Effect of NK4 Transduction in Bone Marrow-Derived Mesenchymal Stem Cells on Biological Characteristics of Pancreatic Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
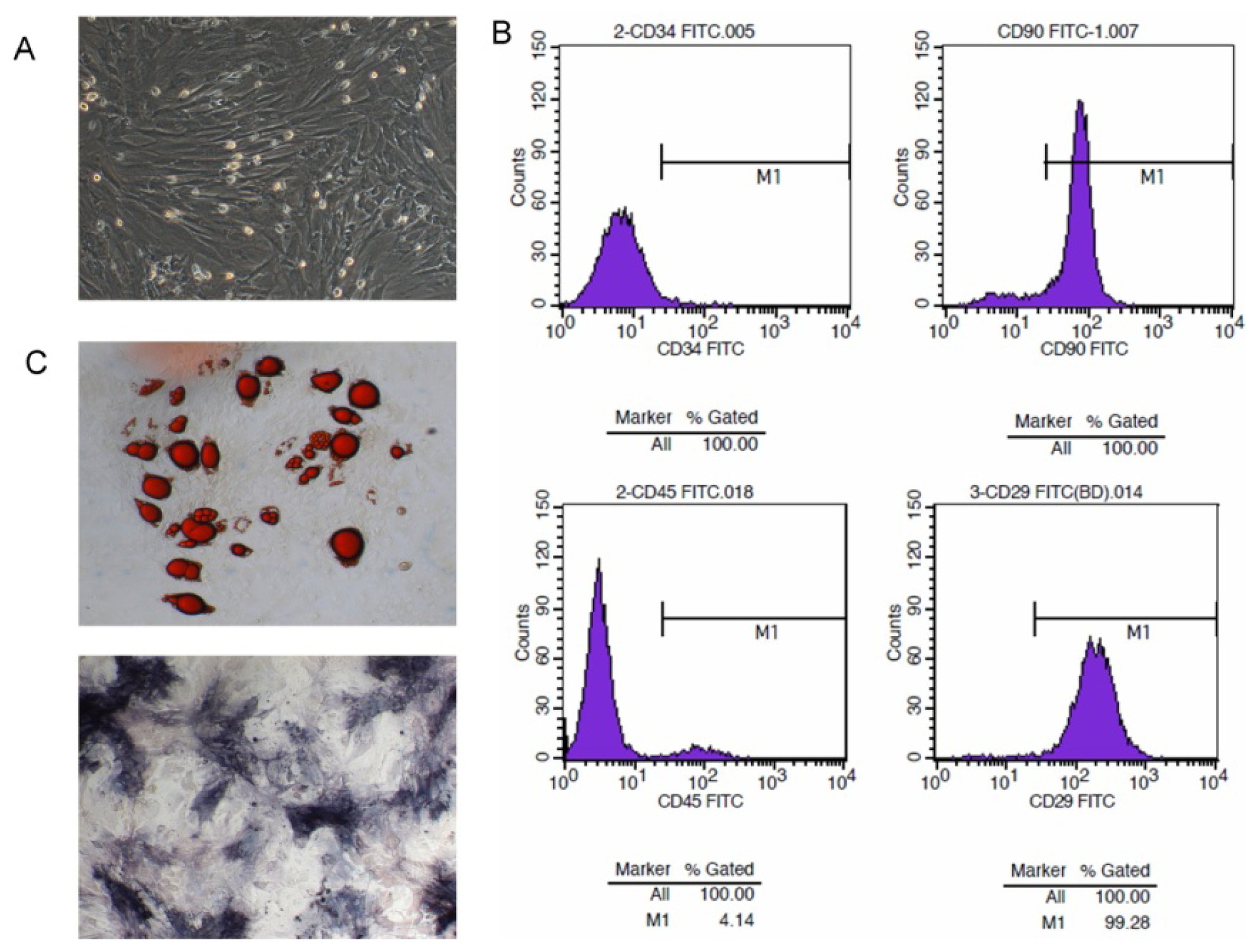
2.1. Isolation and Character Identification of Bone Marrow-Derived MSCs
2.4. NK4 Expression in MSC Cell Supernatant and Oncotropism to Pancreatic Cancer Cells
2.5. Effect of Transfected MSCs on Biological Characteristics of Pancreatic Cancer Cell
2.6. Discussion
3. Experimental Section
3.1. Rat and Cells
3.2. Preparation and Culture of Bone Marrow-Derived MSCs
3.3. Molecular Characterization of MSC
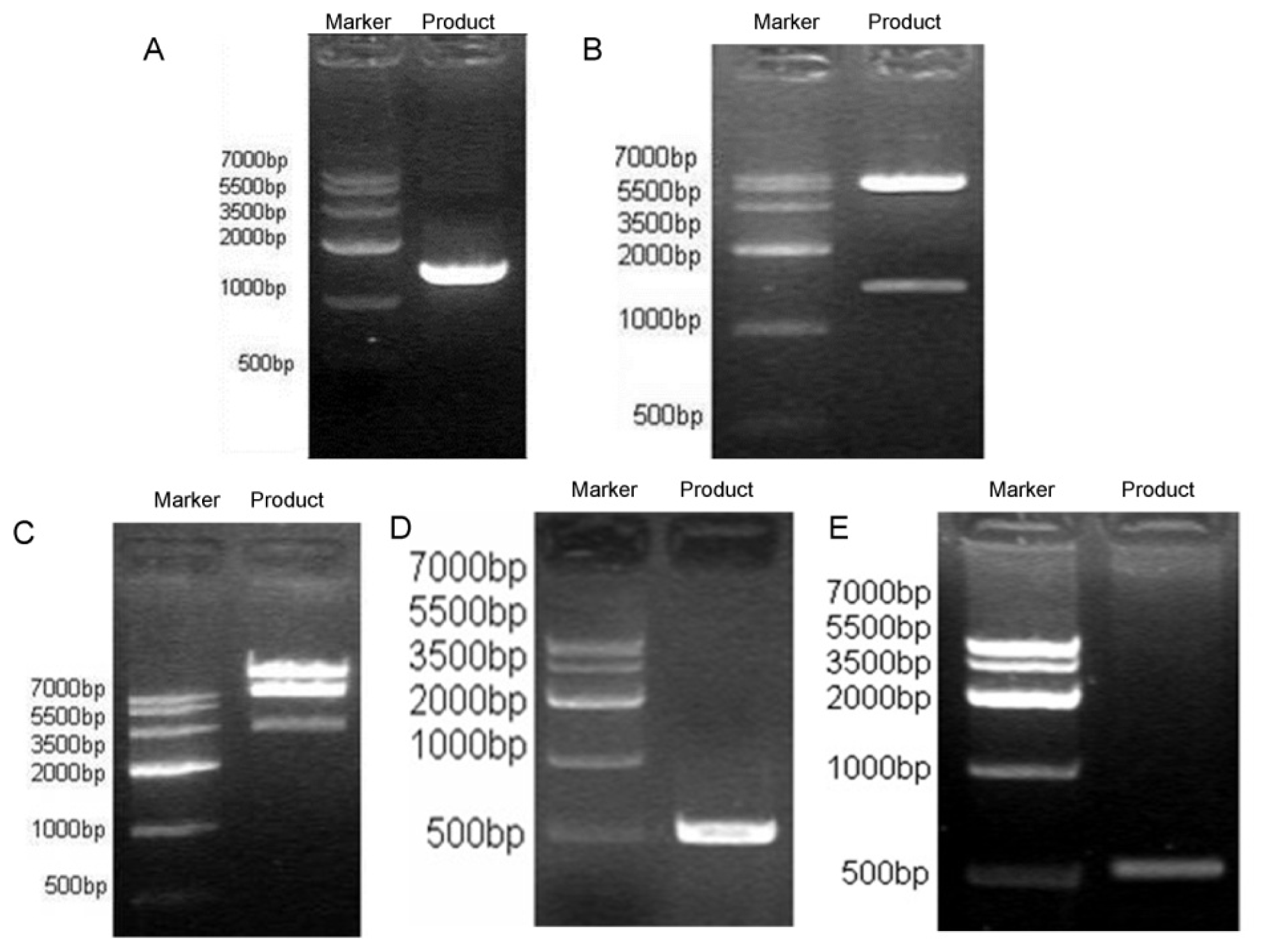
3.4. NK4 Gene Cloning to Adenovirus Shuttle Vector pYr-Adshuttle-6
3.5. Construction of Recombinant Ad Vector DNA Containing Human NK4
3.6. Producing Viral Stocks and Transducing MSCs
3.7. TCID50
3.8. 3-(4,5,-Dimethyl thiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT)
3.9. Western Blot Analysis
3.10. RT-PCR
3.11. Determination of MSC Supernatant NK4 with Enzyme-Linked Immunosorbent Assay
3.12. Co-Culture Migration Assay
3.13. Colony-Forming Assay
3.14. Cell Scatter Assay
3.15. Cell Cycle Detection
3.16. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Saif, M.W. Advancements in the management of pancreatic cancer: 2013. JOP 2013, 14, 112–118. [Google Scholar]
- Mancuso, A.; Calabro, F.; Sternberg, C.N. Current therapies and advances in the treatment of pancreatic cancer. Crit. Rev. Oncol. Hematol. 2006, 58, 231–241. [Google Scholar]
- Manabe, T.; Mizumoto, K.; Nagai, E.; Matsumoto, K.; Nakamura, T.; Nukiwa, T.; Tanaka, M.; Matsuda, T. Cell-based protein delivery system for the inhibition of the growth of pancreatic cancer: Nk4 gene-transduced oral mucosal epithelial cell sheet. Clin. Cancer Res. 2003, 9, 3158–3166. [Google Scholar]
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar]
- Sato, N.; Goggins, M. The role of epigenetic alterations in pancreatic cancer. J. Hepatobiliary Pancreat. Surg. 2006, 13, 286–295. [Google Scholar]
- Jimeno, A.; Hidalgo, M. Molecular biomarkers: Their increasing role in the diagnosis characterization and therapy guidance in pancreatic cancer. Mol. Cancer Ther. 2006, 5, 787–796. [Google Scholar]
- MacKenzie, M.J. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol. 2004, 5, 541–549. [Google Scholar]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar]
- Studeny, M.; Marini, F.C.; Dembinski, J.L.; Zompetta, C.; Cabreira-Hansen, M.; Bekele, B.N.; Champlin, R.E.; Andreeff, M. Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 2004, 96, 1593–1603. [Google Scholar]
- Nakamizo, A.; Marini, F.; Amano, T.; Khan, A.; Studeny, M.; Gumin, J.; Chen, J.; Hentschel, S.; Vecil, G.; Dembinski, J.; et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005, 65, 3307–3318. [Google Scholar]
- Pereboeva, L.; Komarova, S.; Mikheeva, G.; Krasnykh, V.; Curiel, D.T. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells 2003, 21, 389–404. [Google Scholar]
- Montesano, R.; Matsumoto, K.; Nakamura, T.; Orci, L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 1991, 67, 901–908. [Google Scholar]
- Matsumoto, K.; Kataoka, H.; Date, K.; Nakamura, T. Cooperative interaction between α- and β-chains of hepatocyte growth factor on c-met receptor confers ligand-induced receptor tyrosine phosphorylation and multiple biological responses. J. Biol. Chem. 1998, 273, 22913–22920. [Google Scholar]
- Bellusci, S.; Moens, G.; Gaudino, G.; Comoglio, P.; Nakamura, T.; Thiery, J.P.; Jouanneau, J. Creation of an hepatocyte growth factor/scatter factor autocrine loop in carcinoma cells induces invasive properties associated with increased tumorigenicity. Oncogene 1994, 9, 1091–1099. [Google Scholar]
- Weidner, K.M.; Hartmann, G.; Sachs, M.; Birchmeier, W. Properties and functions of scatter factor/hepatocyte growth factor and its receptor c-met. Am. J. Respir. Cell Mol. Biol. 1993, 8, 229–237. [Google Scholar]
- Cao, R.; Bjorndahl, M.A.; Gallego, M.I.; Chen, S.; Religa, P.; Hansen, A.J.; Cao, Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood 2006, 107, 3531–3536. [Google Scholar]
- Maulik, G.; Shrikhande, A.; Kijima, T.; Ma, P.C.; Morrison, P.T.; Salgia, R. Role of the hepatocyte growth factor receptor c-met in oncogenesis and potential for therapeutic inhibition. Cytokine Growth Factor Rev. 2002, 13, 41–59. [Google Scholar]
- Date, K.; Matsumoto, K.; Kuba, K.; Shimura, H.; Tanaka, M.; Nakamura, T. Inhibition of tumor growth and invasion by a four-kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene 1998, 17, 3045–3054. [Google Scholar]
- Lai, R.X.; Yuan, S.Z.; Nakamura, T. Effect of transferred nk4 gene on biological characteristics of human pancreatic cancer cell line sw1990. Ai Zheng 2004, 23, 1134–1138. [Google Scholar]
- Bruckner, S.; Tautenhahn, H.M.; Winkler, S.; Stock, P.; Jonas, S.; Dollinger, M.; Christ, B. Isolation and hepatocyte differentiation of mesenchymal stem cells from porcine bone marrow— “surgical waste” as a novel msc source. Transplant Proc. 2013, 45, 2056–2058. [Google Scholar]
- Pelekanos, R.A.; Li, J.; Gongora, M.; Chandrakanthan, V.; Scown, J.; Suhaimi, N.; Brooke, G.; Christensen, M.E.; Doan, T.; Rice, A.M.; et al. Comprehensive transcriptome and immunophenotype analysis of renal and cardiac msc-like populations supports strong congruence with bone marrow msc despite maintenance of distinct identities. Stem Cell Res. 2012, 8, 58–73. [Google Scholar]
- Chow, K.S.; Jun, D.; Helm, K.M.; Wagner, D.H.; Majka, S.M. Isolation & characterization of hoechst(low) cd45(negative) mouse lung mesenchymal stem cells. J. Vis. Exp. 2011, e3159. [Google Scholar]
- Ooi, Y.Y.; Ramasamy, R.; Vidyadaran, S. Mouse bone marrow mesenchymal stem cells acquire cd45-cd106+ immunophenotype only at later passages. Med. J. Malays. 2008, 63, 65–66. [Google Scholar]
- Kaiser, S.; Hackanson, B.; Follo, M.; Mehlhorn, A.; Geiger, K.; Ihorst, G.; Kapp, U. Bm cells giving rise to msc in culture have a heterogeneous cd34 and cd45 phenotype. Cytotherapy 2007, 9, 439–450. [Google Scholar]
- Lin, C.S.; Ning, H.; Lin, G.; Lue, T.F. Is cd34 truly a negative marker for mesenchymal stromal cells? Cytotherapy 2012, 14, 1159–1163. [Google Scholar]
- Yue, D.; Wang, Y.; Ma, P.; Li, Y.Y.; Chen, H.; Wang, P.; Ren, C.S. Effects of transferred NK4 gene on proliferation migration invasion and apoptosis of human prostate cancer du145 cells. Asian J. Androl. 2010, 12, 381–389. [Google Scholar]
- Suzuki, Y.; Sakai, K.; Ueki, J.; Xu, Q.; Nakamura, T.; Shimada, H.; Matsumoto, K. Inhibition of MET/HGF receptor and angiogenesis by NK4 leads to suppression of tumor growth and migration in malignant pleural mesothelioma. Int. J. Cancer 2010, 127, 1948–1957. [Google Scholar]
- Nakamura, T.; Sakai, K.; Matsumoto, K. Anti-cancer approach with NK4: Bivalent action and mechanisms. Anticancer Agents Med. Chem. 2010, 10, 36–46. [Google Scholar]
- Nakamura, K.; Ito, Y.; Kawano, Y.; Kurozumi, K.; Kobune, M.; Tsuda, H.; Bizen, A.; Honmou, O.; Niitsu, Y.; Hamada, H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004, 11, 1155–1164. [Google Scholar]
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G.; et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of kaposi’s sarcoma. J. Exp. Med. 2006, 203, 1235–1247. [Google Scholar]
- Ji, J.F.; He, B.P.; Dheen, S.T.; Tay, S.S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004, 22, 415–427. [Google Scholar]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated sdf-1/cxcl12 secretion. Cell 2005, 121, 335–348. [Google Scholar]
- Burger, J.A.; Kipps, T.J. Cxcr4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006, 107, 1761–1767. [Google Scholar]
- Maemondo, M.; Narumi, K.; Saijo, Y.; Usui, K.; Tahara, M.; Tazawa, R.; Hagiwara, K.; Matsumoto, K.; Nakamura, T.; Nukiwa, T. Targeting angiogenesis and hgf function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol. Ther. 2002, 5, 177–185. [Google Scholar]
- Kanehira, M.; Xin, H.; Hoshino, K.; Maemondo, M.; Mizuguchi, H.; Hayakawa, T.; Matsumoto, K.; Nakamura, T.; Nukiwa, T.; Saijo, Y. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007, 14, 894–903. [Google Scholar]
- Matsumoto, K.; Nakamura, T. Nk4 gene therapy targeting hgf-met and angiogenesis. Front. Biosci. 2008, 13, 1943–1951. [Google Scholar]
- Hung, C.M.; Kuo, D.H.; Chou, C.H.; Su, Y.C.; Ho, C.T.; Way, T.D. Osthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-met/Akt/Mtor pathway in human breast cancer cells. J. Agric. Food Chem. 2011, 59, 9683–9690. [Google Scholar]
- Chang, H.Y.; Kao, M.C.; Way, T.D.; Ho, C.T.; Fu, E. Diosgenin suppresses hepatocyte growth factor (hgf)-induced epithelial-mesenchymal transition by down-regulation of mdm2 and vimentin. J. Agric. Food Chem. 2011, 59, 5357–5363. [Google Scholar]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-kinase akt pathway in human cancer. Nat. Rev. Cancer 2002, 2, 489–501. [Google Scholar]
- Roche, S.; Downward, J.; Raynal, P.; Courtneidge, S.A. A function for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in fibroblasts during mitogenesis: Requirement for insulin- and lysophosphatidic acid-mediated signal transduction. Mol. Cell. Biol. 1998, 18, 7119–7129. [Google Scholar]
- Choudhury, G.G. Akt serine threonine kinase regulates platelet-derived growth factor-induced DNA synthesis in glomerular mesangial cells: Regulation of c-fos and p27(kip1) gene expression. J. Biol. Chem. 2001, 276, 35636–35643. [Google Scholar]
- Gong, R.; Rifai, A.; Dworkin, L.D. Activation of Pi3k-Akt-Gsk3β pathway mediates hepatocyte growth factor inhibition of rantes expression in renal tubular epithelial cells. Biochem. Biophys. Res. Commun. 2005, 330, 27–33. [Google Scholar]
- Grimes, C.A.; Jope, R.S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog. Neurobiol. 2001, 65, 391–426. [Google Scholar]
- da Meirelles, L.S.; Nardi, N.B. Murine marrow-derived mesenchymal stem cell: Isolation in vitro expansion and characterization. Br. J. Haematol. 2003, 123, 702–711. [Google Scholar]
- Grigorov, B.; Rabilloud, J.; Lawrence, P.; Gerlier, D. Rapid titration of measles and other viruses: Optimization with determination of replication cycle length. PLoS One 2011, 6, e24135. [Google Scholar]



| Gene name | Primer | 5′-3′ |
|---|---|---|
| NK4 | Sense | ATCCAAGGTCAAGGAGAAGGCT |
| Anti-sense | TTCACAACGAGAAATAGGGCA | |
| GAPDH | Sense | ACCACAGTCCATGCCATCAC |
| Anti-sense | TCCACCACCCTGTTGCTGTA | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sun, Y.-P.; Zhang, B.-L.; Duan, J.-W.; Wu, H.-H.; Wang, B.-Q.; Yu, Z.-P.; Yang, W.-J.; Shan, Y.-F.; Zhou, M.-T.; Zhang, Q.-Y. Effect of NK4 Transduction in Bone Marrow-Derived Mesenchymal Stem Cells on Biological Characteristics of Pancreatic Cancer Cells. Int. J. Mol. Sci. 2014, 15, 3729-3745. https://doi.org/10.3390/ijms15033729
Sun Y-P, Zhang B-L, Duan J-W, Wu H-H, Wang B-Q, Yu Z-P, Yang W-J, Shan Y-F, Zhou M-T, Zhang Q-Y. Effect of NK4 Transduction in Bone Marrow-Derived Mesenchymal Stem Cells on Biological Characteristics of Pancreatic Cancer Cells. International Journal of Molecular Sciences. 2014; 15(3):3729-3745. https://doi.org/10.3390/ijms15033729
Chicago/Turabian StyleSun, Yun-Peng, Ben-Long Zhang, Jian-Wen Duan, Huan-Huan Wu, Ben-Quan Wang, Zheng-Ping Yu, Wen-Jun Yang, Yun-Feng Shan, Meng-Tao Zhou, and Qi-Yu Zhang. 2014. "Effect of NK4 Transduction in Bone Marrow-Derived Mesenchymal Stem Cells on Biological Characteristics of Pancreatic Cancer Cells" International Journal of Molecular Sciences 15, no. 3: 3729-3745. https://doi.org/10.3390/ijms15033729




