Nanotoxicity Overview: Nano-Threat to Susceptible Populations
Abstract
:1. Introduction
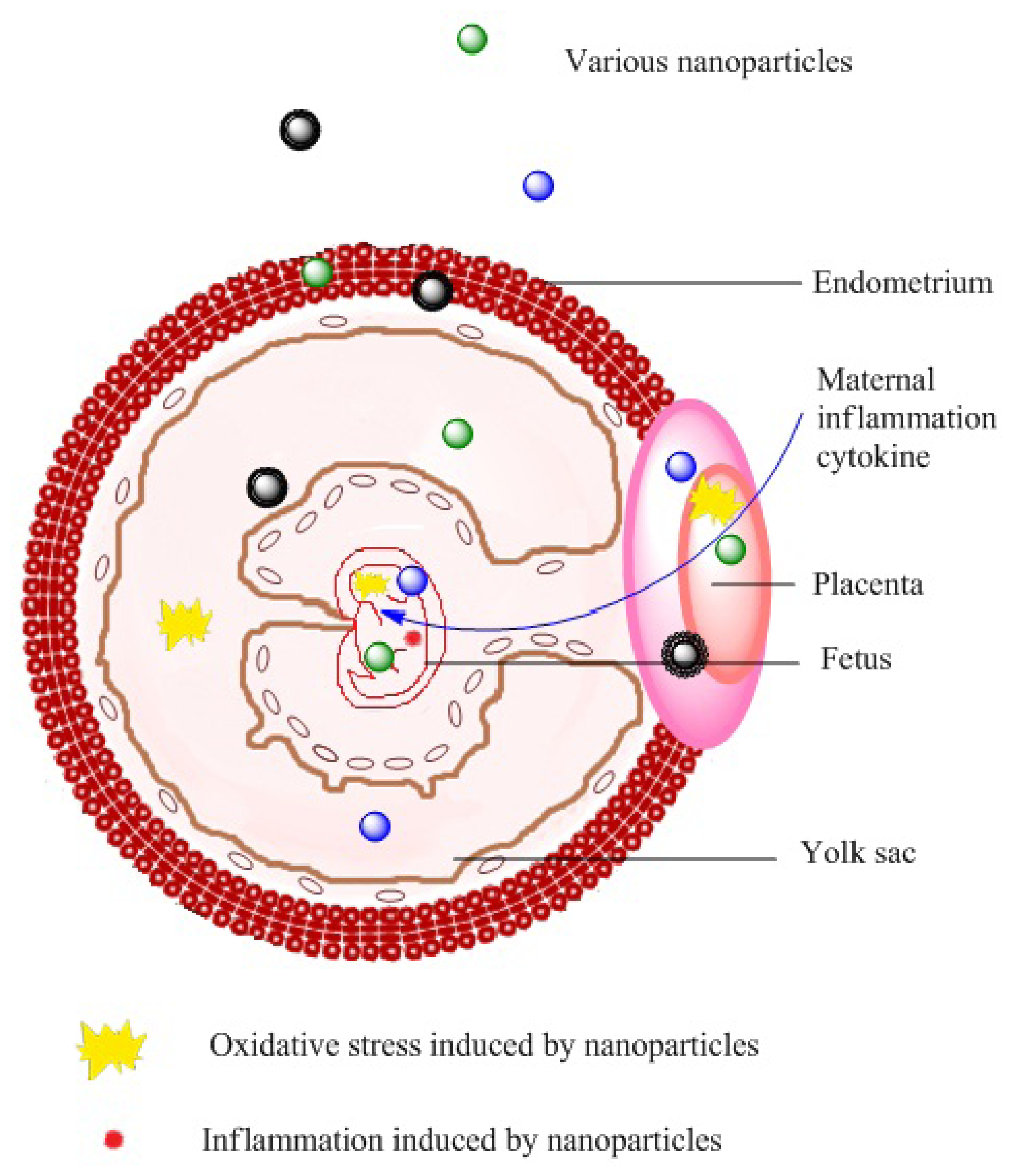
2. Nanotoxicity in Pregnant Females and Neonates
2.1. Effects on Health during Pregnancy
2.2. Effects on Fetal Development
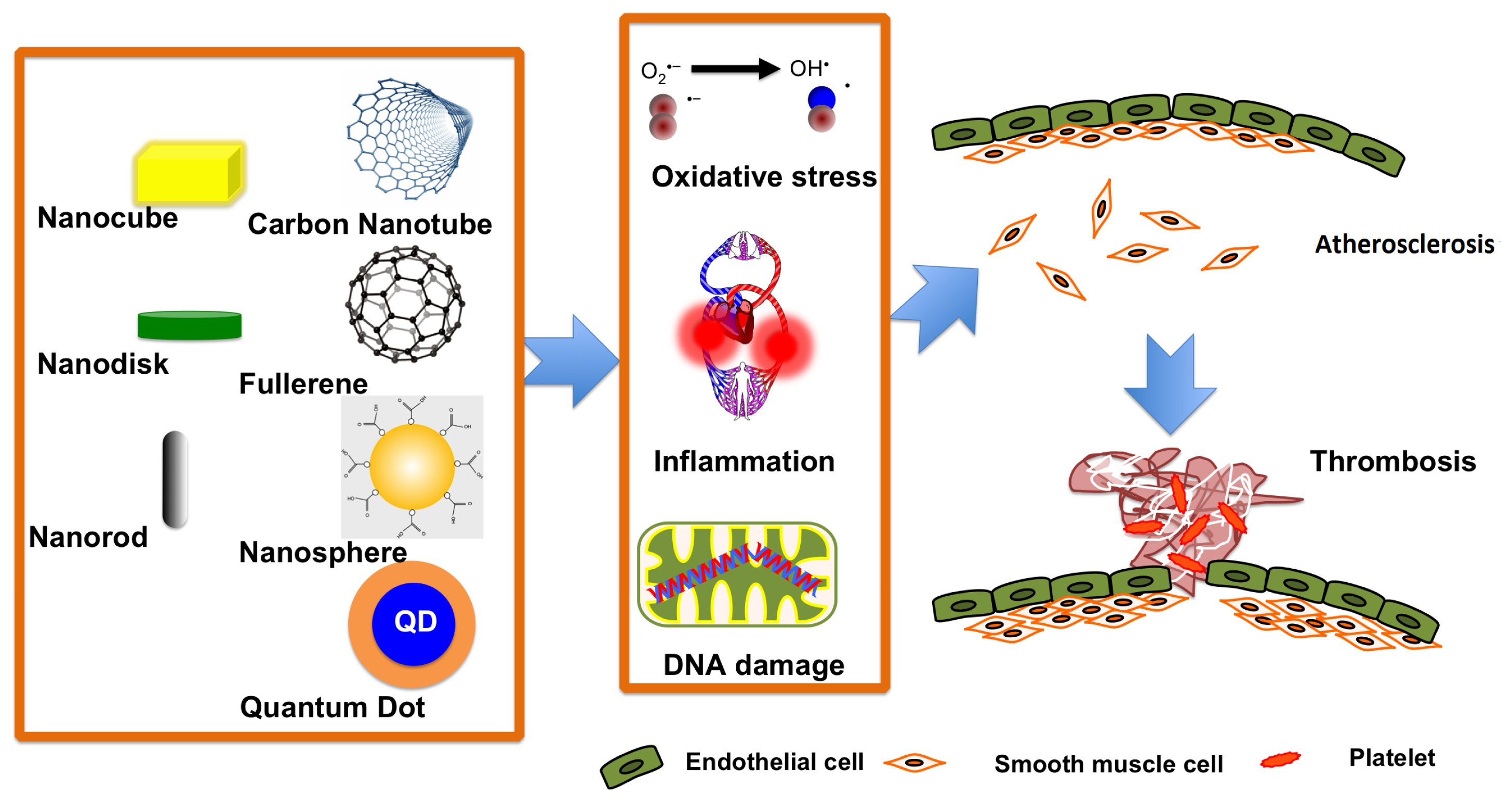
3. Nanoparticle Toxicity in Diseased Populations
3.1. The Effects of Nanoparticles on Subjects with Cardiovascular Diseases
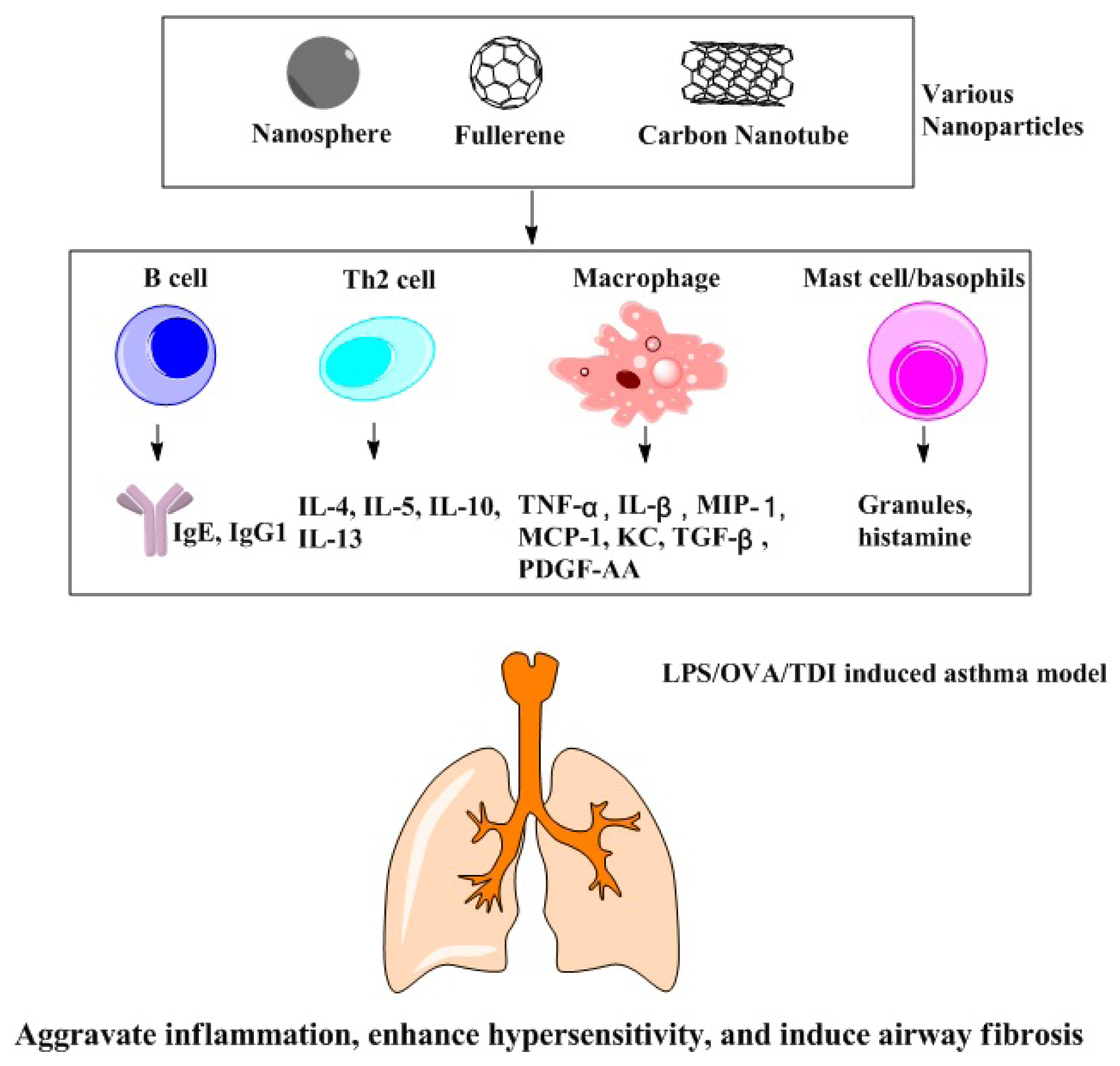
3.2. Effects of Nanoparticles on Populations with Chronic Respiratory Disease
3.2.1. LPS-Induced Animal Asthma Model
3.2.2. OVA-Induced Animal Asthma Model
3.2.3. Chemically Induced Animal Asthma Model
3.3. Effects of Nanoparticles on Hepatitis Patients
4. Nanoparticle Toxicity in the Elderly Population
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lindberg, H.K.; Falck, G.C.M.; Suhonen, S.; Vippola, M.; Vanhala, E.; Catalan, J.; Savolainen, K.; Norppa, H. Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicol. Lett. 2009, 186, 166–173. [Google Scholar]
- Singh, N.; Manshian, B.; Jenkins, G.J.S.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar]
- Sharma, V.; Singh, S.K.; Anderson, D.; Tobin, D.J.; Dhawan, A. Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J. Nanosci. Nanotechnol. 2011, 11, 3782–3788. [Google Scholar]
- Hussain, S.; Vanoirbeek, J.A.J.; Hoet, P.H.M. Interactions of nanomaterials with the immune system. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2012, 4, 169–183. [Google Scholar]
- Truong, L.; Tilton, S.C.; Zaikova, T.; Richman, E.; Waters, K.M.; Hutchison, J.E.; Tanguay, R.L. Surface functionalities of gold nanoparticles impact embryonic gene expression responses. Nanotoxicology 2013, 7, 192–201. [Google Scholar]
- Tkach, A.V.; Shurin, G.V.; Shurin, M.R.; Kisin, E.R.; Murray, A.R.; Young, S.H.; Star, A.; Fadeel, B.; Kagan, V.E.; Shvedova, A.A. Direct effects of carbon nanotubes on dendritic cells induce immune suppression upon pulmonary exposure. ACS Nano 2011, 5, 5755–5762. [Google Scholar]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar]
- Gordon, M. Maternal Physiology. In Obstetrics: Normal and Problem Pregnancies, 5th ed.; Mark, B.L., Ed.; Churchill Livingstone: Philadelphia, PA, USA, 2007; pp. 55–84. [Google Scholar]
- Feldt–Rasmussen, U.; Mathiesen, E.R. Endocrine disorders in pregnancy: Physiological and hormonal aspects of pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 875–884. [Google Scholar]
- Chan, A.L.; Juarez, M.M.; Gidwani, N.; Albertson, T.E. Management of critical asthma syndrome during pregnancy. Clin. Rev. Allergy Immunol. 2013. [Google Scholar] [CrossRef]
- Chu, M.; Wu, Q.; Yang, H.; Yuan, R.; Hou, S.; Yang, Y.; Zou, Y.; Xu, S.; Xu, K.; Ji, A. Transfer of quantum dots from pregnant mice to pups across the placental barrier. Small 2010, 6, 670–678. [Google Scholar]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.-A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432. [Google Scholar]
- Kulvietis, V.; Žalgevičienė, V.; Didžiapetrienė, J.; Bulotienė, D.; Rotomskis, R. Distribution of nanoparticles in the pregnant rat: The morphologic and spectroscopic study. Pap. Anthropol. 2011, 20, 218–228. [Google Scholar]
- Refuerzo, J.S.; Godin, B.; Bishop, K.; Srinivasan, S.; Shah, S.K.; Amra, S.; Ramin, S.M.; Ferrari, M. Size of the nanovectors determines the transplacental passage in pregnancy: Study in rats. Am. J. Obstet. Gynecol. 2011, 204, 546.e5–546.e9. [Google Scholar]
- Burton, G.J.; Hempstock, J.; Jauniaux, E. Oxygen early embryonic metabolism and free radical-mediated embryopathies. Reprod. Biomed. Online 2003, 6, 84–96. [Google Scholar]
- Myllynen, P.K.; Loughran, M.J.; Howard, C.V.; Sormunen, R.; Walsh, A.A.; Vahakangas, K.H. Kinetics of gold nanoparticles in the human placenta. Reprod. Toxicol. 2008, 26, 130–137. [Google Scholar]
- Tian, F.; Razansky, D.; Estrada, G.G.; Semmler-Behnke, M.; Beyerle, A.; Kreyling, W.; Ntziachristos, V.; Stoeger, T. Surface modification and size dependence in particle translocation during early embryonic development. Inhal. Toxicol. 2009, 21, 92–96. [Google Scholar]
- Fedulov, A.V.; Leme, A.; Yang, Z.; Dahl, M.; Lim, R.; Mariani, T.J.; Kobzik, L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am. J. Respir. Cell Mol. Biol. 2008, 38, 57. [Google Scholar]
- Lamoureux, D.P.; Kobzik, L.; Fedulov, A.V. Customized PCR-array analysis informed by gene-chip microarray and biological hypothesis reveals pathways involved in lung inflammatory response to titanium dioxide in pregnancy. J. Toxicol. Environ. Health Part A 2010, 73, 596–606. [Google Scholar]
- Weinberg, E.D. Pregnancy-associated depression of cell-mediated immunity. Rev. Infect. Dis. 1984, 6, 814–831. [Google Scholar]
- Jackson, P.; Hougaard, K.S.; Boisen, A.M.Z.; Jacobsen, N.R.; Jensen, K.A.; Møller, P.; Brunborg, G.; Gutzkow, K.B.; Andersen, O.; Loft, S. Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: Effects on liver DNA strand breaks in dams and offspring. Nanotoxicology 2012, 6, 486–500. [Google Scholar]
- Lim, J.H.; Kim, S.H.; Shin, I.S.; Park, N.H.; Moon, C.; Kang, S.S.; Kim, S.H.; Park, S.C.; Kim, J.C. Maternal exposure to mult-wall carbon nanotubes does not induce embryo–fetal developmental toxicity in rats. Birth Defects Res. Part B: Dev. Reprod. Toxicol. 2011, 92, 69–76. [Google Scholar]
- Park, E.-J.; Kim, H.; Kim, Y.; Park, K. Effects of platinum nanoparticles on the postnatal development of mouse pups by maternal exposure. Environ. Health Toxicol. 2010, 25, 279–286. [Google Scholar]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar]
- Philbrook, N.A.; Walker, V.K.; Afrooz, A.; Saleh, N.B.; Winn, L.M. Investigating the effects of functionalized carbon nanotubes on reproduction and development in Drosophila melanogaster and CD-1 mice. Reprod. Toxicol. 2011, 32, 442–448. [Google Scholar]
- Blum, J.L.; Xiong, J.Q.; Hoffman, C.; Zelikoff, J.T. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol. Sci. 2012, 126, 478–486. [Google Scholar]
- Austin, C.A.; Umbreit, T.H.; Brown, K.M.; Barber, D.S.; Dair, B.J.; Francke-Carroll, S.; Feswick, A.; Saint-Louis, M.A.; Hikawa, H.; Siebein, K.N.; et al. Distribution of silver nanoparticles in pregnant mice and developing embryos. Nanotoxicology 2012, 6, 912–922. [Google Scholar]
- Pietroiusti, A.; Massimiani, M.; Fenoglio, I.; Colonna, M.; Valentini, F.; Palleschi, G.; Camaioni, A.; Magrini, A.; Siracusa, G.; Bergamaschi, A. Low doses of pristine and oxidized single-wall carbon nanotubes affect mammalian embryonic development. ACS Nano 2011, 5, 4624–4633. [Google Scholar]
- Li, P.-W.; Kuo, T.-H.; Chang, J.-H.; Yeh, J.-M.; Chan, W.-H. Induction of cytotoxicity and apoptosis in mouse blastocysts by silver nanoparticles. Toxicol. Lett. 2010, 197, 82–87. [Google Scholar]
- Chan, W.H.; Shiao, N.H. Cytotoxic effect of CdSe quantum dots on mouse embryonic development. Acta Pharmacol. Sin. 2008, 29, 259–266. [Google Scholar]
- Park, M.V.; Annema, W.; Salvati, A.; Lesniak, A.; Elsaesser, A.; Barnes, C.; McKerr, G.; Howard, C.V.; Lynch, I.; Dawson, K.A. In vitro developmental toxicity test detects inhibition of stem cell differentiation by silica nanoparticles. Toxicol. Appl. Pharmacol. 2009, 240, 108–116. [Google Scholar]
- Sumner, S.C.; Fennell, T.R.; Snyder, R.W.; Taylor, G.F.; Lewin, A.H. Distribution of carbon-14 labeled C60 ([14C] C60) in the pregnant and in the lactating dam and the effect of C60 exposure on the biochemical profile of urine. J. Appl. Toxicol. 2010, 30, 354–360. [Google Scholar]
- Gao, X.; Yin, S.; Tang, M.; Chen, J.; Yang, Z.; Zhang, W.; Chen, L.; Yang, B.; Li, Z.; Zha, Y. Effects of developmental exposure to TiO2 nanoparticles on synaptic plasticity in hippocampal dentate gyrus area: An in vivo study in anesthetized rats. Biol. Trace Element Res. 2011, 143, 1616–1628. [Google Scholar]
- Boisen, A.M.Z.; Shipley, T.; Jackson, P.; Hougaard, K.S.; Wallin, H.; Yauk, C.L.; Vogel, U. NanoTiO2 (UV-Titan) does not induce ESTR mutations in the germline of prenatally exposed female mice. Part. Fibre Toxicol. 2012, 9, 1–5. [Google Scholar]
- Žalgevičienė, V.; Kulvietis, V.; Bulotienė, D.; Didžiapetrienė, J.; Rotomskis, R. The effect of nanoparticles in rats during critical periods of pregnancy. Medicina (Kaunas) 2012, 48, 256–264. [Google Scholar]
- Jonakait, G.M. The effects of maternal inflammation on neuronal development: Possible mechanisms. Int. J. Dev. Neurosci. 2007, 25, 415–425. [Google Scholar]
- Meyer, U.; Feldon, J.; Fatemi, S.H. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci. Biobehav. Rev. 2009, 33, 1061–1079. [Google Scholar]
- Hougaard, K.S.; Jackson, P.; Jensen, K.A.; Sloth, J.J.; Löschner, K.; Larsen, E.H.; Birkedal, R.K.; Vibenholt, A.; Boisen, A.-M.Z.; Wallin, H. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan) A study in mice. Part. Fibre Toxicol. 2010, 7, 1–15. [Google Scholar]
- Shimizu, M.; Tainaka, H.; Oba, T.; Mizuo, K.; Umezawa, M.; Takeda, K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part. Fibre Toxicol. 2009, 6, 20. [Google Scholar]
- Jackson, P.; Vogel, U.; Wallin, H.; Hougaard, K.S. Prenatal exposure to carbon black (Printex 90): Effects on sexual development and neurofunction. Basic Clin. Pharmacol. Toxicol. 2011, 109, 434–437. [Google Scholar]
- Umezawa, M.; Tainaka, H.; Kawashima, N.; Shimizu, M.; Takeda, K. Effect of fetal exposure to titanium dioxide nanoparticle on brain development-brain region information. J. Toxicol. Sci. 2011, 37, 1247–1252. [Google Scholar]
- Takeda, K.; Suzuki, K.-I.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Health Sci. 2009, 55, 95–102. [Google Scholar]
- Takahashi, Y.; Mizuo, K.; Shinkai, Y.; Oshio, S.; Takeda, K. Prenatal exposure to titanium dioxide nanoparticles increases dopamine levels in the prefrontal cortex and neostriatum of mice. J. Toxicol. Sci. 2010, 35, 749–756. [Google Scholar]
- Hoelting, L.; Scheinhardt, B.; Bondarenko, O.; Schildknecht, S.; Kapitza, M.; Tanavde, V.; Tan, B.; Lee, Q.Y.; Mecking, S.; Leist, M. A 3-dimensional human embryonic stem cell (hESC)-derived model to detect developmental neurotoxicity of nanoparticles. Arch. Toxicol. 2012, 1–13. [Google Scholar]
- Yoshida, S.; Hiyoshi, K.; Oshio, S.; Takano, H.; Takeda, K.; Ichinose, T. Effects of fetal exposure to carbon nanoparticles on reproductive function in male offspring. Fertil. Steril. 2010, 93, 1695–1699. [Google Scholar]
- Noori, A.; Parivar, K.; Modaresi, M.; Messripour, M.; Yousefi, M.H.; Amiri, G.R. Effect of magnetic iron oxide nanoparticles on pregnancy and testicular development of mice. Afr. J. Biotechnol. 2011, 10, 1221–1227. [Google Scholar]
- Jackson, P.; Halappanavar, S.; Hougaard, K.S.; Williams, A.; Madsen, A.M.; Lamson, J.S.; Andersen, O.; Yauk, C.; Wallin, H.; Vogel, U. Maternal inhalation of surface-coated nanosized titanium dioxide (UV-Titan) in C57BL/6 mice: Effects in prenatally exposed offspring on hepatic DNA damage and gene expression. Nanotoxicology 2013, 7, 85–96. [Google Scholar]
- Kyjovska, Z.O.; Boisen, A.M.Z.; Jackson, P.; Wallin, H.; Vogel, U.; Hougaard, K.S. Daily sperm production: Application in studies of prenatal exposure to nanoparticles in mice. Reprod. Toxicol. 2013, 36, 88–97. [Google Scholar]
- Umezawa, M.; Kudo, S.; Yanagita, S.; Shinkai, Y.; Niki, R.; Oyabu, T.; Takeda, K.; Ihara, T.; Sugamata, M. Maternal exposure to carbon black nanoparticle increases collagen type VIII expression in the kidney of offspring. J. Toxicol. Sci. 2011, 36, 461–468. [Google Scholar]
- Jackson, P.; Hougaard, K.S.; Vogel, U.; Wu, D.; Casavant, L.; Williams, A.; Wade, M.; Yauk, C.L.; Wallin, H.; Halappanavar, S. Exposure of pregnant mice to carbon black by intratracheal instillation: Toxicogenomic effects in dams and offspring. Mutat. Res. 2012, 745, 73–83. [Google Scholar]
- Barton, H.A.; Cogliano, V.J.; Flowers, L.; Valcovic, L.; Setzer, R.W.; Woodruff, T.J. Assessing susceptibility from early-life exposure to carcinogens. Environ. Health Perspect. 2005, 113, 1125. [Google Scholar]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar]
- Almeida, J.P.M.; Chen, A.L.; Foster, A.; Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 2011, 6, 815–835. [Google Scholar]
- Mathers, C.D.; Fat, D.M.; Boerma, J. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Nogueira, J.B. Air pollution and cardiovascular disease. Port. J. Cardiol. 2009, 28, 715. [Google Scholar]
- Sun, Q.; Hong, X.; Wold, L.E. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 2010, 121, 2755–2765. [Google Scholar]
- Nurkiewicz, T.R.; Porter, D.W.; Hubbs, A.F.; Cumpston, J.L.; Chen, B.T.; Frazer, D.G.; Castranova, V. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part. Fibre Toxicol. 2008, 5. [Google Scholar] [CrossRef]
- Minarchick, V.C.; Stapleton, P.A.; Porter, D.W.; Wolfarth, M.G.; Çiftyürek, E.; Barger, M.; Sabolsky, E.M.; Nurkiewicz, T.R. Pulmonary cerium dioxide nanoparticle exposure differentially impairs coronary and mesenteric arteriolar reactivity. Cardiovasc. Toxicol. 2013, 1–15. [Google Scholar]
- Zhu, M.-T.; Wang, B.; Wang, Y.; Yuan, L.; Wang, H.-J.; Wang, M.; Ouyang, H.; Chai, Z.-F.; Feng, W.-Y.; Zhao, Y.-L. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol. Lett. 2011, 203, 162–171. [Google Scholar]
- Agmon, Y.; Khandheria, B.K.; Meissner, I.; Schwartz, G.L.; Petterson, T.M.; O’Fallon, W.M.; Whisnant, J.P.; Wiebers, D.O.; Seward, J.B. Relation of coronary artery disease and cerebrovascular disease with atherosclerosis of the thoracic aorta in the general population. Am. J. Cardiol. 2002, 89, 262–267. [Google Scholar]
- Klaus, D. Atherosclerosis and arteriosclerosis in hypertension. Nieren Hochdruckkrankh. 2000, 29, 1–16. [Google Scholar]
- Taute, B.M.; Feller, S.; Hansgen, K.; Podhaisky, H. Carotid atherosclerosis in patients with peripheral arterial disease. Perfusion 2002, 15, 183–188. [Google Scholar]
- Meir, K.S.; Leitersdorf, E. Atherosclerosis in the Apolipoprotein E–Deficient Mouse A Decade of Progress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1006–1014. [Google Scholar]
- Piedrahita, J.A.; Zhang, S.H.; Hagaman, J.R.; Oliver, P.M.; Maeda, N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 1992, 89, 4471–4475. [Google Scholar]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setälä, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992, 71, 343–353. [Google Scholar]
- Kang, G.S.; Gillespie, P.A.; Gunnison, A.; Moreira, A.L.; Tchou-Wong, K.-M.; Chen, L.-C. Long-term inhalation exposure to nickel nanoparticles exacerbated atherosclerosis in a susceptible mouse model. Environ. Health Perspect. 2011, 119, 176. [Google Scholar]
- Li, Z.; Hulderman, T.; Salmen, R.; Chapman, R.; Leonard, S.S.; Young, S.-H.; Shvedova, A.; Luster, M.I.; Simeonova, P.P. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ. Health Perspect. 2007, 115, 377. [Google Scholar]
- Pang, J.; Xu, Q.; Xu, X.; Yin, H.; Xu, R.; Guo, S.; Hao, W.; Wang, L.; Chen, C.; Cao, J.-M. Hexarelin suppresses high lipid diet and vitamin D3-induced atherosclerosis in the rat. Peptides 2010, 31, 630–638. [Google Scholar]
- Xu, Y.-Y.; Yang, J.; Shen, T.; Zhou, F.; Xia, Y.; Fu, J.-Y.; Meng, J.; Zhang, J.; Zheng, Y.-F.; Yang, J. Intravenous administration of multi-walled carbon nanotubes affects the formation of atherosclerosis in sprague-dawley rats. J. Occup. Health 2012, 54, 361–369. [Google Scholar]
- Inoue, K.-I.; Takano, H.; Yanagisawa, R.; Hirano, S.; Sakurai, M.; Shimada, A.; Yoshikawa, T. Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ. Health Perspect. 2006, 114, 1325. [Google Scholar]
- Inoue, K.-I.; Takano, H.; Koike, E.; Yanagisawa, R.; Sakurai, M.; Tasaka, S.; Ishizaka, A.; Shimada, A. Effects of pulmonary exposure to carbon nanotubes on lung and systemic inflammation with coagulatory disturbance induced by lipopolysaccharide in mice. Exp. Biol. Med. 2008, 233, 1583–1590. [Google Scholar]
- Inoue, K.; Takano, H.; Ohnuki, M.; Yanagisawa, R.; Sakurai, M.; Shimada, A.; Mizushima, K.; Yoshikawa, T. Size effects of nanomaterials on lung inflammation and coagulatory disturbance. Int. J. Immunopathol. Pharmacol. 2007, 21, 197–206. [Google Scholar]
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7–11. [Google Scholar]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar]
- Ballinger, S.W.; Patterson, C.; Knight-Lozano, C.A.; Burow, D.L.; Conklin, C.A.; Hu, Z.; Reuf, J.; Horaist, C.; Lebovitz, R.; Hunter, G.C. Mitochondrial integrity and function in atherogenesis. Circulation 2002, 106, 544–549. [Google Scholar]
- Ballinger, S.W.; Patterson, C.; Yan, C.-N.; Doan, R.; Burow, D.L.; Young, C.G.; Yakes, F.M.; van Houten, B.; Ballinger, C.A.; Freeman, B.A. Hydrogen peroxide–and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ. Res. 2000, 86, 960–966. [Google Scholar]
- Choksi, K.; Boylston, W.; Rabek, J.; Widger, W.; Papaconstantinou, J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim. Biophys. Acta 2004, 1688, 95–101. [Google Scholar]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar]
- Guo, Y.-Y.; Zhang, J.; Zheng, Y.-F.; Yang, J.; Zhu, X.-Q. Cytotoxic and genotoxic effects of multi-wall carbon nanotubes on human umbilical vein endothelial cells in vitro. Mutat. Res. 2011, 721, 184–191. [Google Scholar]
- Su, L.; Han, L.; Ge, F.; Zhang, S.L.; Zhang, Y.; Zhao, B.X.; Zhao, J.; Miao, J.Y. The effect of novel magnetic nanoparticles on vascular endothelial cell function in vitro and in vivo. J. Hazard. Mater. 2012, 235–236, 316–25. [Google Scholar]
- Massberg, S.; Brand, K.; Grüner, S.; Page, S.; Müller, E.; Müller, I.; Bergmeier, W.; Richter, T.; Lorenz, M.; Konrad, I.; et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002, 196, 887–896. [Google Scholar]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar]
- Peters, D.; Kastantin, M.; Kotamraju, V.R.; Karmali, P.P.; Gujraty, K.; Tirrell, M.; Ruoslahti, E. Targeting atherosclerosis by using modular multifunctional micelles. Proc. Natl. Acad. Sci. USA 2009. [Google Scholar] [CrossRef]
- Dvir, T.; Bauer, M.; Schroeder, A.; Tsui, J.H.; Anderson, D.G.; Langer, R.; Liao, R.; Kohane, D.S. Nanoparticles Targeting the Infarcted Heart. Nano Lett. 2011, 11, 4411–4414. [Google Scholar]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar]
- The 10 Leading Causes of Death in the World, 2000 and 2011. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 1 July 2013).
- Agrawal, D.K.; Shao, Z. Pathogenesis of allergic airway inflammation. Curr. Allergy Asthma Rep. 2010, 10, 39–48. [Google Scholar]
- Jin, C.; Shelburne, C.P.; Li, G.J.; Potts, E.N.; Riebe, K.J.; Sempowski, G.D.; Foster, W.M.; Abraham, S.N. Particulate allergens potentiate allergic asthma in mice through sustained IgE-mediated mast cell activation. J. Clin. Investig. 2011, 121, 941–955. [Google Scholar]
- Brunton, S.; Carmichael, B.P.; Colgan, R.; Feeney, A.S.; Fendrick, A.M.; Quintiliani, R.; Scott, G. Acute exacerbation of chronic bronchitis: A primary care consensus guideline. Am. J. Manag. Care 2004, 10, 689–696. [Google Scholar]
- Card, J.W.; Zeldin, D.C.; Bonner, J.C.; Nestmann, E.R. Pulmonary applications and toxicity of engineered nanoparticles. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 295, L400–L411. [Google Scholar]
- Kim, C.S.; Kang, T.C. Comparative measurement of lung deposition of inhaled fine particles in normal subjects and patients with obstructive airway disease. Am. J. Respir. Crit. Care Med. 1997, 155, 899–905. [Google Scholar]
- Chalupa, D.C.; Morrow, P.E.; Oberdörster, G.; Utell, M.J.; Frampton, M.W. Ultrafine particle deposition in subjects with asthma. Environ. Health Perspect. 2004, 112, 879. [Google Scholar]
- Kamata, H.; Tasaka, S.; Inoue, K.-I.; Miyamoto, K.; Nakano, Y.; Shinoda, H.; Kimizuka, Y.; Fujiwara, H.; Ishii, M.; Hasegawa, N. Carbon black nanoparticles enhance bleomycin-induced lung inflammatory and fibrotic changes in mice. Exp. Biol. Med. 2011, 236, 315–324. [Google Scholar]
- Takano, H.; Yanagisawa, R.; Ichinose, T.; Sadakane, K.; Yoshino, S.; Yoshikawa, T.; Morita, M. Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines chemokines and intercellular adhesion molecule-1. Am. J. Respir. Crit. Care Med. 2002, 165, 1329–1335. [Google Scholar]
- Inoue, K.-I.; Takano, H.; Yanagisawa, R.; Hirano, S.; Kobayashi, T.; Fujitani, Y.; Shimada, A.; Yoshikawa, T. Effects of inhaled nanoparticles on acute lung injury induced by lipopolysaccharide in mice. Toxicology 2007, 238, 99–110. [Google Scholar]
- Santhanam, P.; Wagner, J.G.; Elder, A.; Gelein, R.; Carter, J.M.; Driscoll, K.E.; Oberdorster, G.; Harkema, J.R. Effects of subchronic inhalation exposure to carbon black nanoparticles in the nasal airways of laboratory rats. Int. J. Nanotechnol. 2008, 5, 30–54. [Google Scholar]
- Sung, J.H.; Ji, J.H.; Yoon, J.U.; Kim, D.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; Chung, Y.H.; Kim, J.; et al. Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal. Toxicol. 2008, 20, 567–574. [Google Scholar]
- Grassian, V.H.; O’Shaughnessy, P.T.; Adamcakova-Dodd, A.; Pettibone, J.M.; Thorne, P.S. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007, 115, 397–402. [Google Scholar]
- Ambalavanan, N.; Stanishevsky, A.; Bulger, A.; Halloran, B.; Steele, C.; Vohra, Y.; Matalon, S. Titanium oxide nanoparticle instillation induces inflammation and inhibits lung development in mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2013, 304, L152–L161. [Google Scholar]
- Ruenraroengsak, P.; Novak, P.; Berhanu, D.; Thorley, A.J.; Valsami-Jones, E.; Gorelik, J.; Korchev, Y.E.; Tetley, T.D. Respiratory epithelial cytotoxicity and membrane damage (holes) caused by amine-modified nanoparticles. Nanotoxicology 2012, 6, 94–108. [Google Scholar]
- Khatri, M.; Bello, D.; Pal, A.K.; Cohen, J.M.; Woskie, S.; Gassert, T.; Lan, J.; Gu, A.Z.; Demokritou, P.; Gaines, P. Evaluation of cytotoxic genotoxic and inflammatory responses of nanoparticles from photocopiers in three human cell lines. Part. Fibre Toxicol. 2013, 10, 42. [Google Scholar]
- Inoue, K.-I.; Koike, E.; Yanagisawa, R.; Hirano, S.; Nishikawa, M.; Takano, H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol. Appl. Pharmacol. 2009, 237, 306–316. [Google Scholar]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar]
- Takano, H.; Yoshikawa, T.; Ichinose, T.; Miyabara, Y.; Imaoka, K.; Sagai, M. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am. J. Respir. Crit. Care Med. 1997, 156, 36–42. [Google Scholar]
- Inoue, K.-I.; Koike, E.; Takano, H.; Yanagisawa, R.; Ichinose, T.; Yoshikawa, T. Effects of diesel exhaust particles on antigen-presenting cells and antigen-specific Th immunity in mice. Exp. Biol. Med. 2009, 234, 200–209. [Google Scholar]
- Al-Humadi, N.H.; Siegel, P.D.; Lewis, D.M.; Barger, M.W.; Ma, J.Y.; Weissman, D.N.; Ma, J.K. The effect of diesel exhaust particles (DEP) and carbon black (CB) on thiol changes in pulmonary ovalbumin allergic sensitized Brown Norway rats. Exp. Lung Res. 2002, 28, 333–349. [Google Scholar]
- Inoue, K.-I.; Takano, H.; Yanagisawa, R.; Sakurai, M.; Ichinose, T.; Sadakane, K.; Yoshikawa, T. Effects of nano particles on antigen-related airway inflammation in mice. Respir. Res. 2005, 6, 106. [Google Scholar]
- Inoue, K.-I.; Takano, H.; Yanagisawa, R.; Koike, E.; Shimada, A. Size effects of latex nanomaterials on lung inflammation in mice. Toxicol. Appl. Pharmacol. 2009, 234, 68–76. [Google Scholar]
- De Haar, C.; Hassing, I.; Bol, M.; Bleumink, R.; Pieters, R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin. Exp. Allergy 2006, 36, 1469–1479. [Google Scholar]
- Ryman-Rasmussen, J.P.; Tewksbury, E.W.; Moss, O.R.; Cesta, M.F.; Wong, B.A.; Bonner, J.C. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am. J. Respir. Cell Mol. Biol. 2009, 40, 349. [Google Scholar]
- Nygaard, U.C.; Hansen, J.S.; Samuelsen, M.; Alberg, T.; Marioara, C.D.; Løvik, M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol. Sci. 2009, 109, 113–123. [Google Scholar]
- Inoue, K.-I.; Yanagisawa, R.; Koike, E.; Nishikawa, M.; Takano, H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: Possible role of oxidative stress. Free Radic. Biol. Med. 2010, 48, 924–934. [Google Scholar]
- Yanagisawa, R.; Takano, H.; Inoue, K.; Ichinose, T.; Sadakane, K.; Yoshino, S.; Yamaki, K.; Kumagai, Y.; Uchiyama, K.; Yoshikawa, T. Enhancement of acute lung injury related to bacterial endotoxin by components of diesel exhaust particles. Thorax 2003, 58, 605–612. [Google Scholar]
- Cesta, M.F.; Ryman-Rasmussen, J.P.; Wallace, D.G.; Masinde, T.; Hurlburt, G.; Taylor, A.J.; Bonner, J.C. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am. J. Respir. Cell Mol. Biol. 2010, 43, 142. [Google Scholar]
- Hussain, S.; Vanoirbeek, J.A.; Luyts, K.; de Vooght, V.; Verbeken, E.; Thomassen, L.C.; Martens, J.A.; Dinsdale, D.; Boland, S.; Marano, F. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur. Respir. J. 2011, 37, 299–309. [Google Scholar]
- Nikula, K.J.; Green, F.H. Animal models of chronic bronchitis and their relevance to studies of particle-induced disease. Inhal. Toxicol. 2001, 12, 123–153. [Google Scholar]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nano 2007, 2, 469–478. [Google Scholar]
- Li, N.; Wang, M.; Bramble, L.A.; Schmitz, D.A.; Schauer, J.J.; Sioutas, C.; Harkema, J.R.; Nel, A.E. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ. Health Perspect. 2009, 117, 1116–1123. [Google Scholar]
- Warheit, D.B.; Laurence, B.; Reed, K.L.; Roach, D.; Reynolds, G.; Webb, T. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004, 77, 117–125. [Google Scholar]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar]
- Meredith, S.; Bugler, J.; Clark, R. Isocyanate exposure and occupational asthma: A case-referent study. Occup. Environ. Med. 2000, 57, 830–836. [Google Scholar]
- Matheson, J.M.; Johnson, V.J.; Vallyathan, V.; Luster, M.I. Exposure and immunological determinants in a murine model for toluene diisocyanate (TDI) asthma. Toxicol. Sci. 2005, 84, 88–98. [Google Scholar]
- Roberts, R.A.; Ganey, P.E.; Ju, C.; Kamendulis, L.M.; Rusyn, I.; Klaunig, J.E. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol. Sci. 2007, 96, 2–15. [Google Scholar]
- Hollinger, F.B.; Liang, T.J. Hepatitis B virus. In Fields Virology, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 2971–3036. [Google Scholar]
- Schuppan, D.; Schattenberg, J.M. Non-alcoholic steatohepatitis: Pathogenesis and novel therapeutic approaches. J. Gastroenterol. Hepatol. 2013, 28, 68–76. [Google Scholar]
- Ahmad, J.; Ahamed, M.; Akhtar, M.J.; Alrokayan, S.A.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol. Appl. Pharmacol. 2012, 259, 160–168. [Google Scholar]
- Abdelhalim, M.A.K.; Jarrar, B.M. Gold nanoparticles administration induced prominent inflammatory central vein intima disruption fatty change and Kupffer cells hyperplasia. Lipids Health Dis. 2011, 10, 133. [Google Scholar]
- Bastús, N.G.; Sánchez-Tilló, E.; Pujals, S.; Farrera, C.; López, C.; Giralt, E.; Celada, A.; Lloberas, J.; Puntes, V. Homogeneous conjugation of peptides onto gold nanoparticles enhances macrophage response. ACS Nano 2009, 3, 1335–1344. [Google Scholar]
- Chen, Q.; Xue, Y.; Sun, J. Kupffer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 1129. [Google Scholar]
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano 2012, 6, 8767–8777. [Google Scholar]
- Hwang, J.H.; Kim, S.J.; Kim, Y.H.; Noh, J.R.; Gang, G.T.; Chung, B.H.; Song, N.W.; Lee, C.H. Susceptibility to gold nanoparticle-induced hepatotoxicity is enhanced in a mouse model of nonalcoholic steatohepatitis. Toxicology 2012, 294, 27–35. [Google Scholar]
- Boonstra, A.; Woltman, A.M.; Janssen, H.L. Immunology of hepatitis B and hepatitis C virus infections. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 1049–1061. [Google Scholar]
- Guidotti, L. Pathogenesis of viral hepatitis. J. Biol. Regul. Homeost. Agents 2003, 17, 115. [Google Scholar]
- Lee, W.M. Drug-induced hepatotoxicity. N. Engl. J. Med. 2003, 349, 474–485. [Google Scholar]
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar]
- Seidell, J.C. Epidemiology of obesity. In Seminars in Vascular Medicine, 2005; Thieme Medical Publishers, Inc.: New York, NY, USA, 2005; pp. 3–14. [Google Scholar]
- Fratiglioni, L.; Winblad, B.; von Strauss, E. Prevention of alzheimer’s disease and dementia major findings from the kungsholmen project. Physiol. Behav. 2007, 92, 98–104. [Google Scholar]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y. Global prevalence of dementia: A Delphi consensus study. Lancet 2006, 366, 2112–2117. [Google Scholar]
- Liao, D.; Creason, J.; Shy, C.; Williams, R.; Watts, R.; Zweidinger, R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ. Health Perspect. 1999, 107, 521. [Google Scholar]
- Creason, J.; Neas, L.; Walsh, D.; Williams, R.; Sheldon, L.; Liao, D.; Shy, C. Particulate matter and heart rate variability among elderly retirees: The Baltimore 1998 PM study. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 116. [Google Scholar]
- Neupane, B.; Jerrett, M.; Burnett, R.T.; Marrie, T.; Arain, A.; Loeb, M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am. J. Respir. Crit. Care Med. 2010, 181, 47–53. [Google Scholar]
- Chen, Z.; Meng, H.; Xing, G.; Yuan, H.; Zhao, F.; Liu, R.; Chang, X.; Gao, X.; Wang, T.; Jia, G. Age-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: Nanotoxicity has susceptible population. Environ. Sci. Technol. 2008, 42, 8985–8992. [Google Scholar]
- Barandeh, F.; Nguyen, P.-L.; Kumar, R.; Iacobucci, G.J.; Kuznicki, M.L.; Kosterman, A.; Bergey, E.J.; Prasad, P.N.; Gunawardena, S. Organically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivo. PLoS One 2012, 7, e29424. [Google Scholar]
- Garcia-Bennett, A.E.; Kozhevnikova, M.; König, N.; Zhou, C.; Leao, R.; Knöpfel, T.; Pankratova, S.; Trolle, C.; Berezin, V.; Bock, E. Delivery of differentiation factors by mesoporous silica particles assists advanced differentiation of transplanted murine embryonic stem cells. Stem Cells Transl. Med. 2013, 2, 906–915. [Google Scholar]
- Beausejour, C.M.; Campisi, J. Ageing: Balancing regeneration and cancer. Nature 2006, 443, 404–405. [Google Scholar]
- Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2009, 41, 67–76. [Google Scholar]
| Type | Materials | Animals/cells | Mechanism of exposure | Findings | Ref. |
|---|---|---|---|---|---|
| In vivo | CdTe/CdS core/shell QDs (1.7, 2.6, 3.2 nm) | Kun Ming mice | Intravenous injection of PBS (pH 7.4)-diluted QDs containing 20, 50, 86, or 125 μg Cd 20–22 days after female mice were housed with male mice | QDs were transferred to the fetuses across the placental barrier, smaller QDs transferred more easily, the number of QDs transferred was dose dependent | [11] |
| In vivo | PEG-coated CdSe/ZnS QDS | Wistar rats | Intraperitoneal injection of 0.8 μmol/L QDS on GD 18 | QDs were not detected in fetal tissues | [13] |
| In vitro | Gold nanoparticles coated with PEG (15 and 30 nm) | Human placenta | Open perfusion for 5 min, 7.9 × 1011 for 15-nm particles and 7.8 × 1010 for 30-nm particles | Detection of high levels of nanoparticles soon after perfusion in maternal outflow, no detection of nanoparticles in fetal outflow | [16] |
| In vitro | Gold nanoparticles coated with PEG (10 and 15 nm) | Human placenta | Recirculating perfusion for 6 h, 9.1 × 109 for 10-nm particles and 2.0 × 109 for 15-nm particles | No transplacental transfer of nanoparticles | [16] |
| In vitro | Polystyrene beads (50.80, 240, 500 nm) | Human placenta | Open perfusion for 20 min at 25 μg/mL | Polystyrene beads with diameters up to 240 nm crossed the placental barrier | [12] |
| In vivo | Silicon nanovectors (519, 834, 1000 nm) | Sprague Dawley rats | Intravenous injection on GD 20 at 1.2 × 10−9 g/mouse | Fetal silicon levels were higher only in the 519 nm SNV group | [14] |
| In vitro | Amine-modified polystyrene beads (PS; 200 nm), carboxyl-modified PS (20, 100, 500 nm) | BALB/c mice blastocysts | Micro injection of 0.6 (20 nm carboxyl PS), 0.6 (100 nm carboxyl PS), 1.25 (200 nm amine PS), 8 μL (500 nm carboxyl PS) PS via extraembryonic tissue on GD 7.5 | 20-nm carboxylic PS and 200-nm amine-modified PS were detected in the embryos, while 100- and 500-nm PS were not | [17] |
| Type | Materials | Animals/cells | Mechanism of exposure | Findings | Ref. |
|---|---|---|---|---|---|
| In vivo | Cadmium oxide nanoparticles (11 and 15 nm) | CD-1 mice | Inhalation of 100 μg CdO/m3/2 days or 230 μg CdO/m3/day on 4.5 days post coitus (dpc) to 16.5 dpc | Fetal length and neonatal growth rate decreased | [26] |
| In vivo | TiO2 nanoparticles (20.6 nm) | C57BL/6 mice | Inhalation of 42.4 mg UV-Titan/m3 1 h/day on GD 8–18 | F2 female descendants’ ESTR germline mutation rates unchanged | [34] |
| In vivo | p-SWCNTs, o-SWCNTs, uo-SWCNTs | CD-1 mice | Intravenous injection of 10 ng, 100 ng, 300 ng, 3 μg, or 30 μg/mouse on 5.5 dpc | Early miscarriages and fetal malformations | [28] |
| In vitro | Silver nanoparticles (13 nm) | ICR mice blastocysts | Incubation of 25 or 50 μmol/L silver nanoparticles on GD 3 | Apoptosis and developmental retardation in blastocysts | [29] |
| In vitro | CdSe-core QDs (3.5 nm) | ICR mice blastocysts and morulas | Incubation at 125, 250, or 500 nmol/L for 24 h | Number of apoptotic cells of blastocysts at 250 and 500 nmol/L increased, development of morulas into blastocysts at 250 and 500 nmol/L was blocked, blastocyst development at 125 nmol/L and higher was retarded | [30] |
| In vitro | Amine-modified polystyrene beads (200 nm), carboxyl-modified PS (20, 100, or 500 nm) | BALB/c mice blastocysts | Micro injection via extraembryonic tissue of 0.6, 0.6, 1.25, or 8 μL PS on GD 7.5 | Growth inhibition of embryos was detected; translocation in embryos was associated with surface modification and size | [17] |
| In vitro | Silica nanoparticles (10 or 30 nm) | Mouse embryonic stem cells | Incubation at 1, 3, 10, 30, 100 μg/mL for 24 h or 10 days | Inhibition of differentiation of stem cells was detected below cytotoxic concentrations | [31] |
| In vivo | CdSe/ZnS QDs, CdTe QDs | Wistar rat | Intraperitoneal injection on the 6th, 13th, and 18th days of embryogenesis at 5 mg/kg | QDs did not cause any direct embryotoxic or teratogenic effects | [35] |
| Type | Materials | Animals/cells | Methods of exposure | Findings | Ref. |
|---|---|---|---|---|---|
| In vivo | TiO2 nanoparticles (97 nm) | C57BL/6BomTac mice | Inhalation of 42.4 mg UV-Titan/m3 1 h/day on GD 8–18 | Moderate neurobehavioral alterations in offspring | [38] |
| In vivo | Anantase TiO2 nanopowder (2570 nm) | ICR mice | Subcutaneous injection of 100 μg/mouse/time on GD 6, 9, 12, and 15 | Alterations in expression of genes related to brain development, central neural system function, and inflammation in offspring | [39] |
| In vivo | Carbon black nanoparticles (Printex 90; 140 nm) | C57BL/6BomTac mice | Instillation of 11, 54, and 268 μg Printex 90/animal on GD 7, 10, 15, and 18 | Altered habituation pattern in the open field test | [40] |
| In vivo | Anantase TiO2 nanoparticles (25–70 nm) | ICR mice | Subcutaneous injection of 100 μg/mouse/time on GD 6, 9, 12, and 15 | Alterations in the cerebral cortex, olfactory bulb, and some regions related to dopamine systems | [41] |
| In vivo | Anantase TiO2 nanoparticles (25–70 nm) | ICR mice | Subcutaneous injection of 100 μg/mouse/time on 3, 7, 10, and 14 dpc | Apoptosis in the olfactory bulb of the brain | [42] |
| In vivo | Anantase TiO2 nanoparticles (25–70 nm) | ICR mice | Subcutaneous injection at 0.1 mg/mouse/time on GD 6, 9, 12, 15, and 18 | Dopamine levels in the prefrontal cortex and neostriatum increased | [43] |
| In vivo | Anantase TiO2 nanoparticles (<25 nm) | Sprague-Dawley rats | Oral administration at 100 mg/kg on prenatal day 2–21 or postnatal day 2–21 | Short and long-term synaptic plasticity in the rat hippocampal DG area was impaired | [33] |
| In vitro | Polyethylene nanoparticles (33 nm) | Human embryonic stem cells | Incubation at 360 μg/mL for 48 h | Downstream neuronal precursor genes and a patterning marker gene were reduced in expression | [44] |
| Type | Materials | Animals/cells | Method of exposure | Findings | Ref. |
|---|---|---|---|---|---|
| In vivo | Carbon black nanoparticles (14 nm) | ICR mice | Instillation at 0.2 mg/mouse on GD 7 and 14 | Seminiferous tubule vacuolation, decreased DSP, reduced cellular adhesion of seminiferous epithelia | [45] |
| In vivo | DMSA-coated Fe3O4 nanoparticles (3–9 nm) | Balb/C mice | Intraperitoneal injection at 50, 100, 200, and 300 mg/kg on GD8 | Infant growth decreased, testes development was disrupted | [46] |
| In vivo | Titanium dioxide (UV-Titan) nanoparticles (17 nm) | C57BL/6BomTac | Inhalation of 42 mg UV-Titan/m3 on GD 8–18 1 h/day | Changes in gene expression related to the retinoic acid signaling pathway in female offspring | [47] |
| In vivo | UV-Titan (20.6 nm), Printex 90 (14 nm) | C57BL/6J mice | Inhalation and intratracheal instillation of 42 mg/m3 UV-Titan or 67 μg/animal Printex 90 on GD 8–18 at 1 h/day (UV-Titan) or on GD 7, 10, 15, and 18 (Printex 90) | UV-Titan reduced sperm counts in the F1 generation, time-to-first F2 litter increased in male offspring | [48] |
| In vivo | Anantase TiO2 nanoparticles (25–70 nm) | ICR mice | Subcutaneous injection at 100 μg/mouse/time on 3, 7, 10, and 14 dpc | Daily sperm production reduced | [42] |
| Type | Materials | Animal/cell model | Mechanism of exposure | Findings | Ref. |
|---|---|---|---|---|---|
| In vivo | DEP | OVA-induced asthma ICR mice model | Intratracheal injection of 100 μg DEP once a week for 6 weeks | OVA-specific IgG and IgE production were enhanced; IL-5, IL-4, GM-CSF, and IL-2 expression increased; ovalbumin-induced airway inflammation was aggravated | [105] |
| In vivo | DEP | OVA-induced ICR asthma mice model | Intratracheal injection of 100 μg DEP every 2 weeks for 4 weeks (a total of 3 injections) | DEP promoted local and systemic dysregulation of Th immunity in mice by 1. enhancement of antigen-presenting cell (APC) activity including dendritic cells (DC) and 2. enhancement of extrathoracic antigen-specific Th responses | [106] |
| In vivo and In vitro | DEP, carbon black (CB) | In vivo: OVA-induced Brown Norway asthma rat model. In vitro: bone marrow-derived dendritic cells (BMDC) | In vivo: Intratracheal instillation of 5 mg/kg DEP or CB once In vitro: Exposed to different concentrations of DEP (1–10 μg/mL) for 24 h | Pulmonary inflammation was enhanced; serum OVA-specific IgG and IgE levels increased significantly; glutathione (GSH) levels in lymphocytes were reduced; IL-4 mRNA levels in lung tissue increased | [107] |
| In vivo | Carbon black NP | OVA-induced ICR asthma mice model | Intratracheal injection of 50 μg DEP once a week for 6 weeks | Accelerated OVA-induced expression of IL-5 and activated Th2-like lymphocytes, which together caused eosinophilic inflammation; smaller CB had more prominent aggravation effects | [108] |
| In vivo | Latex nanoparticles (25, 50, and 100 nm) | OVA-induced ICR asthma mice model | Intratracheal injection of 50 or 100 μg latex nanoparticles every week for 6 weeks | Latex nanoparticles enhanced neutrophilic, but not eosinophilic lung inflammation in a size-dependent manner | [109] |
| In vivo | Titanium dioxide nanoparticles (TiO2; 250, 260, 29 and 14 nm) | OVA-induced BALB/cANN Crl asthma mice model | Intranasal droplet application on days 0, 1, and 2 (total 200 μg) | Lung-draining peribronchial lymph node cell numbers increased, and OVA-specific Th2 cytokines (IL-4, IL-5, IL-10, and IL-13) were produced | [110] |
| In vivo and In vitro | MWCNTs | In vivo: OVA-induced ICR asthma mice model In vitro: BMDCs | In vivo: Intratracheal injection of 25 or 50 μg MWCNT once a week for 6 weeks In vitro: exposure to different concentrations of MWCNT (0.1–1 μg/mL) for 24 h | MWCNTs aggravated allergen-induced airway inflammation, Th cytokine and chemokine levels increased, IgG1 and IgE levels increased, syngeneic T-cell proliferation increased, and APCs including DC were activated | [103] |
| In vivo | MWCNTs | OVA-induced C57BL/6 asthma mice model | Inhalation of 100 mg/m3 MWCNT for 6 h | PDGF, TGF-β1, and IL-5 mRNA levels were elevated, airway fibrosis was induced | [111] |
| In vivo | MWCNTs, SWCNTs | OVA-induced BALB/cAnN Crl asthma mice model | Injection model: subcutaneous injection of 200 μg (single dose) MWCNT or SWCNT into the mouse footpad Intranasal model: Intranasal administration of 400 μg (133 μg per day for 3 days) MWCNT or SWCNT | Serum OVA-specific IgE levels increased, the number of eosinophils in bronchoalveolar lavage fluid (BALF) increased, Th2-associated cytokines in the mediastinal lymph node (MLN) increased, IgG2a levels, TNF-α levels and neutrophil cell numbers increased only in the MWCNT group | [112] |
| In vivo and In vitro | SWCNTs | In vivo: OVA-induced ICR asthma mice model In vitro: BMDCs | In vivo: intratracheal administration of 25 or 50 μg SWCNT once a week for 6 weeks In vitro: exposed to various concentrations of SWCNT (0.1–10 μg/mL) | Aggravated allergen-induced airway inflammation with mucus hyperplasia, OVA-specific IgG1 and IgE and Th cytokine and chemokine levels increased, oxidative stress level was accentuated, dendritic cells were activated | [113] |
| In vivo | DEP | LPS-induced ICR asthma mice model | Intratracheal instillation of 250 μg DEP once | DEP enhanced neutrophilic lung inflammation by the induction of proinflammatory molecules including p65-containing dimer(s) of NF-κB and Toll-like receptors | [95] |
| In vivo | DEP | LPS-induced ICR asthma mice model | Inhalation of DEP at a concentration of 15, 36, or 169 μg/m3 once | DEP exacerbated lung inflammation by production of IL-1β and keratinocyte chemoattractant | [96] |
| In vivo | Washed DEP, organic chemicals of DEP (DEP-OC) | LPS-induced ICR asthma mice model | Intratracheal instillation of 125 μg washed DEP or DEP-OC once | Residual carbonaceous DEP nuclei mainly contribute to the aggravation of LPS-induced lung inflammation | [114] |
| In vivo | MWCNTs, CB nanoparticles | LPS-induced Sprague-Dawley asthma rat model | Intratracheal instillation at 4 mg/kg once | MWCNTs but not CB caused more obvious lung injury and led to the formation of pulmonary fibrosis in rats with pre-existing inflammatory conditions | [115] |
| In vivo | SWCNTs, MWCNTs | LPS-induced ICR asthma mice model | Intratracheal instillation at dose of 4 mg/kg once | Both CNTs enhanced LPS-stimulated expression of inflammatory cytokines and chemokines in lung tissue and in circulation, including IL-1β, MIP-1α, MCP-1, and keratinocyte-derived chemo-attractants; the effects were more prominent with SWCNT than with MWCN | [71] |
| In vivo | CB nanoparticles (14, 56 nm) | LPS-induced ICR asthma mice model | Intratracheal administration at dose of 4 mg/kg once | CB nanoparticles of 14 nm but not 56 nm aggravated lung inflammation and pulmonary edema by inducing the expression of IL-1β, MIP-1α and keratinocyte chemoattractant | [70] |
| In vivo | Latex nanoparticles (25, 50, and 100 nm) | LPS-induced ICR asthma mice model | Intratracheal injection of 50 or 100 μg latex nanoparticles every week for 6 weeks | Latex nanoparticles aggravated lung inflammation induced by LPS; the enhancement was greater with smaller nanoparticles | [109] |
| In vivo | TiO2 nanoparticles, gold nanoparticles | TDI-induced BALB/c asthma mice model | Intratracheal instillation at dose of 0.8 mg/kg once | TiO2 and Au nanoparticles increased pulmonary inflammation and airway hyperreactivity | [116] |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, Y.; Zhang, Y.; Yan, B. Nanotoxicity Overview: Nano-Threat to Susceptible Populations. Int. J. Mol. Sci. 2014, 15, 3671-3697. https://doi.org/10.3390/ijms15033671
Li Y, Zhang Y, Yan B. Nanotoxicity Overview: Nano-Threat to Susceptible Populations. International Journal of Molecular Sciences. 2014; 15(3):3671-3697. https://doi.org/10.3390/ijms15033671
Chicago/Turabian StyleLi, Yang, Yi Zhang, and Bing Yan. 2014. "Nanotoxicity Overview: Nano-Threat to Susceptible Populations" International Journal of Molecular Sciences 15, no. 3: 3671-3697. https://doi.org/10.3390/ijms15033671




