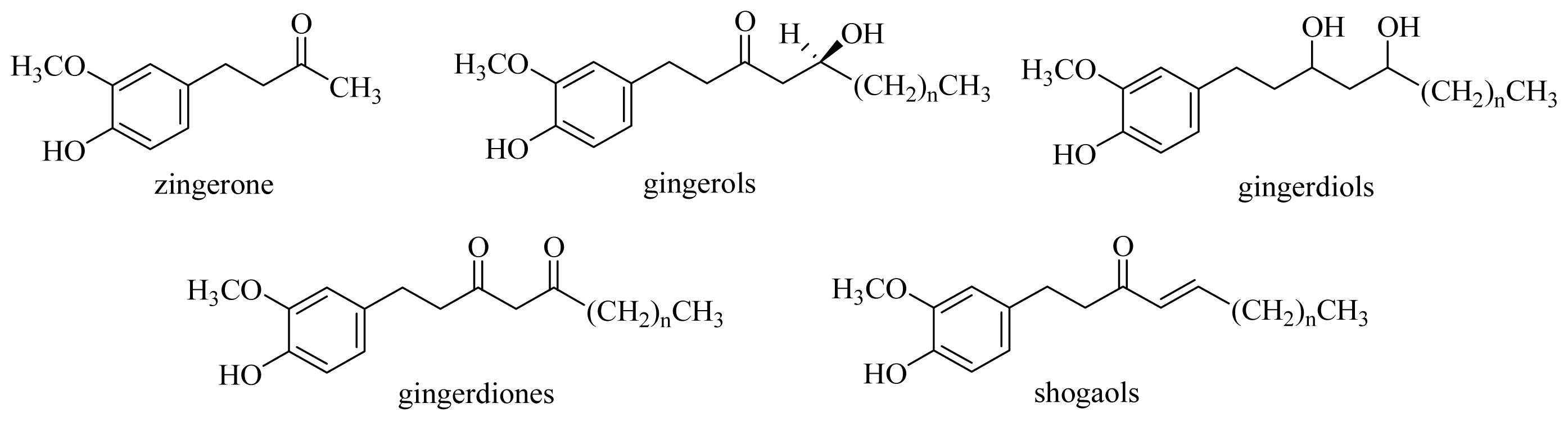
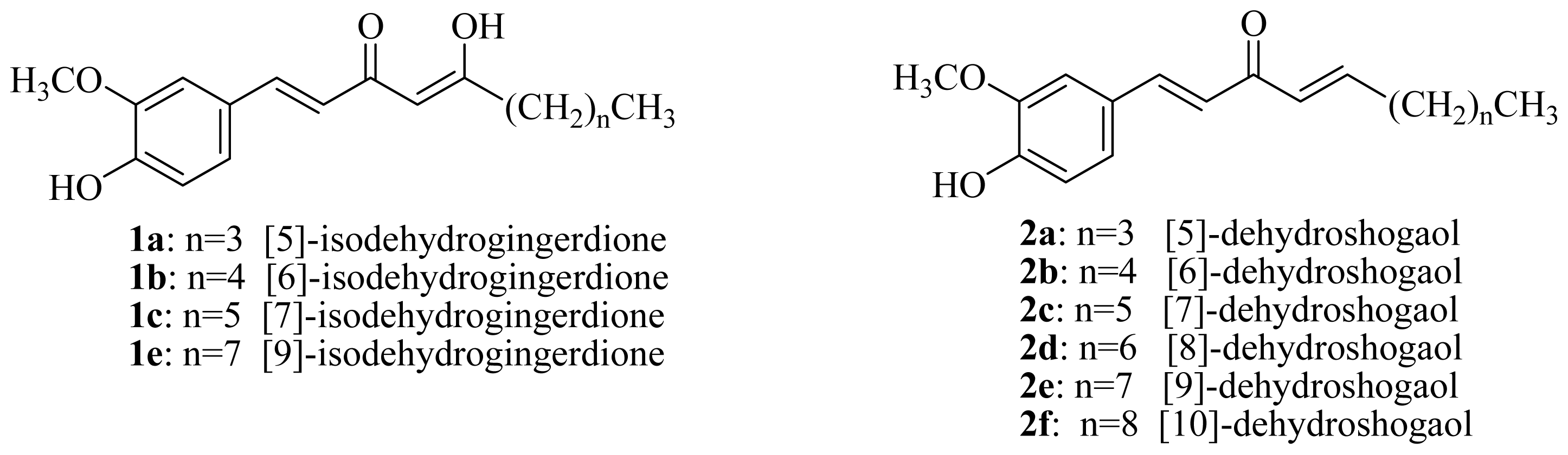
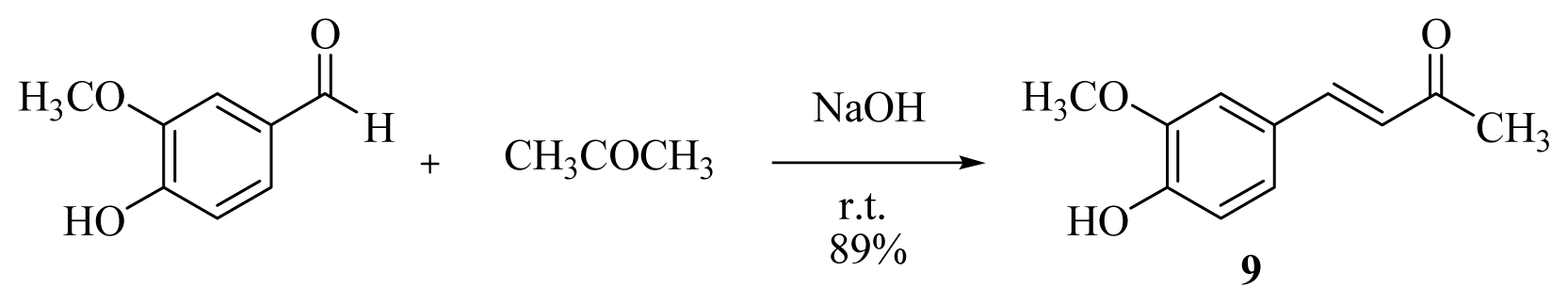3.2.2. General Procedure for the Synthesis of [n]-Dehydroshogaols (2a–f) and [n]-Dehydrogingerols (3a–f)
A 1.0 M THF solution of lithium bis(trimethylsilyl)amide (20.8 mL) was added dropwise to a solution of dehydrozingerone (9) (2.0 g, 10.4 mmol) in dry THF (10 mL) at 0 °C under argon. After the mixture had been stirred for 1 h, the appropriate aldehyde (10.5 mmol) was added and stirred for another 15 min. The reaction was then quenched with 5% HCl(aq) at 0 °C and extracted with EtOAc (4 × 20 mL). The organic layers were combined, washed with brine, dried over MgSO4, and concentrated under reduced pressure. Products 2 and 3 were isolated using silica gel column chromatography (EtOAc/CH2Cl2 = 1/16).
[5]-Dehydroshogaol (2a): yellow syrup (9%); UV (MeOH) λ
max (log ɛ) 356 (4.14), 255 (4.03) nm; IR (neat) ν
max 3325, 2958, 2860, 1652, 1625, 1579, 1514, 1460, 1276, 1126, 1031 cm
−1; 1H-NMR (CDCl
3) δ 7.57 (1H, d,
J = 15.8 Hz, H-1), 7.13 (1H, dd,
J = 8.2, 2.0 Hz, H-6′), 7.06 (1H, d,
J = 2.0 Hz, H-2′), 6.99 (1H, dt,
J = 15.6, 6.8 Hz, H-5), 6.92 (1H, d,
J = 8.2 Hz, H-5′), 6.80 (1H, d,
J = 15.8 Hz, H-2), 6.44 (1H, dt,
J = 15.6, 1.4 Hz, H-4), 6.04 (1H, br s, -OH), 3.93 (3H, s, -OCH
3), 2.28 (2H, tdd,
J = 6.8, 6.8, 1.4 Hz, H-6), 1.57–1.26 (4H, m, H-7, -8), 0.92 (3H, t,
J = 7.0 Hz, H-9);
13C-NMR (CDCl
3) δ 189.3, 148.2, 148.0, 146.8, 143.3, 129.0, 127.4, 123.3, 122.8, 114.8, 109.7, 56.0, 32.4, 30.3, 22.3, 13.8; EIMS
m/
z (
rel. int.) 260 (M
+, 66), 259 (26), 217 (56), 177 (80), 168 (45), 152 (65), 151 (100), 137 (40), 123 (21), 111 (35), 97 (35), 91 (23), 71 (44), 69 (55), 57 (94), 55 (85); HREIMS
m/
z 260.1410 [M]
+ (Calcd for C
16H
20O
3, 260.1412).
[5]-Dehydrogingerol (3a): yellow needles (65%), mp 143–144 °C (lit. 144–146 °C) [
22]; UV (MeOH) λ
max (log ɛ) 340 (4.46), 271 (sh) (3.62), 244 (4.11) nm; IR (KBr) ν
max 3341, 3150, 2926, 2855, 1626, 1583, 1514, 1284, 1031, 972, 816 cm
−1; 1H-NMR (CDCl
3) δ 7.50 (1H, d,
J = 16.2 Hz, H-1), 7.11 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 7.05 (1H, d,
J = 1.8 Hz, H-2′), 6.93 (1H, d,
J = 8.0 Hz, H-5′), 6.58 (1H, d,
J = 16.2 Hz, H-2), 6.02 (1H, br s, -OH), 4.13(1H, m, H-5), 3.93 (3H, s, -OCH
3), 2.88 (1H, dd,
J = 17.0, 2.8 Hz, H-4), 2.73 (1H, dd,
J = 17.0, 8.8 Hz, H-4), 1.58–1.35 (6H, m, H-6~8), 0.92 (3H, t,
J = 6.6 Hz, H-9);
13C-NMR (CDCl
3) δ 200.9, 148.5, 146.8, 143.8, 126.6, 124.1, 123.7, 114.8, 109.4, 67.9, 56.0, 46.4, 36.2, 27.7, 22.6, 14.0; EIMS
m/
z (
rel. int.) 278 (M
+, 33), 192 (37), 177 (100), 150 (38), 145 (37), 137 (38), 89 (14); Anal. Calcd for C
16H
20O
4: C, 69.06%; H, 7.91%; Found: C, 69.09%; H, 7.85%.
[6]-Dehydroshogaol (2b): yellow syrup (15%); UV (MeOH) λ
max (log ɛ) 355 (4.02), 258 (3.93) nm; IR(neat) ν
max 3354, 2956, 2856, 1654, 1625, 1581, 1514, 1267, 1207, 1033 cm
−1; 1H-NMR (CDCl
3) δ 7.58 (1H, d,
J = 15.8 Hz, H-1), 7.14 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.07 (1H, d,
J = 1.8 Hz, H-2′), 7.00 (1H, dt,
J = 15.6, 7.0 Hz, H-5), 6.94 (1H, d,
J = 8.2 Hz, H-5′), 6.81 (1H, d,
J = 15.8 Hz, H-2), 6.43 (1H, dt,
J = 15.6, 1.4 Hz, H-4), 5.97 (1H, br s, -OH), 3.94 (3H, s, -OCH
3), 2.27 (2H, tdd,
J = 7.0, 6.8, 1.4 Hz, H-6), 1.57–1.25 (6H, m, H-7~9), 0.90 (3H, t,
J = 6.7 Hz, H-10);
13C-NMR (CDCl
3) δ 189.3, 148.1, 148.0, 147.2, 143.3, 129.0, 127.4, 123.3, 122.8, 114.8, 109.7, 56.0, 32.7, 31.4, 27.9, 22.4, 14.0; EIMS
m/
z (
rel. int.) 274 (M
+, 100), 273 (36), 217 (82), 177 (81), 152 (21), 151 (36), 145 (20), 137 (45), 57 (36), 55 (44); HREIMS
m/
z 274.1571 [M]
+ (Calcd for C
17H
22O
3, 274.1568).
[6]-Dehydrogingerol (3b): yellow needles (59%), mp 123–124 °C (lit. 134–136 °C) [
22]; UV (MeOH) λ
max (log ɛ) 341 (4.34), 270 (sh) (3.59), 247 (3.98) nm; IR (KBr) ν
max 3460, 3161, 2962, 2858, 1675, 1589, 1517, 1433, 1281, 1223, 1174, 1076, 872 cm
−1; 1H-NMR (CDCl
3) δ 7.50 (1H, d,
J = 16.0 Hz, H-1), 7.11 (1H, dd,
J = 8.0, 2.0 Hz, H-6′), 7.05 (1H, d,
J = 2.0 Hz, H-2′), 6.93 (1H, d,
J = 8.2 Hz, H-5′), 6.58 (1H, d,
J = 16.0 Hz, H-2), 4.14 (1H, m, H-5), 3.92 (3H, s, -OCH
3), 2.88 (1H, dd,
J = 17.2, 3.2 Hz, H-4), 2.72 (1H, dd,
J = 17.2, 8.6 Hz, H-4), 1.51–1.25 (8H, m, H-6~9), 0.89 (3H, t,
J = 6.4 Hz, H-10);
13C-NMR (CDCl
3) δ 200.1, 150.2, 148.7, 143.8, 127.6, 125.1, 124.1, 116.1, 111.4, 68.5, 56.2, 48.4, 38.0, 32.6, 26.0, 23.3, 14.3; EIMS
m/
z (
rel. int.) 292 (M
+, 51), 192 (20), 177 (100), 150 (47), 137 (40), 89 (10).
[7]-Dehydroshogaol (2c): yellow syrup (13%); UV (MeOH) λ
max (log ɛ) 357 (3.91), 261 (3.95) nm; IR (neat) ν
max 3384, 2954, 2856, 1654, 1625, 1583, 1514, 1274, 1124, 1033 cm
−1; 1H-NMR (CDCl
3) δ 7.58 (1H, d,
J = 15.8 Hz, H-1), 7.14 (1H, dd,
J = 8.1, 1.8 Hz, H-6′), 7.08 (1H, d,
J = 1.8 Hz, H-2′), 7.00 (1H, dt,
J = 15.6, 6.9 Hz, H-5), 6.93 (1H, d,
J = 8.1 Hz, H-5′), 6.81 (1H, d,
J = 15.8 Hz, H-2), 6.43 (1H, dt,
J = 15.6, 1.4 Hz, H-4), 5.92 (1H, br s, -OH), 3.94 (3H, s, -OCH
3), 2.27 (2H, tdd,
J = 6.9, 6.9, 1.4 Hz, H-6), 1.54–1.25 (8H, m, H-7~10), 0.89 (3H, t,
J = 6.7 Hz, H-11);
13C-NMR (CDCl
3) δ 189.3, 148.2, 148.0, 146.8, 143.4, 129.0, 127.3, 123.3, 122.7, 114.8, 109.7, 55.9, 32.7, 31.6, 28.9, 28.1, 22.5, 14.0; EIMS
m/
z (
rel. int.) 288 (M
+, 100), 287 (48), 217 (82), 204 (27), 177 (49), 137 (33); HREIMS
m/
z 288.1725 [M]
+ (Calcd for C
18H
24O
3, 288.1725).
[7]-Dehydrogingerol (3c): yellow needles (56%), mp 108–109 °C (lit. 110–112 °C) [
22]; UV (MeOH) λ
max (log ɛ) 340 (4.63), 271 (sh) (4.23), 252 (4.39) nm; IR (KBr) ν
max 3447, 3258, 2926, 2855, 1694, 1589, 1512, 1437, 1279, 1221, 1053, 812 cm
−1; 1H-NMR (CDCl
3) δ 7.46 (1H, d,
J = 16.0 Hz, H-1), 7.04 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.00 (1H, d,
J = 1.8 Hz, H-2′), 6.88 (1H, d,
J = 8.0 Hz, H-5′), 6.53 (1H, d,
J = 16.0 Hz, H-2), 4.17–4.06 (1H, m, H-5), 3.87 (3H, s, -OCH
3), 2.85 (1H, dd,
J = 17.0, 3.2 Hz, H-4), 2.71 (1H, dd,
J = 17.0, 8.6 Hz, H-4), 1.58–1.25 (10H, m, H-6~10), 0.85 (3H, t,
J = 6.6 Hz, H-11);
13C-NMR (CDCl
3) δ 201.0, 148.6, 147.0, 143.9, 126.7, 124.1, 123.7, 115.0, 109.5, 68.0, 56.0, 46.5, 36.6, 31.8, 29.3, 25.5, 22.6, 14.0; EIMS
m/
z (
rel. int.) 306 (M
+, 26), 217 (23), 192 (44), 177 (100), 150 (34), 145 (38), 137 (17), 89 (14).
[8]-Dehydroshogaol (2d): yellow syrup (15%); UV (MeOH) λ
max (log ɛ) 357 (4.10), 258 (3.96) nm; IR(neat) ν
max 3395, 2925, 2856, 1660, 1614, 1581, 1514, 1278, 1174, 1033 cm
−1; 1H-NMR (CDCl
3) δ 7.58 (1H, d,
J = 16.0 Hz, H-1), 7.12 (1H, dd,
J = 8.2, 2.0 Hz, H-6′), 7.06 (1H, d,
J = 2.0 Hz, H-2′), 6.99 (1H, dt,
J = 15.6, 6.9 Hz, H-5), 6.93 (1H, d,
J = 8.2 Hz, H-5′), 6.81 (1H, d,
J = 16.0 Hz, H-2), 6.43 (1H, dt,
J = 15.6, 1.4 Hz, H-4), 6.21 (1H, br s, -OH), 3.91 (3H, s, -OCH
3), 2.26 (2H, tdd,
J = 6.9, 6.9, 1.4 Hz, H-6), 1.52–1.27 (10H, m, H-7-11), 0.88 (3H, t,
J = 6.8 Hz, H-12);
13C-NMR (CDCl
3) δ 189.3, 148.2, 148.0, 146.8, 143.3, 129.0, 127.3, 123.2, 122.7, 114.8, 109.7, 55.9, 32.7, 31.7, 29.1, 29.0, 28.1, 22.6, 14.0; EIMS
m/
z (
rel. int.) 302 (M
+, 81), 301 (29), 217 (68), 177 (100), 150 (21), 137 (33), 55 (24); HREIMS
m/
z 302.1879 [M]
+ (Calcd for C
19H
26O
3, 302.1881).
[8]-Dehydrogingerol (3d): yellow needles (66%), mp 83–84 °C (lit. 88–90 °C) [
22]; UV (MeOH) λ
max (log ɛ) 340 (4.57), 270 (sh) (4.25), 247 (4.38) nm; IR (KBr) ν
max 3451, 3215, 2924, 2855, 1680, 1585, 1510, 1433, 1280, 1116, 854 cm
−1; 1H-NMR (CDCl
3) δ 7.51 (1H, d,
J = 16.0 Hz, H-1), 7.10 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.06 (1H, d,
J = 1.8 Hz, H-2′), 6.93 (1H, d,
J = 8.2 Hz, H-5′), 6.59 (1H, d,
J = 16.0 Hz, H-2), 4.17–4.06 (1H, m, H-5), 3.94 (3H, s, -OCH
3), 2.88 (1H, dd,
J = 17.2, 3.1 Hz, H-4), 2.72 (1H, dd,
J = 17.2, 8.7 Hz, H-4), 1.56–1.26 (12H, m, H-6~11), 0.88 (3H, t,
J = 6.4 Hz, H-12);
13C-NMR (CDCl
3) δ 200.2, 150.2, 148.8, 143.9, 127.6, 125.0, 124.2, 116.1, 111.5, 68.6, 56.2, 48.4, 38.1, 32.5, 30.3, 30.0, 26.3, 23.2, 14.3; EIMS
m/
z (
rel. int.) 320 (M
+, 22), 192 (53), 177 (100), 150 (28), 145 (31), 137 (30), 84 (37), 69 (28), 57 (40), 55(55); Anal. Calcd. for C
19H
28O
4: C, 71.25%; H, 8.75%; Found: C, 71.26%; H, 8.79%.
[9]-Dehydroshogaol (2e): yellow syrup (13%); UV (MeOH) λ
max (log ɛ) 355 (4.02), 260 (4.03) nm; IR (neat) ν
max 3358, 2925, 2856, 1641, 1587, 1525, 1274, 1120, 1037 cm
−1; 1H-NMR (CDCl
3) δ 7.57 (1H, d,
J = 15.8 Hz, H-1), 7.11 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.06 (1H, d,
J = 1.8 Hz, H-2′), 6.99 (1H, dt,
J = 15.4, 7.2 Hz, H-5), 6.91 (1H, d,
J = 8.2 Hz, H-5′), 6.80 (1H, d,
J = 15.8 Hz, H-2), 6.42 (1H, dt,
J = 15.4, 1.3 Hz, H-4), 3.90 (3H, s, -OCH
3), 2.25 (2H, tdd,
J = 7.2, 6.8, 1.3 Hz, H-6), 1.41–1.26 (12H, m, H-7~12), 0.86 (3H, t,
J = 6.6 Hz, H-13);
13C-NMR (CDCl
3) δ 189.4, 148.3, 148.1, 146.9, 143.4, 129.0, 127.3, 123.3, 122.7, 114.9, 109.8, 56.0, 32.7, 31.8, 29.3, 29.2, 29.1, 28.2, 22.6, 14.1; EIMS
m/
z (
rel. int.) 316 (M
+, 100), 315 (36), 217 (83), 204 (23), 177 (86), 137 (44), 55 (21); HREIMS
m/
z 316.2040 [M]
+ (Calcd for C
20H
28O
3, 316.2038).
[9]-Dehydrogingerol (3e): yellow needles (58%), mp 93–94 °C (lit. 93–94 °C) [
22]; UV (MeOH) λ
max (log ɛ) 339 (4.50), 270 (sh) (4.10), 250 (4.27) nm; IR (KBr) ν
max 3451, 2926, 2854, 1676, 1583, 1516, 1460, 1280, 1170, 1031, 977, 810 cm
−1; 1H-NMR (CDCl
3) δ 7.50 (1H, d,
J = 16.0 Hz, H-1), 7.10 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.05 (1H, d,
J = 1.8 Hz, H-2′), 6.93 (1H, d,
J = 8.2 Hz, H-5′), 6.59 (1H, d,
J = 16.0 Hz, H-2), 4.17–4.06 (1H, m, H-5), 3.94 (3H, s, -OCH
3), 2.87 (1H, dd,
J = 17.1, 3.0 Hz, H-4), 2.72 (1H, dd,
J = 17.1, 8.7 Hz, H-4), 1.56–1.28 (14H, m, H-6~12), 0.88 (3H, t,
J = 6.4 Hz, H-13);
13C-NMR (CDCl
3) δ 200.1, 150.1, 148.7, 143.8, 127.6, 125.0, 124.1, 116.1, 111.4, 68.5, 56.2, 48.4, 38.0, 32.6, 30.4, 30.3, 30.0, 26.3, 23.2, 14.3; EIMS
m/
z (
rel. int.) 334 (M
+, 33), 316 (27), 217 (22), 192 (50), 177 (100), 150 (30), 145 (27), 137 (46), 57 (20).
[10]-Dehydroshogaol (2f): yellow syrup (6%); UV (MeOH) λ
max (log ɛ) 355 (4.18), 257 (4.04) nm; IR (neat) ν
max 3533, 2925, 2856, 1660, 1614, 1581, 1514, 1278, 1201, 1120, 1031 cm
−1; 1H-NMR (CDCl
3) δ 7.58 (1H, d,
J = 15.9 Hz, H-1), 7.13 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.06 (1H, d,
J = 1.8 Hz, H-2′), 6.99 (1H, dt,
J = 15.6, 6.8 Hz, H-5), 6.91 (1H, d,
J = 8.2 Hz, H-5′), 6.81 (1H, d,
J = 15.9 Hz, H-2), 6.42 (1H, dt,
J = 15.6, 1.4 Hz, H-4), 6.17 (1H, br s, -OH), 3.92 (3H, s, -OCH
3), 2.25 (2H, tdd,
J = 6.8, 6.8, 1.4 Hz, H-6), 1.52–1.26 (14H, m, H-7~13), 0.87 (3H, t,
J = 6.7 Hz, H-14);
13C-NMR (CDCl
3) δ 189.3, 148.2, 148.0, 146.8, 143.4, 129.0, 127.3, 123.3, 122.7, 114.8, 109.7, 55.9, 32.7, 31.8, 29.5, 29.4, 29.3, 29.2, 28.2, 22.7, 14.1; EIMS
m/
z (
rel. int.) 330 (M
+, 47), 217 (37), 177 (100), 152 (53), 150 (22), 137 (35), 97 (26), 85 (23), 71 (36), 57 (80), 55 (61); HREIMS
m/
z 330.2196 [M]
+ (Calcd for C
21H
30O
3, 330.2194).
[10]-Dehydrogingerol (3f): yellow needles (50%), mp 74–75 °C (lit. 76–77.5 °C) [
22]; UV (MeOH) λ
max (log ɛ) 340 (4.27), 273 (sh) (3.68), 239 (4.06) nm; IR (KBr) ν
max 3414, 2926, 2855, 1656, 1587, 1515, 1460, 1281, 1169, 1031, 979, 810 cm
−1; 1H-NMR (CDCl
3) δ 7.51 (1H, d,
J = 16.1 Hz, H-1), 7.11 (1H, dd,
J = 8.1, 1.8 Hz, H-6′), 7.05 (1H, d,
J = 1.8 Hz, H-2′), 6.93 (1H, d,
J = 8.1 Hz, H-5′), 6.59 (1H, d,
J = 16.1 Hz, H-2), 5.97 (1H, br s, -OH), 4.17–4.06 (1H, m, H-5), 3.93 (3H, s, -OCH
3), 2.88 (1H, dd,
J = 17.1, 3.0 Hz, H-4), 2.73 (1H, dd,
J = 17.1, 8.7 Hz, H-4), 1.57–1.27 (16H, m, H-6~13), 0.88 (3H, t,
J = 6.7 Hz, H-14);
13C-NMR (CDCl
3) δ 200.9, 148.4, 146.8, 143.8, 126.7, 124.1, 123.7, 114.8, 109.4, 68.0, 55.9, 46.5, 36.5, 31.8, 29.5 (×2), 29.4, 29.3, 25.5, 22.6, 14.1; EIMS
m/
z (
rel. int.) 348 (M
+, 24), 232 (21), 192 (17), 177 (52), 150 (76), 145 (12), 137 (29), 97 (29), 91 (45), 57 (100).
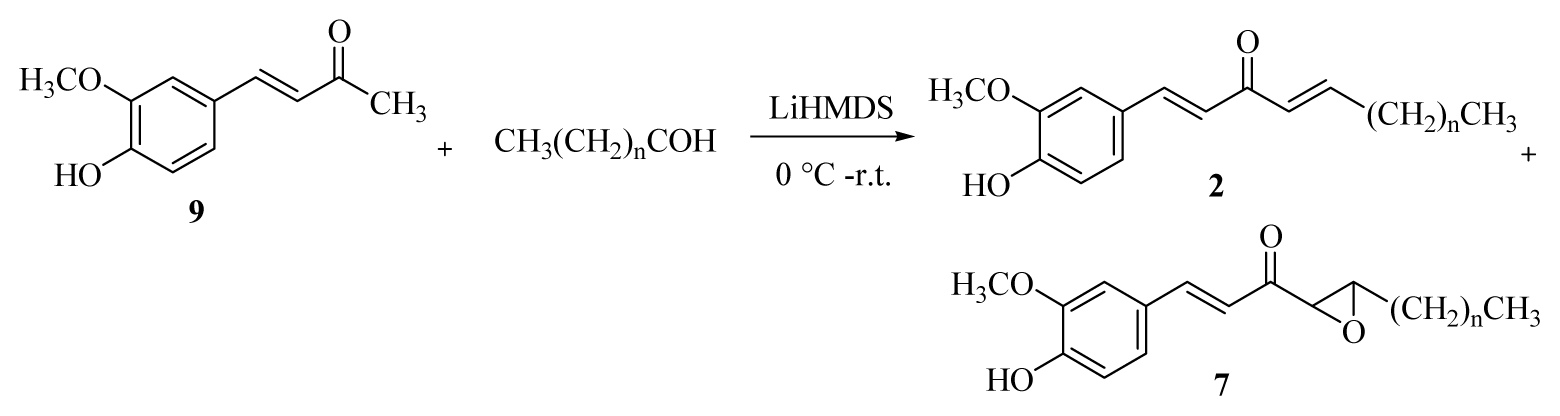
3.2.3. General Procedure for the Synthesis of [n]-Epoxydehydroparadol (7a–f)
A 1.0 M THF solution of lithium bis(trimethylsilyl)amide (20.8 mL) was added dropwise to a solution of dehydrozingerone (9) (2.0 g, 10.4 mmol) in dry THF (10 mL) at 0 °C in an air atmosphere. After the mixture had been stirred for 1 h, the appropriate aldehyde (31.4 mmol) was added and stirred for 3 h. The reaction was then quenched with 5% HCl(aq) at 0 °C and extracted with EtOAc (4 × 20 mL). The organic layers were combined, washed with brine, dried over Na2SO4, and concentrated under reduced pressure. Products 2 and 7 were isolated using C-18 gel column chromatography (water/methanol = 1/2).
1-(4-Hydroxy-3-methoxyphenyl)-4,5-expoxynon-1-en-3-one (7a): yellow syrup (8%); UV (MeOH) λmax (log ɛ) 349 (4.09), 251 (3.75) nm; IR (KBr) νmax 3451, 2956, 2931, 1693, 1587, 1514, 1465, 1271, 1031 cm−1; 1H-NMR (CDCl3) δ 7.71 (1H, d, J = 16.0 Hz, H-1), 7.13 (1H, dd, J = 8.2, 1.8 Hz, H-6′), 7.06 (1H, d, J = 1.8 Hz, H-2′), 6.92 (1H, d, J = 8.2 Hz, H-5′), 6.71 (1H, d, J = 16.0 Hz, H-2), 6.01 (1H, br s, -OH), 3.92 (3H, s, -OCH3), 3.41 (1H, d, J = 2.0 Hz, H-4), 3.11 (1H, td, J = 5.3, 2.0 Hz, H-5), 1.74–1.25 (6H, m, H-6~8), 0.92 (3H, t, J = 6.8 Hz, H-9); 13C-NMR (CDCl3) δ 195.7, 148.7, 146.8, 145.2, 126.8, 124.2, 116.8, 114.8, 109.7, 59.6, 58.4, 56.0, 31.6, 27.9, 22.4, 13.9; EIMS m/z (rel. int.) 276 (M+, 38), 177 (100), 145 (20); HREIMS m/z 276.1363 [M]+ (Calcd for C16H20O4, 276.1361).
1-(4-Hydroxy-3-methoxyphenyl)-4,5-expoxydec-1-en-3-one (7b): yellow syrup (10%); UV (MeOH) λmax (log ɛ) 354 (4.27), 251 (3.93) nm; IR (neat) νmax 3414, 2954, 2862, 1676, 1585, 1512, 1460, 1272, 1031 cm−1; 1H-NMR (CDCl3) δ 7.71 (1H, d, J = 15.9 Hz, H-1), 7.13 (1H, dd, J = 8.1, 1.8 Hz, H-6′), 7.06 (1H, d, J = 1.8 Hz, H-2′), 6.91 (1H, d, J = 8.1 Hz, H-5′), 6.71 (1H, d, J = 15.9 Hz, H-2), 3.92 (3H, s, -OCH3), 3.41 (1H, d, J = 2.0 Hz, H-4), 3.12 (1H, td, J = 5.2, 2.0 Hz, H-5), 1.73–1.24 (8H, m, H-6~9), 0.89 (3H, t, J = 6.8 Hz, H-10); 13C-NMR (CDCl3) δ 195.6, 148.6, 146.7, 145.2, 126.8, 124.2, 116.8, 114.8, 109.7, 59.5, 58.4, 56.0, 31.8, 31.4, 25.4, 22.4, 13.9; EIMS m/z (rel. int.) 290 (M+, 39), 178 (21), 177 (100), 145 (22); HREIMS m/z 290.1516 [M]+ (Calcd for C17H22O4, 290.1518).
1-(4-Hydroxy-3-methoxyphenyl)-4,5-expoxyundec-1-en-3-one (7c): yellow syrup (9%); UV (MeOH) λmax (log ɛ) 352 (4.19), 254 (3.89) nm; IR (neat) νmax 3408, 2927, 2858, 1672, 1585, 1514, 1434, 1276, 1031 cm−1; 1H-NMR (CDCl3) δ 7.71 (1H, d, J = 15.8 Hz, H-1), 7.13 (1H, dd, J = 8.4, 2.0 Hz, H-6′), 7.06 (1H, d, J = 2.0 Hz, H-2′), 6.91 (1H, d, J = 8.4 Hz, H-5′), 6.70 (1H, d, J = 15.8 Hz, H-2), 6.05 (1H, br s, -OH), 3.92 (3H, s, -OCH3), 3.41 (1H, d, J = 2.0 Hz, H-4), 3.13 (1H, td, J = 5.0, 2.0 Hz, H-5), 1.73–1.29 (10H, m, H-6~10), 0.88 (3H, t, J = 6.8 Hz, H-11); 13C-NMR (CDCl3) δ 195.6, 148.6, 146.7, 145.2, 126.8, 124.2, 116.8, 114.8, 109.7, 59.5, 58.4, 56.0, 31.8, 31.6, 28.9, 25.7, 22.4, 13.9 ; EIMS m/z (rel. int.) 304 (M+, 46), 178 (26), 177 (100), 145 (26); HREIMS m/z 304.1673 [M]+ (Calcd for C18H24O4, 304.1674).
1-(4-Hydroxy-3-methoxyphenyl)-4,5-expoxydodec-1-en-3-one (7d): yellow syrup (9%); UV (MeOH) λmax (log ɛ) 351 (4.33), 255 (4.07) nm; IR(neat) νmax 3395, 2925, 2858, 1672, 1581, 1514, 1434, 1172, 1031 cm−1; 1H-NMR (CDCl3) δ 7.70 (1H, d, J = 16.0 Hz, H-1), 7.12 (1H, dd, J = 8.2, 2.0 Hz, H-6′), 7.05 (1H, d, J = 2.0 Hz, H-2′), 6.90 (1H, d, J = 8.2 Hz, H-5′), 6.70 (1H, d, J = 16.0 Hz, H-2), 6.16 (1H, br s, -OH), 3.91 (3H, s, -OCH3), 3.41 (1H, d, J = 2.0 Hz, H-4), 3.12 (1H, td, J = 5.2, 2.0 Hz, H-5), 1.73–1.26 (12H, m, H-6~11), 0.87 (3H, t, J = 6.8 Hz, H-12); 13C-NMR (CDCl3) δ 195.7, 148.7, 146.8, 145.2, 126.8, 124.2, 116.8, 114.8, 109.7, 59.5, 58.4, 56.0, 31.8, 31.7, 29.2, 29.1, 25.8, 22.6, 14.0; EIMS m/z (rel. int.) 318 (M+, 37), 178 (22), 177 (100), 145 (19); HREIMS m/z 318.1834 [M]+ (Calcd for C19H26O4, 318.1831).
1-(4-Hydroxy-3-methoxyphenyl)-4,5-expoxytridec-1-en-3-one (7e): yellow syrup (8%); UV (MeOH) λmax (log ɛ) 353 (4.32), 254 (4.04) nm; IR (neat) νmax 3404, 2925, 2856, 1672, 1583, 1514, 1434, 1276, 1031 cm−1; 1H-NMR (CDCl3) δ 7.72 (1H, d, J = 15.8 Hz, H-1), 7.14 (1H, dd, J = 8.2, 1.8 Hz, H-6′), 7.07 (1H, d, J = 1.8 Hz, H-2′), 6.92 (1H, d, J = 8.2 Hz, H-5′), 6.71 (1H, d, J = 15.8 Hz, H-2), 5.95 (1H, br s, -OH), 3.94 (3H, s, -OCH3), 3.41 (1H, d, J = 2.0 Hz, H-4), 3.13 (1H, td, J = 5.0, 2.0 Hz, H-5), 1.74–1.27 (14H, m, H-6~12), 0.88 (3H, t, J = 6.8 Hz, H-13); 13C-NMR (CDCl3) δ 195.6, 148.7, 146.8, 145.1, 126.9, 124.2, 116.9, 114.8, 109.7, 59.6, 58.4, 56.0, 31.8 (×2), 29.4, 29.3, 29.1, 25.8, 22.6, 14.0; EIMS m/z (rel. int.) 332 (M+, 36), 177 (100), 145 (15), 55 (12); HREIMS m/z 332.1990 [M]+ (Calcd for C20H28O4, 332.1987).
1-(4-Hydroxy-3-methoxyphenyl)-4,5-expoxytetradec-1-en-3-one (7f): yellow syrup (8%); UV (MeOH) λmax (log ɛ) 350 (4.19), 253 (4.17) nm; IR (neat) νmax 3423, 2925, 2854, 1676, 1585, 1512, 1460, 1274, 1031 cm−1; 1H-NMR (CDCl3) δ 7.71 (1H, d, J = 15.8 Hz, H-1), 7.14 (1H, dd, J = 8.2, 1.8 Hz, H-6′), 7.07 (1H, d, J = 1.8 Hz, H-2′), 6.92 (1H, d, J = 8.2 Hz, H-5′), 6.72 (1H, d, J = 15.8 Hz, H-2), 5.90 (1H, br s, -OH), 3.94 (3H, s, -OCH3), 3.41 (1H, d, J = 2.0 Hz, H-4), 3.13 (1H, td, J = 5.4, 2.0 Hz, H-5), 1.74–1.27 (16H, m, H-6~13), 0.88 (3H, t, J = 6.8 Hz, H-14); 13C-NMR (CDCl3) δ 195.7, 148.7, 146.8, 145.2, 126.9, 124.2, 116.8, 114.8, 109.7, 59.6, 58.4, 56.0, 31.9, 31.8, 29.5, 29.4, 29.3, 29.2, 25.8, 22.6, 14.1; EIMS m/z (rel. int.) 346 (M+, 33), 177 (100), 151 (23), 150 (55), 55 (20); HREIMS m/z 346.2145 [M]+ (Calcd for C21H30O4, 346.2144).
3.2.4. General Procedure for the Synthesis of [n]-Paradols (5a–f)
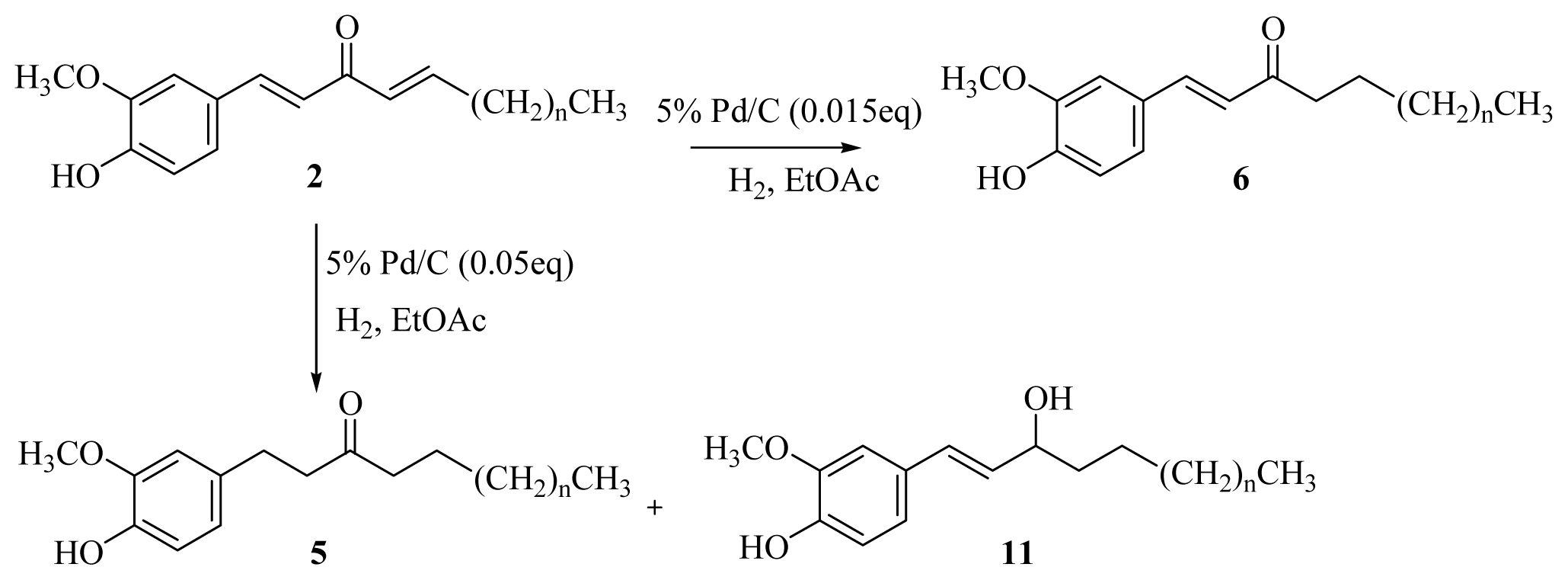
A solution of [n]-dehydroshogaols (2a–f) (0.96 mmol) in ethyl acetate (20 mL) containing palladium-charcoal (5%, 0.05 g) was stirred under hydrogen at atmospheric pressure and room temperature for 30 min. The reaction mixture was monitored by TLC until no starting material remained. The catalyst was removed through celite, and the filtrate was concentrated under reduced pressure. The product was isolated using silica gel column chromatography (EtOAc/hexanes = 1/4).
[5]-Paradol (5a): colorless syrup (79%) [
23]; UV (MeOH) λ
max (log ɛ) 282 (3.41) nm; IR (neat) ν
max 3439, 2939, 2862, 1707, 1608, 1516, 1452, 1365, 1269, 1031, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.79 (1H, d,
J = 8.0 Hz, H-5′), 6.67 (1H, d,
J = 1.8 Hz, H-2′), 6.63 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 3.82 (3H, s, -OCH
3), 2.84–2.63 (4H, m, H-1, -2), 2.35 (2H, t,
J = 7.2 Hz, H-4), 1.58–1.51 (2H, m, H-5), 1.23 (6H, m, H-6~8), 0.87 (3H, t,
J = 6.2 Hz, H-9);
13C-NMR (CDCl
3) δ 210.8, 146.4, 143.8, 132.9, 120.6, 114.3, 111.1, 55.7, 44.5, 43.0, 31.5, 29.4, 28.8, 23.7, 22.4, 14.0; EIMS
m/
z (
rel. int.) 264 (M
+, 58), 179 (19), 151 (22), 137 (100); HREIMS
m/
z 246.1729 [M]
+ (Calcd for C
16H
24O
3, 246.1725).
[6]-Paradol (5b): colorless syrup (78%) [
23]; UV (MeOH) λ
max (log ɛ) 281 (3.34) nm; IR (neat) ν
max 3451, 2940, 2862, 1713, 1516, 1452, 1367, 1267, 1036, 804 cm
−1; 1H-NMR (CDCl
3) δ 6.80 (1H, d,
J = 8.0 Hz, H-5′), 6.67 (1H, d,
J = 1.8 Hz, H-2′), 6.64 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 3.85 (3H, s, -OCH
3), 2.86–2.63 (4H, m, H-1, -2), 2.36 (2H, t,
J = 7.4 Hz, H-4), 1.58–1.51 (2H, m, H-5), 1.24 (8H, m, H-6~9), 0.88 (3H, t,
J = 6.2 Hz, H-10);
13C-NMR (CDCl
3) δ 210.6, 146.3, 143.8, 133.1, 120.7, 114.3, 111.0, 55.8, 44.6, 43.1, 31.6, 29.5, 29.1, 29.0, 23.8, 22.5, 14.0; EIMS
m/
z (
rel. int.) 278 (M
+, 67), 179 (21), 151 (23), 137 (100), 117 (19), 99 (23), 55 (21); HREIMS
m/
z 278.1883 [M]
+ (Calcd for C
17H
26O
3, 278.1881).
[7]-Paradol (5c): colorless syrup (81%) [
23]; UV (MeOH) λ
max (log ɛ) 282 (3.45) nm; IR (neat) ν
max 3543, 2930, 2858, 1707, 1608, 1516, 1452, 1365, 1269, 1034, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.77 (1H, d,
J = 8.0 Hz, H-5′), 6.66 (1H, d,
J = 1.8 Hz, H-2′), 6.61 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 5.48 (1H, br s, -OH), 3.79 (3H, s, -OCH
3), 2.83–2.61 (4H, m, H-1, -2), 2.33 (2H, t,
J = 7.2 Hz, H-4), 1.58–1.51 (2H, m, H-5), 1.22 (10H, m, H-6~10), 0.85 (3H, t,
J = 6.8 Hz, H-11);
13C-NMR (CDCl
3) δ 210.7, 146.5, 143.9, 132.9, 120.6, 114.4, 111.1, 55.7, 44.4, 42.9, 31.7, 29.4, 29.2, 29.1, 29.0, 23.7, 22.5, 14.0; EIMS
m/
z (
rel. int.) 292 (M
+, 36), 179 (17), 151 (21), 137 (100), 119 (10), 55 (11).
[8]-Paradol (5d): colorless powder (77%), mp 42–43 °C (lit. 42–43 °C) [
23]; UV (MeOH) λ
max (log ɛ) 282 (3.46) nm; IR (KBr) ν
max 3541, 2920, 2856, 1707, 1608, 1514, 1365, 1271, 1030, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.82 (1H, d,
J = 7.8 Hz, H-5′), 6.68–6.63 (2H, m, H-2′, -6′), 3.86 (3H, s, -OCH
3), 2.87–2.64 (4H, m, H-1, -2), 2.37 (2H, t,
J = 7.2 Hz, H-4), 1.58–1.51 (2H, m, H-5), 1.25 (12H, m, H-6~11), 0.88 (3H, t,
J = 6.8 Hz, H-12);
13C-NMR (CDCl
3) δ 210.6, 146.4, 143.8, 133.1, 120.7, 114.3, 111.0, 55.8, 44.6, 43.1, 31.8, 29.5, 29.3 (×3), 29.2, 23.8, 22.6, 14.1; EIMS
m/
z (
rel. int.) 306 (M
+, 17), 292 (12), 164 (21), 179 (19), 151 (22), 137 (100), 57 (10); Anal. Calcd. for C
19H
30O
3: C, 74.50%; H, 9.80%; Found: C, 74.57%; H, 9.84%.
[9]-Paradol (5e): colorless powder (80%), mp 49–50 °C (lit. 48–49 °C) [
23]; UV (MeOH) λ
max (log ɛ) 282 (3.41) nm; IR (KBr) ν
max 3516, 2922, 2856, 1712, 1608, 1516, 1361, 1273, 1165, 1028, 856 cm
−1; 1H-NMR (CDCl
3) δ 6.81 (1H, d,
J = 8.0 Hz, H-5′), 6.68–6.63 (2H, m, H-2′, -6′), 3.86 (3H, s, -OCH
3), 2.86–2.64 (4H, m, H-1, -2), 2.36 (2H, t,
J = 7.2 Hz, H-4), 1.58–1.51 (2H, m, H-5), 1.24 (14H, m, H-6~12), 0.87 (3H, t,
J = 6.8 Hz, H-13);
13C-NMR (CDCl
3) δ 210.6, 146.3, 143.8, 133.1, 120.7, 114.2, 111.0, 56.0, 44.5, 43.1, 31.8, 29.5 (×2), 29.4 29.3, 29.2, 29.1, 23.8, 22.6, 14.1; EIMS
m/
z (
rel. int.) 320 (M
+, 80), 179 (19), 151 (21), 137 (100), 119 (8); Anal. Calcd. for C
20H
32O
3: C, 75.00%; H, 10.00%; Found: C, 75.01%; H, 10.01%.
[10]-Paradol (5f): colorless powder (79%), mp 50–51 °C (lit. 50–51 °C) [
23]; UV (MeOH) λ
max (log ɛ) 280 (3.44) nm; IR (KBr) ν
max 3486, 2920, 2856, 1707, 1608, 1512, 1361, 1273, 1165, 1028, 856 cm
−1; 1H-NMR (CDCl
3) δ 6.81 (1H, d,
J = 8.0 Hz, H-5′), 6.68–6.63 (2H, m, H-2′, -6′), 3.85 (3H, s, -OCH
3), 2.86–2.64 (4H, m, H-1, -2), 2.37 (2H, t,
J = 7.2 Hz, H-4), 1.58–1.51 (2H, m, H-5), 1.25 (16H, m, H-6~13), 0.88 (3H, t,
J = 6.6 Hz, H-14);
13C-NMR (CDCl
3) δ 210.6, 146.4, 143.8, 133.0, 120.6, 114.3, 111.0, 55.7, 44.5, 43.0, 31.8, 29.5 (×2), 29.4 29.3 (×2), 29.2, 29.1, 23.7, 22.6, 14.0; Anal. Calcd. for C
21H
34O
3: C, 75.45%; H, 10.18%; Found: C, 75.49%; H, 10.13%.
3.2.5. General Procedure for the Synthesis of [n]-Dehydroparadols (6a–f)
A solution of [n]-dehydroshogaols (2a–f) (0.96 mmol) in ethyl acetate (20 mL) containing palladium-charcoal (5%, 0.015 g) was stirred under hydrogen at atmospheric pressure and room temperature for 40 min. The reaction mixture was monitored using thin layer chromatography (TLC) until no starting material remained. The catalyst was removed through celite, and the filtrate was concentrated under reduced pressure conditions. The product was isolated using silica gel column chromatography (EtOAc/hexanes = 1/3).
[5]-Dehydroparadol (6a): colorless powder (80%), mp 52–53 °C (lit. 52–53 °C) [
23]; UV (MeOH) λ
max (log ɛ) 340 (4.16), 224 (3.79) nm; IR (KBr) ν
max 3400, 2930, 2860, 1666, 1587, 1514, 1460, 1375, 1276, 1033, 979, 812 cm
−1; 1H-NMR (CDCl
3) δ 7.48 (1H, d,
J = 16.1 Hz, H-1), 7.09 (1H, dd,
J = 8.1, 2.0 Hz, H-6′), 7.05 (1H, d,
J = 2.0 Hz, H-2′), 6.92 (1H, d,
J = 8.1 Hz, H-5′), 6.59 (1H, d,
J = 16.1 Hz, H-2), 6.12 (1H, br s, -OH), 3.92 (3H, s, -OCH
3), 2.64 (2H, t,
J = 7.1 Hz, H-4), 1.70–1.59 (2H, m, H-5), 1.41–1.22 (6H, m, H-6~8), 0.88 (3H, t,
J = 6.5 Hz, H-9);
13C-NMR (CDCl
3) δ 200.8, 148.1, 146.8, 142.6, 127.1, 124.1, 123.3, 114.8, 109.4, 55.9, 40.7, 31.6, 29.0, 24.5, 22.5, 14.0; EIMS
m/z (
rel. int.) 262 (M
+, 31), 192 (34), 177 (100), 145 (22), 137 (44), 117 (10), 89 (12).
[6]-Dehydroparadol (6b): colorless powder (76%), mp 47–48 °C (lit. 44–45 °C) [
23]; UV (MeOH) λ
max (log ɛ) 341 (4.04), 224 (3.85) nm; IR (KBr) ν
max 3400, 2926, 2856, 1666, 1601, 1514, 1460, 1375, 1278, 1031, 979, 810 cm
−1; 1H-NMR (CDCl
3) δ 7.47 (1H, d,
J = 16.0 Hz, H-1), 7.08 (1H, dd,
J = 8.1, 1.8 Hz, H-6′), 7.04 (1H, d,
J = 1.8 Hz, H-2′), 6.91 (1H, d,
J = 8.1 Hz, H-5′), 6.58 (1H, d,
J = 16.0 Hz, H-2), 6.19 (1H, br s, -OH), 3.90 (3H, s, -OCH
3), 2.63 (2H, t,
J = 7.2 Hz, H-4), 1.69–1.59 (2H, m, H-5), 1.31–1.27 (8H, m, H-6~9), 0.88 (3H, t,
J = 6.6 Hz, H-10);
13C-NMR (CDCl
3) δ 200.8, 148.1, 146.8, 142.6, 126.9, 123.9, 123.3, 114.8, 109.4, 55.9, 40.6, 31.6, 29.3, 29.0, 24.5, 22.5, 14.0; EIMS
m/z (
rel. int.) 276 (M
+, 31), 192 (40), 177 (100), 145 (19), 137 (71), 117 (10), 89 (11), 55(10); HREIMS
m/z 276.1727 [M]
+ (Calcd for C
17H
24O
3, 276.1725).
[7]-Dehydroparadol (6c): colorless powder (75%), mp 49–50 °C (lit. 45–46 °C) [
23]; UV (MeOH) λ
max (log ɛ) 338 (4.03), 225 (3.80) nm; IR (KBr) ν
max 3401, 2926, 2854, 1676, 1589, 1514, 1460, 1377, 1207, 1033, 979, 810 cm
−1; 1H-NMR (CDCl
3) δ 7.46 (1H, d,
J = 16.1 Hz, H-1), 7.05 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 7.01 (1H, d,
J = 1.8 Hz, H-2′), 6.89 (1H, d,
J = 8.0 Hz, H-5′), 6.57 (1H, d,
J = 16.1 Hz, H-2), 3.86 (3H, s, -OCH
3), 2.61 (2H, t,
J = 7.2 Hz, H-4), 1.67–1.57 (2H, m, H-5), 1.26–1.23 (10H, m, H-6~10), 0.85 (3H, t,
J = 6.8 Hz, H-11);
13C-NMR (CDCl
3) δ 200.9, 148.3, 146.9, 142.8, 126.8, 123.8, 123.3, 114.9, 109.5, 55.8, 40.5, 31.7, 29.3 (×2), 29.1, 24.5, 22.6, 14.0; EIMS
m/z (
rel. int.) 290 (M
+, 15), 205 (12), 192 (28), 177 (63), 137 (100), 91 (11), 55(12); HREIMS
m/z 290.1885 [M]
+ (Calcd for C
18H
26O
3, 290.1881).
[8]-Dehydroparadol (6d): colorless powder (73%), mp 58–59 °C (lit. 57–58 °C) [
23]; UV (MeOH) λ
max (log ɛ) 339 (4.09), 223 (3.96) nm; IR (KBr) ν
max 3401, 2925, 2854, 1675, 1589, 1514, 1460, 1272, 1033, 979, 810 cm
−1; 1H-NMR (CDCl
3) δ 7.48 (1H, d,
J = 16.0 Hz, H-1), 7.06 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 7.04 (1H, d,
J = 1.8 Hz, H-2′), 6.91 (1H, d,
J = 8.0 Hz, H-5′), 6.59 (1H, d,
J = 16.0 Hz, H-2), 6.19 (1H, br s, -OH), 3.91 (3H, s, -OCH
3), 2.63 (2H, t,
J = 7.0 Hz, H-4), 1.67–1.62 (2H, m, H-5), 1.28–1.25 (12H, m, H-6~11), 0.86 (3H, t,
J = 6.8 Hz, H-12);
13C-NMR (CDCl
3) δ 200.8, 148.1, 146.8, 142.7, 127.0, 123.9, 123.3, 114.8, 109.4, 55.9, 40.6, 31.8, 29.4 (×2), 29.3, 29.2, 24.5, 22.6, 14.0; EIMS
m/z (
rel. int.) 304 (M
+, 27), 205 (13), 192 (34), 177 (66), 151 (18), 137 (100), 91 (10), 55(12); Anal. Calcd for C
19H
28O
3: C, 75.00%; H, 9.21%; Found: C, 74.99%; H, 9.25%.
[9]-Dehydroparadol (6e): colorless powder (74%), mp 56–58 °C (lit. 53–54 °C) [
23]; UV (MeOH) λ
max (log ɛ) 337 (3.96), 224 (3.74) nm; IR (KBr) ν
max 3395, 2925, 2854, 1666, 1589, 1516, 1460, 1277, 1033, 979, 812 cm
−1; 1H-NMR (CDCl
3) δ 7.47 (1H, d,
J = 16.0 Hz, H-1), 7.08 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 7.03 (1H, d,
J = 1.8 Hz, H-2′), 6.90 (1H, d,
J = 8.0 Hz, H-5′), 6.58 (1H, d,
J = 16.0 Hz, H-2), 6.22 (1H, br s, -OH), 3.90 (3H, s, -OCH
3), 2.63 (2H, t,
J = 7.2 Hz, H-4), 1.69–1.62 (2H, m, H-5), 1.28–1.24 (14H, m, H-6~12), 0.86 (3H, t,
J = 6.6 Hz, H-13);
13C-NMR (CDCl
3) δ 200.8, 148.1, 146.8, 142.6, 126.9, 123.9, 123.3, 114.8, 109.4, 55.8, 40.6, 31.8, 29.5, 29.4, 29.3 (×2), 29.2, 24.5, 22.5, 14.0; EIMS
m/z (
rel. int.) 318 (M
+, 27), 192 (57), 177 (100), 153 (22), 137 (39), 55(23).
[10]-Dehydroparadol (6f): colorless powder (79%), mp 72–73 °C (lit. 76–77 °C) [
23]; UV (MeOH) λ
max (log ɛ) 339 (3.99), 225 (3.78) nm; IR(KBr) ν
max 3412, 2920, 2854, 1666, 1589, 1512, 1460, 1277, 1033 cm
−1; 1H-NMR (CDCl
3) δ 7.47 (1H, d,
J = 16.0 Hz, H-1), 7.10–7.04 (2H, m, H-2′,6′), 6.89 (1H, d,
J = 8.2 Hz, H-5′), 6.58 (1H, d,
J = 16.0 Hz, H-2), 6.25 (1H, br s, -OH), 3.90 (3H, s, -OCH
3), 2.63 (2H, t,
J = 7.2 Hz, H-4), 1.69–1.59 (2H, m, H-5), 1.28–1.25 (16H, m, H-6~13), 0.86 (3H, t,
J = 6.4 Hz, H-14);
13C-NMR (CDCl
3) δ 200.8, 148.1, 146.8, 142.6, 126.9, 123.9, 123.3, 114.8, 109.4, 55.8, 40.5, 31.8, 29.5 (×2), 29.4, 29.3 (×2), 29.2, 24.5, 22.6, 14.0.
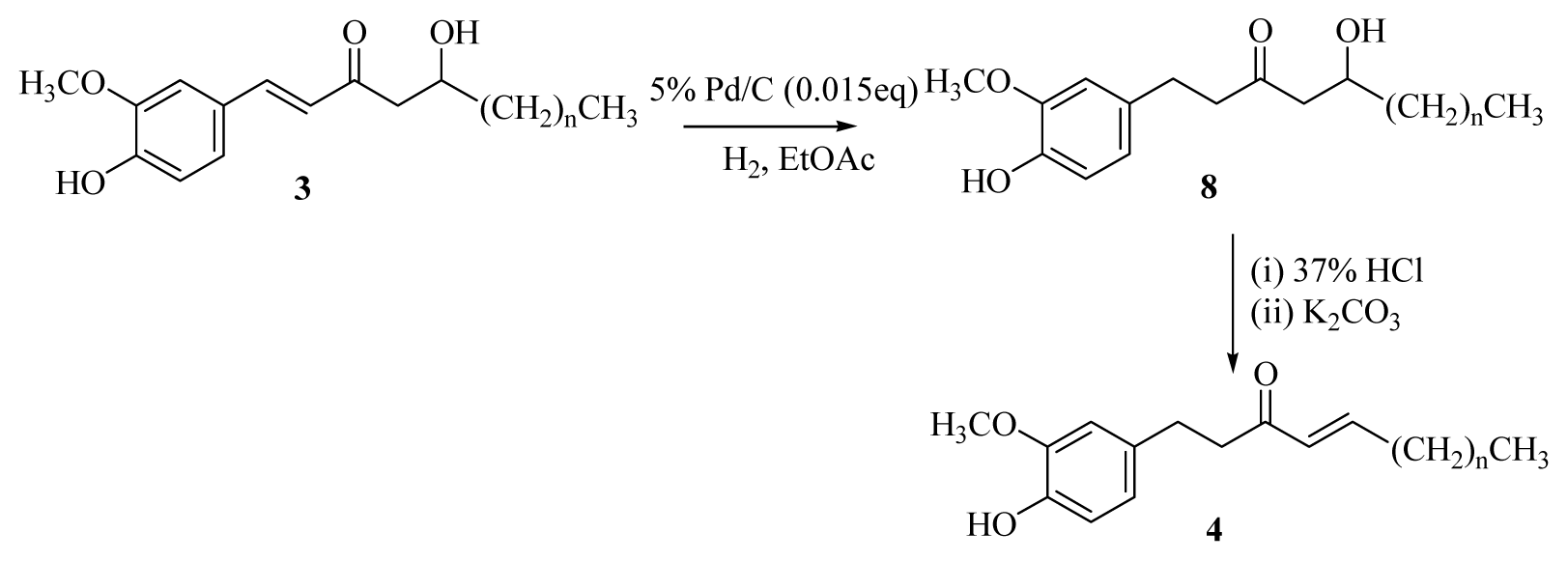
3.2.6. General Procedure for the Synthesis of [n]-Gingerols (8a–f)
A solution of [n]-dehydrogingerols (3a–f) (1.1 mmol) in ethyl acetate (20 mL) containing palladium-charcoal (5%, 0.04 g) was stirred under hydrogen at atmospheric pressure and room temperature for 40 min. The reaction mixture was monitored using TLC until no starting material remained. The catalyst was removed through celite, and the filtrate was concentrated under reduced pressure. The product was isolated using silica gel column chromatography (EtOAc/hexanes = 1/2).
[5]-Gingerol (8a): colorless powder (85%), mp 44–45 °C (lit. 45–46 °C); UV (MeOH) λ
max (log ɛ) 282 (3.44), 224 (3.85) nm; IR (KBr) ν
max 3460, 2943, 2864, 1704, 1612, 1138, 1371, 1271, 1138, 1034, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.78 (1H, d,
J = 7.8 Hz, H-5′), 6.64 (1H, d,
J = 1.8 Hz, H-2′), 6.61 (1H, dd,
J = 7.8, 1.8 Hz, H-6′), 4.34 (1H, br s, -OH), 4.06–3.94 (1H, m, H-5), 3.81 (3H, s, -OCH
3), 2.48–2.65 (4H, m, H-1, -2), 2.52–2.48 (2H, m, H-4), 1.50–1.22 (6H, m, H-6~8), 0.86 (3H, t,
J = 7.0 Hz, H-9);
13C-NMR (CDCl
3) δ 211.4, 147.0, 144.0, 132.6, 120.7, 114.4, 111.0, 67.6, 55.9, 49.3, 45.4, 36.1, 29.2, 27.6, 22.6, 14.3; EIMS
m/
z (
rel. int.) 280 (M
+, 31), 205 (9), 150 (50), 137 (100), 91 (10); HREIMS
m/
z 280.1677 [M]
+ (Calcd for C
16H
24O
4, 280.1674).
[6]-Gingerol (8b): colorless syrup (84%); UV (MeOH) λ
max (log ɛ) 282 (3.51) nm; IR (KBr) ν
max 3469, 2937, 2860, 1705, 1608, 1516, 1371, 1140, 1036, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.79 (1H, d,
J = 7.8 Hz, H-5′), 6.66–6.60 (2H,m, H-2′, -6′), 4.05–3.99 (1H, m, H-5), 3.83 (3H, s, -OCH
3), 2.86–2.66 (4H, m, H-1, -2), 2.53–2.48 (2H, m, H-4), 1.49–1.24 (8H, m, H-6~9), 0.86 (3H, t,
J = 6.2 Hz, H-10);
13C-NMR (CDCl
3) δ 211.4, 146.4, 143.9, 132.5, 120.6, 114.4, 110.9, 67.6, 55.7, 49.2, 45.3, 36.3, 31.6, 29.1, 25.6, 22.5, 14.0; EIMS
m/
z (
rel. int.) 294 (M
+, 18), 205 (7), 194 (14), 150 (40), 137 (100), 91 (11); HREIMS
m/
z 294.1831 [M]
+ (Calcd for C
17H
26O
4, 294.1831).
[7]-Gingerol (8c): colorless powder (86%), mp 101–102 °C; UV (MeOH) λ
max (log ɛ) 281 (3.67), 223 (3.95) nm; IR (KBr) ν
max 3524, 2926, 2858, 1705, 1608, 1516, 1369, 1271, 1036, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.80 (1H, d,
J = 8.0 Hz, H-5′), 6.66–6.60 (2H, m, H-2′, -6′), 4.06–3.95 (1H, m, H-5), 3.84 (3H, s, -OCH
3), 2.86–2.66 (4H, m, H-1, -2), 2.52–2.48 (2H, m, H-4), 1.51–1.25 (10H, m, H-6~10), 0.86 (3H, t,
J = 6.6 Hz, H-11);
13C-NMR (CDCl
3) δ 211.5, 146.5, 144.0, 132.6, 120.7, 114.5, 111.1, 67.7, 55.8, 49.3, 45.4, 36.5, 31.8, 29.2, 29.1, 25.4, 22.6, 14.1; EIMS
m/
z (
rel. int.) 308 (M
+, 16), 290 (15), 205 (21), 150 (32), 137 (100), 91 (13), 55 (24); Anal. Calcd. for C
18H
28O
4: C, 70.12%; H, 9.09%; Found: C, 70.14%; H, 9.04%.
[8]-Gingerol (8d): colorless syrup (83%); UV (MeOH) λ
max (log ɛ) 282 (3.63), 222 (3.93) nm; IR (neat) ν
max 3516, 2928, 2858, 1705, 1608, 1516, 1452, 1271, 1035, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.80 (1H, d,
J = 7.8 Hz, H-5′), 6.66–6.61 (2H, m, H-2′, -6′), 4.05–3.95 (1H, m, H-5), 3.85 (3H, s, -OCH
3), 2.86–2.67 (4H, m, H-1, -2), 2.53–2.48 (2H, m, H-4), 1.49–1.25 (12H, m, H-6~11), 0.86 (3H, t,
J = 6.2 Hz, H-12);
13C-NMR (CDCl
3) δ 211.3, 146.4, 143.9, 132.5, 120.6, 114.3, 110.9, 67.6, 55.7, 49.2, 45.3, 36.4, 31.7, 29.4, 29.1, 25.3, 22.5, 14.0; EIMS
m/
z (
rel. int.) 322 (M
+, 38), 150 (50), 137 (100), 55 (9).
[9]-Gingerol (8e): colorless syrup (84%); UV (MeOH) λ
max (log ɛ) 281 (3.33), 224 (3.71) nm; IR (neat) ν
max 3535, 2924, 2856, 1704, 1608, 1514, 1369, 1271, 1031, 804 cm
−1; 1H-NMR (CDCl
3) δ 6.80 (1H, d,
J = 7.8 Hz, H-5′), 6.66–6.60 (2H, m, H-2′, -6′), 4.07–3.99 (1H, m, H-5), 3.84 (3H, s, -OCH
3), 2.86–2.66 (4H, m, H-1, -2), 2.53–2.48 (2H, m, H-4), 1.38–1.25 (14H, m, H-6~12), 0.87 (3H, t,
J = 6.8 Hz, H-13);
13C-NMR (CDCl
3) δ 211.4, 146.5, 143.9, 132.6, 120.7, 114.5, 111.0, 67.7, 55.8, 49.3, 45.4, 36.5, 31.8, 29.5 (×2), 29.2 (×2), 25.4, 22.6, 14.0; EIMS
m/
z (
rel. int.) 336 (M
+, 32), 318 (14), 205 (17), 150 (36), 137 (100), 55 (9); HREIMS
m/
z: 336.2300 [M]
+ (Calcd for C
20H
32O
4, 336.2300).
[10]-Gingerol (8f): colorless syrup (85%); UV (MeOH) λ
max (log ɛ) 282 (3.43), 224 (3.75) nm; IR (neat) ν
max 3439, 2920, 2854, 1706, 1608, 1514, 1369, 1271, 1031, 804 cm
−1; 1H-NMR (CDCl
3) δ 6.78 (1H, d,
J = 7.8 Hz, H-5′), 6.65–6.59 (2H, m, H-2′, -6′), 4.05–3.99 (1H, m, H-5), 3.82 (3H, s, -OCH
3), 2.83–2.65 (4H, m, H-1, -2), 2.53–2.49 (2H, m, H-4), 1.38–1.25 (16H, m, H-6~13), 0.87 (3H, t,
J = 6.6 Hz, H-14);
13C-NMR (CDCl
3) δ 211.3, 146.4, 143.8, 132.4, 120.5, 114.4, 110.9, 67.6, 55.6, 49.2, 45.2, 36.3, 31.7, 29.4 (×3), 29.2, 29.1, 25.3, 22.5, 14.0.
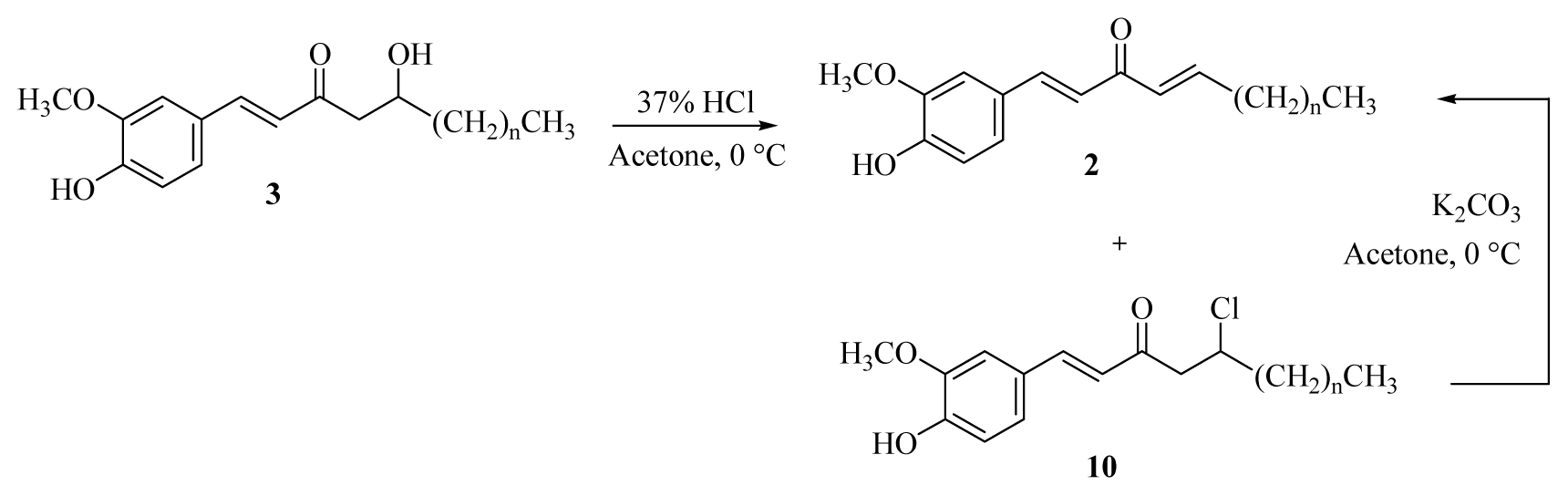
3.2.7. General Procedure for the Synthesis of [n]-Shogaols (4a–f)
Conc. HCl (0.1 mL) was added dropwise to a solution of [n]-gingerols (8a–f) (0.54 mmol) in acetone (10 mL) at room temperature. The reaction mixture was stirred for 15 min and then cooled to 0 °C in an ice bath, neutralized by saturated sodium bicarbonate, and extracted with CH2Cl2 (3 × 10 mL). The organic layers were combined, washed with brine, dried over MgSO4, and concentrated under reduced pressure. The crude product was diluted with acetone (10 mL) and then potassium carbonate (0.81 mmol) was added at room temperature. The reaction mixture was stirred for 6h, then cooled to 0 °C in an ice bath, neutralized by 5% HCl(aq), and extracted with CH2Cl2 (3 × 10 mL). The organic layers were combined, washed with brine, dried over MgSO4, and concentrated under reduced pressure. The product was isolated using silica gel column chromatography (ethyl acetate/hexanes = 1/3).
[5]-Shogaol (4a): yellow syrup (86%) [
24]; UV (MeOH) λ
max (log ɛ) 281 (3.51), 225 (4.31) nm; IR (neat) ν
max 3451, 2932, 2862, 1685, 1629, 1514, 1456, 1271, 1034, 984, 806 cm
−1; 1H-NMR (CDCl
3) δ 6.89–6.65 (4H, m, H-2′, -5′, -6′, -5), 6.07 (1H, dt,
J = 15.8, 1.6 Hz, H-4), 5.50 (1H, br s, -OH), 3.87 (3H, s, -OCH
3), 2.87–2.79 (4H, m, H-1, -2), 2.25–2.14 (2H, m, H-6), 1.49–1.23 (4H, m, H-7, -8), 0.90 (3H, t,
J = 6.8 Hz, H-9);
13C-NMR (CDCl
3) δ 199.8, 147.8, 146.3, 143.8, 133.2, 130.3, 120.8, 114.3, 111.1, 55.8, 42.0, 32.1, 30.1, 29.9, 22.2, 13.8; EIMS
m/
z (
rel. int.) 262 (M
+, 46), 205 (42), 151 (16), 137 (100), 55 (22).
[6]-Shogaol (4b): yellow syrup (85%) [
24]; UV (MeOH) λ
max (log ɛ) 282 (3.47), 224 (4.25) nm; IR (neat) ν
max 3424, 2928, 2860, 1662, 1616, 1514, 1456, 1271, 1034, 982, 808 cm
−1; 1H-NMR (CDCl
3) δ 6.89–6.65 (4H, m, H-2′, -5′, -6′, -5), 6.08 (1H, dt,
J = 16.0, 1.4 Hz, H-4), 5.54 (1H, br s, -OH), 3.86 (3H, s, -OCH
3), 2.89–2.79 (4H, m, H-1, -2), 2.24–2.13 (2H, m, H-6), 1.51–1.26 (6H, m, H-7~9), 0.88 (3H, t,
J = 6.5 Hz, H-10);
13C-NMR (CDCl
3) δ 199.8, 147.8, 146.3, 143.8, 133.2, 130.2, 120.7, 114.2, 111.0, 55.8, 41.9, 32.4, 31.3, 29.8, 27.1, 22.4, 13.9; EIMS
m/
z (
rel. int.) 276 (M
+, 43), 205 (52), 151 (16), 137 (100), 119 (10), 55 (18).
[7]-Shogaol (4c): yellow syrup (83%) [
24]; UV (MeOH) λ
max (log ɛ) 282 (3.44), 225 (4.18) nm; IR (neat) ν
max 3450, 2927, 2856, 1691, 1626, 1516, 1460, 1367, 1271, 1036, 978, 815 cm
−1; 1H-NMR (CDCl
3) δ 6.89–6.65 (4H, m, H-2′, -5′, -6′, -5), 6.08 (1H, dt,
J = 16.0, 1.5 Hz, H-4), 3.86 (3H, s, -OCH
3), 2.86–2.82 (4H, m, H-1, -2), 2.24–2.14 (2H, m, H-6), 1.47–1.26 (8H, m, H-7~10), 0.88 (3H, t,
J = 6.7 Hz, H-11);
13C-NMR (CDCl
3) δ 199.8, 147.9, 146.4, 143.8, 133.2, 130.3, 120.8, 114.3, 111.1, 55.8, 42.0, 32.5, 31.5, 29.8, 28.8, 28.0, 22.5, 14.0; EIMS
m/
z (
rel. int.) 290 (M
+, 27), 205 (28), 151 (14), 137 (100), 55 (9).
[8]-Shogaol (4d): yellow syrup (79%) [
24]; UV (MeOH) λ
max (log ɛ) 282 (3.72), 225 (4.52) nm; IR (neat) ν
max 3433, 2926, 2856, 1675, 1629, 1514, 1456, 1271, 1034, 980, 808 cm
−1; 1H-NMR (CDCl
3) δ 6.89–6.65 (4H, m, H-2′, -5′, -6′, -5), 6.08 (1H, dt,
J = 15.8, 1.6 Hz, H-4), 3.86 (3H, s, -OCH
3), 2.85–2.82 (4H, m, H-1, -2), 2.24–2.13 (2H, m, H-6), 1.47–1.27 (10H, m, H-7~11), 0.88 (3H, t,
J = 6.7 Hz, H-12);
13C-NMR (CDCl
3) δ 199.8, 147.9, 146.4, 143.8, 133.2, 130.2, 120.7, 114.3, 111.1, 55.8, 41.9, 32.4, 31.7, 29.8, 29.1, 28.0, 22.6, 14.0; EIMS
m/
z (
rel. int.) 304 (M
+, 34), 205 (51), 151 (18), 137 (100), 69 (20), 55 (26).
[9]-Shogaol (4e): yellow syrup (85%) [
24]; UV (MeOH) λ
max (log ɛ) 282 (3.55), 226 (4.36) nm; IR (neat) ν
max 3432, 2926, 2856, 1685, 1638, 1514, 1471, 1271, 1034, 982, 808 cm
−1; 1H-NMR (CDCl
3) δ 6.91–6.66 (4H, m, H-2′, -5′, -6′, -5), 6.08 (1H, dt,
J = 15.7, 1.4 Hz, H-4), 3.87 (3H, s, -OCH
3), 2.87–2.80 (4H, m, H-1, -2), 2.25–2.14 (2H, m, H-6), 1.47–1.27 (12H, m, H-7~12), 0.88 (3H, t,
J = 6.6 Hz, H-13);
13C-NMR (CDCl
3) δ 199.8, 147.9, 146.4, 143.9, 133.2, 130.3, 120.8, 114.3, 111.1, 55.9, 42.0, 32.5, 31.8, 29.9, 29.3, 29.1, 28.1, 22.6, 14.1; EIMS
m/
z (
rel. int.) 318 (M
+, 35), 205 (58), 151 (16), 137 (100), 55 (15).
[10]-Shogaol (4f): yellow syrup (88%) [
24]; UV (MeOH) λ
max (log ɛ) 284 (3.45), 225 (4.16) nm; IR (neat) ν
max 3513, 2924, 2852, 1688, 1638, 1512, 1471, 1271, 1034, 982, 808 cm
−1; 1H-NMR (CDCl
3) δ 6.89–6.64 (4H, m, H-2′, -5′, -6′, -5), 6.08 (1H, dt,
J = 15.6, 1.4 Hz, H-4), 3.85 (3H, s, -OCH
3), 2.84–2.82 (4H, m, H-1, -2), 2.19–2.13 (2H, m, H-6), 1.43–1.25 (14H, m, H-7~13), 0.87 (3H, t,
J = 6.4 Hz, H-14);
13C-NMR (CDCl
3) δ 199.9, 147.9, 146.4, 143.9, 133.2, 130.3, 120.7, 114.3, 111.1, 55.8, 41.9, 32.5, 31.8, 29.8, 29.4, 29.3, 29.2, 29.1, 28.1, 22.6, 14.1.
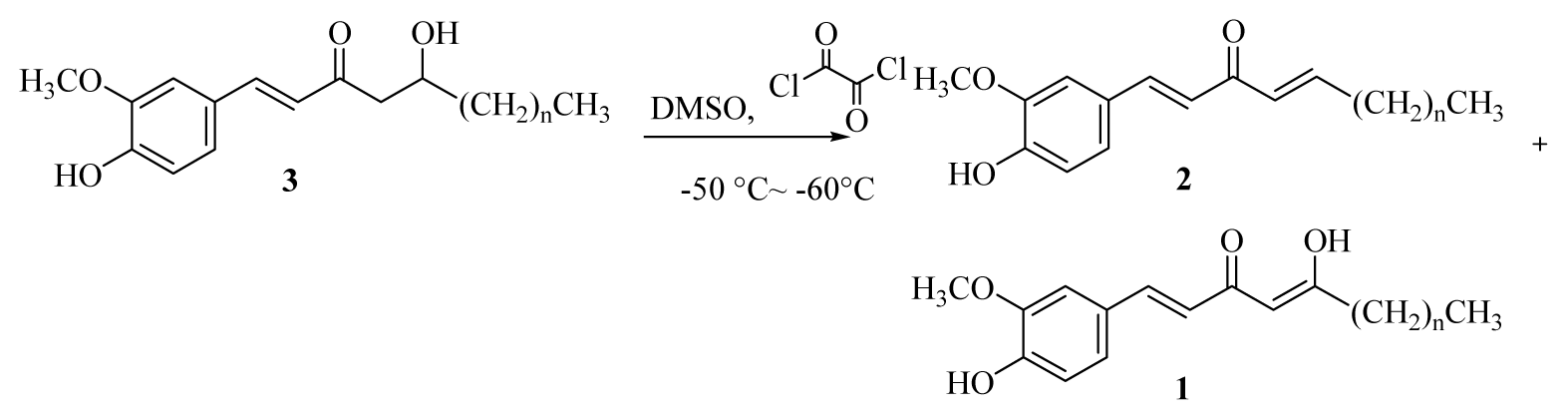
3.2.8. General Procedure for the Synthesis of [n]-Isodehydrogingerdiones (1a–f)
DMSO (0.15 mL, 2.16 mmol) was added dropwise to a solution of oxalyl chloride (0.12 mL, 1.40 mmol) in acetone (10 mL) at −50–60 °C under argon. The reaction mixture was stirred for 3 min, and then a solution of [n]-dehydrogingerols (3a–f) (1.08 mmol) in CH2Cl2 (5 mL) was slowly added. The reaction mixture was stirred for another 15 min, Et3N was added to the mixture; the temperature was changed to 0 °C in an ice bath for 20 min, and the reaction mixture was then neutralized using 5% HCl(aq) and extracted with CH2Cl2 (3 × 10 mL). The organic layers were combined, washed with brine, dried over MgSO4, and concentrated under reduced pressure. The product [n]-isodehydrogingerdiones (1a–f) and [n]-dehydroshogaols (2a–f) were isolated using silica gel column chromatography (ethyl acetate/hexanes = 1/3).
[5]-Isodehydrogingerdione (1a): yellow syrup (51%); UV (MeOH) λ
max (log ɛ) 369 (4.39), 255 (3.71) nm; IR (KBr) ν
max 3358, 2958, 2866, 1634, 1576, 1512, 1427, 1273, 1030, 966, 837 cm
−1; 1H-NMR (CDCl
3) δ 7.51 (1H, d,
J = 15.8 Hz, H-1), 7.07 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.00 (1H, d,
J = 1.8 Hz, H-2′), 6.90 (1H, d,
J = 8.2 Hz, H-5′), 6.34 (1H, d,
J = 15.8 Hz, H-2), 5.62 (1H, s, H-4), 3.91 (3H, s, -OCH
3), 2.38 (2H, t,
J = 7.2 Hz, H-6), 1.70–1.58 (2H, m, H-7), 1.46–1.28 (2H, m, H-8), 0.92 (3H, t,
J = 7.2 Hz, H-9);
13C-NMR (CDCl
3) δ 200.2, 178.0, 147.6, 146.8, 139.8, 127.6, 122.6, 120.5, 114.8, 109.4, 100.1, 55.9, 39.8, 27.7, 22.4, 13.8.
[6]-Isodehydrogingerdione (1b): yellow syrup (49%); UV (MeOH) λ
max (log ɛ) 369 (4.37), 256 (3.70) nm; IR (KBr) ν
max 3418, 2956, 2864, 1634, 1591, 1512, 1427, 1271, 1032, 970, 816 cm
−1; 1H-NMR (CDCl
3) δ 7.51 (1H, d,
J = 15.8 Hz, H-1), 7.06 (1H, dd,
J = 8.0, 1.8 Hz, H-6′), 7.01 (1H, d,
J = 1.8 Hz, H-2′), 6.90 (1H, d,
J = 8.0 Hz, H-5′), 6.34 (1H, d,
J = 15.8 Hz, H-2), 5.61 (1H, s, H-4), 3.94 (3H, s, -OCH
3), 2.37 (2H, t,
J = 7.4 Hz, H-6), 1.69–1.57 (2H, m, H-7), 1.35–1.28 (2H, m, H-8~9), 0.90 (3H, t,
J = 6.2 Hz, H-10);
13C-NMR (CDCl
3) δ 200.2, 178.0, 147.6, 146.7, 139.8, 127.7, 122.6, 120.5, 114.8, 109.4, 100.1, 55.9, 40.1, 31.4, 25.3, 22.4, 13.9.
[7]-Isodehydrogingerdione (1c): yellow syrup (59%); UV (MeOH) λ
max (log ɛ) 368 (4.17), 257 (3.62) nm; IR (KBr) ν
max 3423, 2928, 2858, 1634, 1582, 1512, 1427, 1273, 1031, 974, 814 cm
−1; 1H-NMR (CDCl
3) δ 7.52 (1H, d,
J = 15.8 Hz, H-1), 7.08 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.02 (1H, d,
J = 1.8 Hz, H-2′), 6.91 (1H, d,
J = 8.2 Hz, H-5′), 6.33 (1H, d,
J = 15.8 Hz, H-2), 5.94 (1H, br s, -OH), 5.62 (1H, s, H-4), 3.93 (3H, s, -OCH
3), 2.37 (2H, t,
J = 7.6 Hz, H-6), 1.64–1.61 (2H, m, H-7), 1.30–1.24 (6H, m, H-8~10), 0.89 (3H, t,
J = 6.6 Hz, H-11);
13C-NMR (CDCl
3) δ 200.2, 178.0, 147.7, 146.8, 139.8, 127.7, 122.6, 120.5, 114.8, 109.5, 100.1, 55.9, 40.1, 31.6, 29.0, 25.6, 22.5, 14.0.
[9]-Isodehydrogingerdione (1e): yellow syrup (48%); UV (MeOH) λ
max (log ɛ) 369 (4.37), 254 (3.72) nm; IR (KBr) ν
max 3423, 2955, 2854, 1634, 1583, 1512, 1427, 1271, 1031, 970, 816 cm
−1; 1H-NMR (CDCl
3) δ 7.52 (1H, d,
J = 15.6 Hz, H-1), 7.08 (1H, dd,
J = 8.2, 1.8 Hz, H-6′), 7.01 (1H, d,
J = 1.8 Hz, H-2′), 6.91 (1H, d,
J = 8.2 Hz, H-5′), 6.34 (1H, d,
J = 15.6 Hz, H-2), 5.91 (1H, br s, -OH), 5.62 (1H, s, H-4), 3.93 (3H, s, -OCH
3), 2.37 (2H, t,
J = 7.2 Hz, H-6), 1.67–1.60 (2H, m, H-7), 1.29–1.27 (10H, m, H-8~12), 0.88 (3H, t,
J = 6.2 Hz, H-13);
13C-NMR (CDCl
3) δ 200.1, 177.9, 147.6, 146.7, 140.0, 127.6, 122.5, 120.5, 114.7, 109.4, 100.1, 55.9, 40.1, 31.8, 29.3, 29.1, 25.6, 22.6, 14.0.