Three-Dimensional Stratification of Bacterial Biofilm Populations in a Moving Bed Biofilm Reactor for Nitritation-Anammox
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reactor Conditions
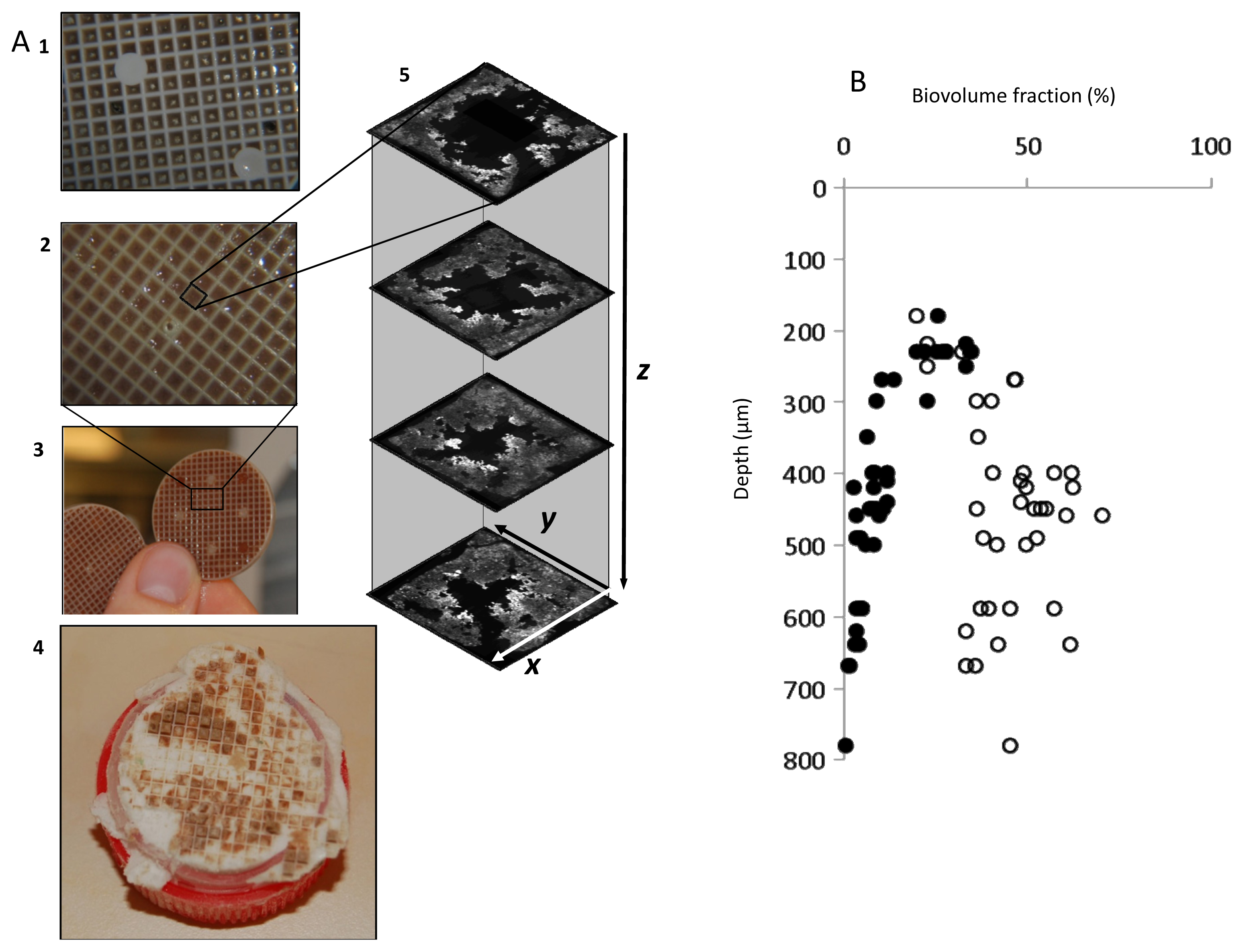
2.2. Biofilm Retrieval and Sectioning
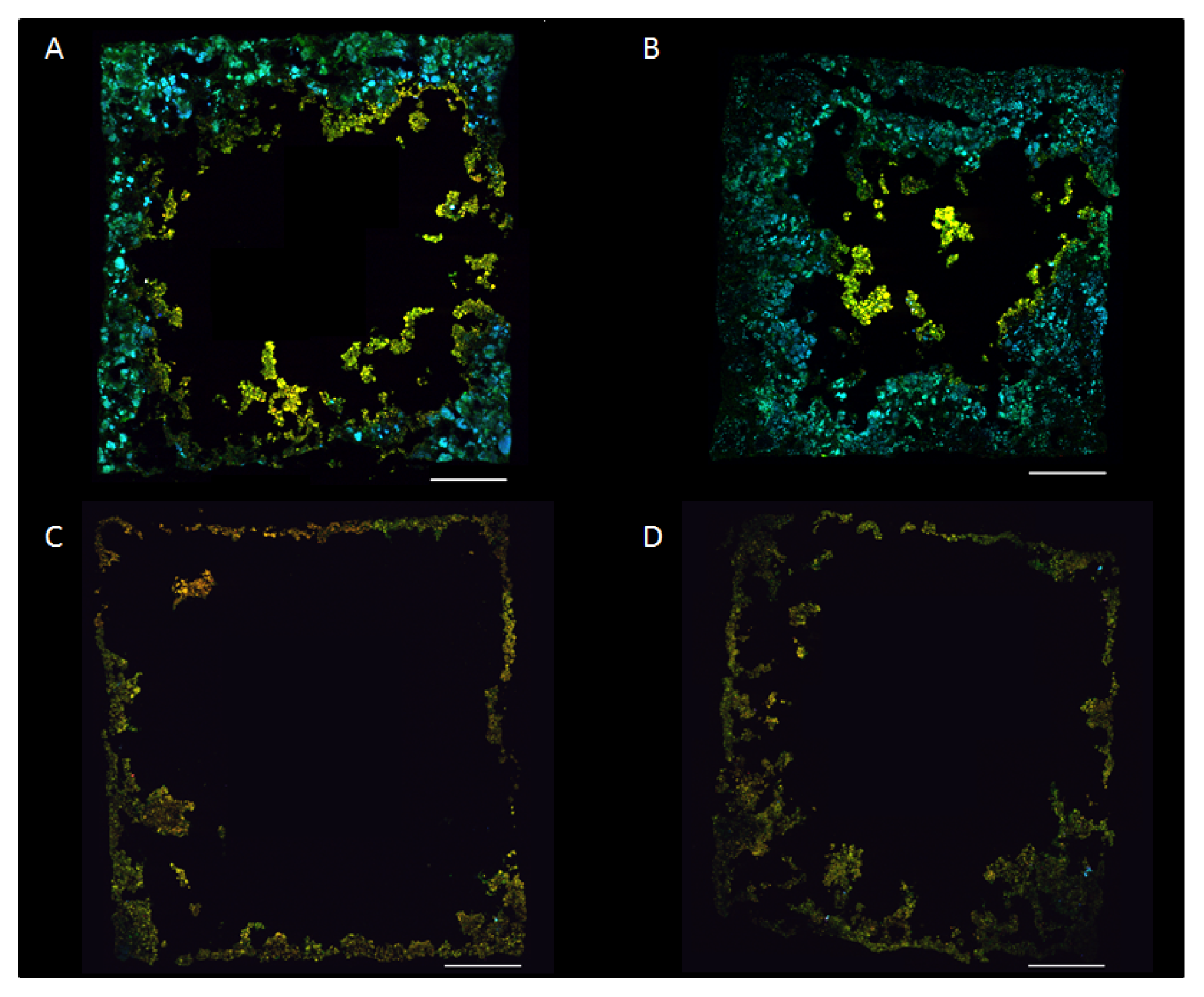
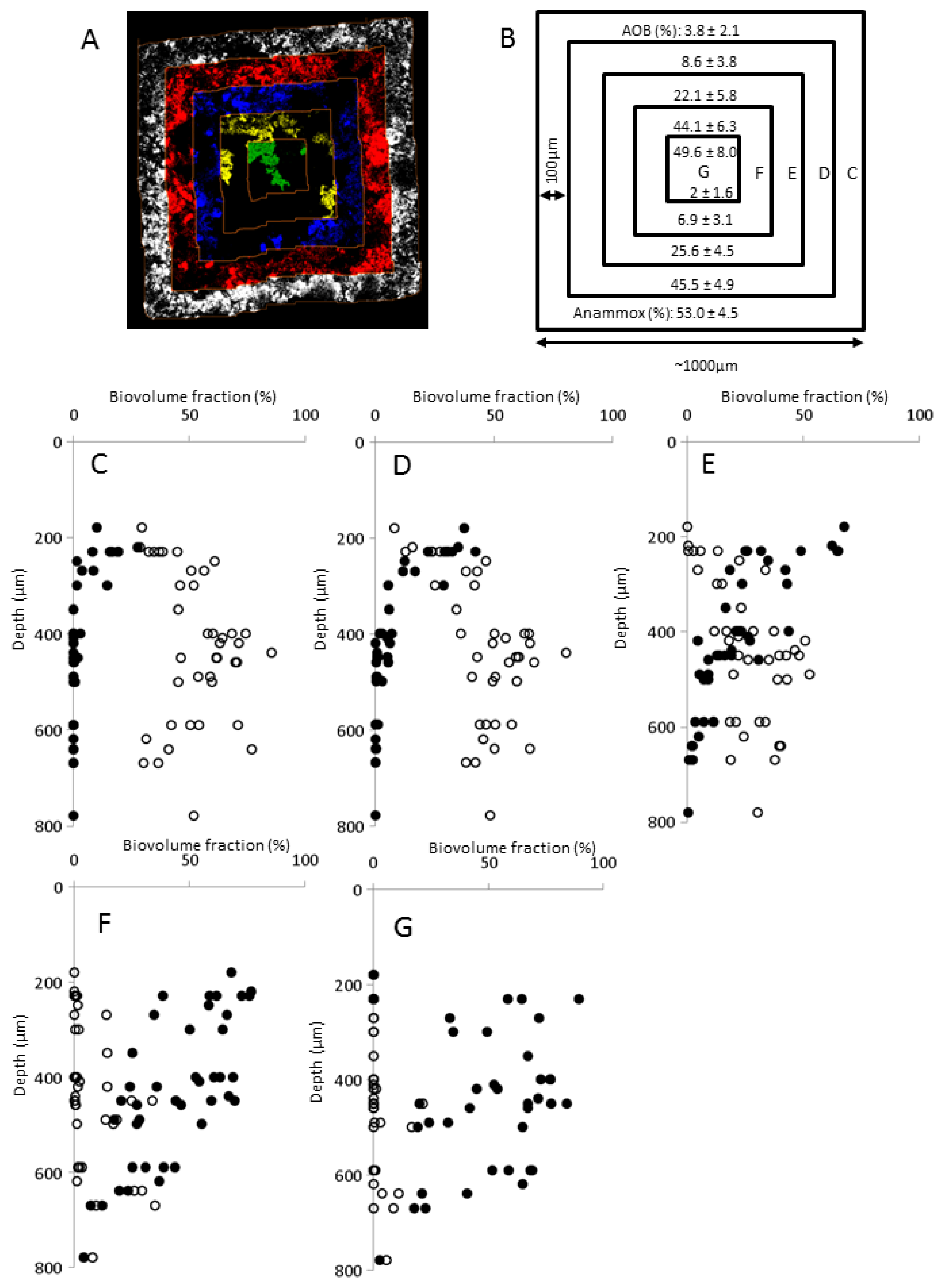
2.3. Bacterial Community Composition
2.4. Biofilm Establishment on New Carriers
2.5. Biofilm Structure
3. Experimental Procedures
3.1. Reactor Conditions
3.2. Biofilm Sampling and Preparation
3.3. Fluorescence in Situ Hybridization
3.4. Confocal Laser Scanning Microscopy
3.5. Digital Image Analysis
4. Conclusions
Supplementary Information
1. Reactor Medium
2. Measurement of Biofilm Carrier Compartment Size
3. Digital Image Analysis of Biofilms
3.1. Estimation of Biofilm Shrinkage
3.2. Estimation of Mature Biofilm Thickness
3.3. Estimation of Biovolume Fraction of Populations
| Carrier specifics | Type 1 1 | Type 2 1 |
|---|---|---|
| Depth of carrier compartments (i.e., z dimension in figures) | 2 mm | 3 mm |
| Diameter of the carrier | 30 mm | 30 mm |
| Protected surface area per carrier | 2.73 × 10−3 m2 | 4.10 × 10−3 m2 |
| Number of square compartments | 325 | 325 |
| Probe name 1 | Target | Sequence (5′–3′) | FA 2 [%] |
|---|---|---|---|
| Eub338 3 | Most Bacteria | GCT GCC TCC CGT AGG AGT | 0–50 |
| Amx820 | Anaerobic ammonium-oxidizing bacteria, Candidatus “Brocadia anammoxidans” and Candidatus “Kuenenia stuttgartiensis” | AAA ACC CCT CTA CTT AGT GCC C | 40 |
| Apr820 | Candidatus Anammoxoglobus propionicus | AAA CCC CTC TAC CGA GTG CCC | 40 |
| Ban162 | Candidatus Brocadia anammoxidans | CGG TAG CCC CAA TTG CTT | 40 |
| BS820 | Candidatus Scalindua wagneri, Candidatus Scalindua sorokinii | TAA TTC CCT CTA CTT AGT GCC C | 20 |
| Bfu613 | Candidatus Brocadia fulgida | GGA TGC CGT TCT TCC GTT AAG CGG | 30 |
| Sca1309 | Genus Candidatus Scalindua | TGG AGG CGA ATT TCA GCC TCC | 5 |
| Scabr1114 | Candidatus Scalindua brodae | CCC GCT GGT AAC TAA AAA CAA G | 20 |
| Pla46 | Planctomycetales | GAC TTG CAT GCC TAA TCC | 30 |
| Nso1225 | Most beta-proteobacterial AOB | CGC CAT TGT ATT ACG TGT GA | 35 |
| Nse1472 | Nitrosomonas europea, N. halophila, N. eutropha, Kraftisried-Isolat Nm103 | ACC CCA GTC ATG ACC CCC | 50 |
| Ntspa662 4 | Genus Nitrospira | GGA ATT CCG CGC TCC TCT | 35 |
| Ntspa1151 | Sublineage II of the genus Nitrospira | TTC TCC TGG GCA GTC TCT CC | 35–40 |
| Ntspa1431 | Sublineage I of the genus Nitrospira | TTG GCT TGG GCG ACT TCA | 35 |
| Ntg840 | Nitrotoga arctica | CTA AGG AAG TCT CCT CCC | 10–20 |
| Nit3 4 | Genus Nitrobacter | CCT GTG CTC CAT GCT CCG | 40 |
| NmII | Nitrosomonas communis lineage | TTA AGA CAC GTT CCG ATG TA | 35 |
| Alf968 | Alphaproteobacteria, except of Rickettsiales | GGT AAG GTT CTG CGC GTT | 20 |
| CF319a | Most Flavobacteria, some Bacteroidetes, some Sphingobacteria | TGG TCC GTG TCT CAG TAC | 35 |
| Gam42a 4 | Gammaproteobacteria | GCC TTC CCA CAT CGT TT | 35 |
Acknowledgments
Conflicts of Interest
- Author ContributionsM.H., M.C., R.A., F.S. initiated the project. M.H., R.A., F.S. designed the experiment and R.A., M.E., M.C. carried out the laboratory work. RA did biofilm retrieval and preparation, microscopy and image analyses, R.A., H.D., M.H., F.P., F.S. analyzed data and R.A., F.P., M.H., H.D,. B.M.W. wrote the manuscript.
References
- Vlaeminck, S.E.; de Clippeleir, H.; Verstraete, W. Microbial resource management of one-stage partial nitritation/anammox. Microb. Biotechnol 2012, 5, 433–448. [Google Scholar] [Green Version]
- Sliekers, A.O.; Derwort, N.; Gomez, J.L.C.; Strous, M.; Kuenen, J.G.; Jetten, M.S.M. Completely autotrophic nitrogen removal over nitrite in one single reactor. Water Res 2002, 36, 2475–2482. [Google Scholar]
- Pynaert, K.; Smets, B.F.; Wyffels, S.; Beheydt, D.; Siciliano, S.D.; Verstraete, W. Characterization of an autotrophic nitrogen-removing biofilm from a highly loaded lab-scale rotating biological contactor. Appl. Environ. Microbiol 2003, 69, 3626–3635. [Google Scholar]
- Vlaeminck, S.E.; Terada, A.; Smets, B.F.; de Clippeleir, H.; Schaubroeck, T.; Bolca, S.; Demeestere, L.; Mast, J.; Boon, N.; Carballa, M.; Verstraete, W. Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and anammox. Appl. Environ. Microbiol 2010, 76, 900–909. [Google Scholar]
- Vazquez-Padin, J.; Mosquera-Corral, A.; Campos, J.L.; Mendez, R.; Revsbech, N.P. Microbial community distribution and activity dynamics of granular biomass in a canon reactor. Water. Res 2010, 44, 4359–4370. [Google Scholar]
- Winkler, M.K.H.; Kleerebezem, R.; Kuenen, J.G.; Yang, J.J.; van Loosdrecht, M.C.M. Segregation of biomass in cyclic anaerobic/aerobic granular sludge allows the enrichment of anaerobic ammonium oxidizing bacteria at low temperatures. Environ. Sci. Technol 2011, 45, 7330–7337. [Google Scholar]
- Ni, B.J.; Chen, Y.P.; Liu, S.Y.; Fang, F.; Xie, W.M.; Yu, H.Q. Modeling a granule-based anaerobic ammonium oxidizing (anammox) process. Biotechnol. Bioeng 2009, 103, 490–499. [Google Scholar]
- Volcke, E.I.P.; Picioreanu, C.; de Baets, B.; van Loosdrecht, M.C.M. The granule size distribution in an anammox-based granular sludge reactor affects the conversion-implications for modeling. Biotechnol. Bioeng 2012, 109, 1629–1636. [Google Scholar]
- Vlaeminck, S.E.; Terada, A.; Smets, B.F.; van der Linden, D.; Boon, N.; Verstraete, W.; Carballa, M. Nitrogen removal from digested black water by one-stage partial nitritation and anammox. Environ. Sci. Technol 2009, 43, 5035–5041. [Google Scholar]
- Qiao, S.; Nishiyama, T.; Fujii, T.; Bhatti, Z.; Furukawa, K. Rapid startup and high rate nitrogen removal from anaerobic sludge digester liquor using a snap process. Biodegradation 2012, 23, 157–164. [Google Scholar]
- Liu, T.; Li, D.; Zeng, H.; Li, X.; Liang, Y.; Chang, X.; Zhang, J. Distribution and genetic diversity of functional microorganisms in different canon reactors. Bioresour. Technol 2012, 123, 574–580. [Google Scholar]
- Chen, H.; Liu, S.; Yang, F.; Xue, Y.; Wang, T. The development of simultaneous partial nitrification, anammox and denitrification (snad) process in a single reactor for nitrogen removal. Bioresour. Technol 2009, 100, 1548–1554. [Google Scholar]
- Cho, S.; Fujii, N.; Lee, T.; Okabe, S. Development of a simultaneous partial nitrification and anaerobic ammonia oxidation process in a single reactor. Bioresour. Technol 2011, 102, 652–659. [Google Scholar]
- Tsushima, I.; Ogasawara, Y.; Kindaichi, T.; Satoh, H.; Okabe, S. Development of high-rate anaerobic ammonium-oxidizing (anammox) biofilm reactors. Water Res 2007, 41, 1623–1634. [Google Scholar]
- Plaza, E.; Stridh, S.; Örnmark, J.; Kanders, L.; Trela, J. Swedish experiences of the deammonification process in a biofilm system. Proceedings of WEF-IWA Conference on Nutrient Recovery and Management, Miami, FL, USA, 9–12 January 2011.
- Christensson, M.; Ekström, S.; Andersson Chan, A.; Le Vaillant, E.; Lemaire, R. Experience from start-ups of the first anita mox plants. Water Sci. Technol 2013, 67, 2677–2684. [Google Scholar]
- Persson, F.; Sultana, R.; Suarez, M.; Hermansson, M.; Plaza, E.; Wilén, B.-M. Structure and composition of biofilm communities in a moving bed biofilm reactor for nitritation-anammox at low temperatures. Bioresour. Technol 2014, 154, 267–273. [Google Scholar]
- Alpkvist, E.; Bengtsson, J.; Overgaard, N.C.; Christensson, M.; Heyden, A. Simulation of nitrification of municipal wastewater in a Moving Bed™ biofilm process: A bottom-up approach based on a 2D-continuum model for growth and detachment. Water Sci. Technol 2007, 55, 247–255. [Google Scholar]
- Masic, A.; Bengtsson, J.; Christensson, M. Measuring and modeling the oxygen profile in a nitrifying moving bed biofilm reactor. Math. Biosci 2010, 227, 1–11. [Google Scholar]
- Almstrand, R.; Daims, H.; Persson, F.; Sorensson, F.; Hermansson, M. New methods for analysis of spatial distribution and coaggregation of microbial populations in complex biofilms. Appl. Environ. Microbiol 2013, 79, 5978–5987. [Google Scholar]
- Kindaichi, T.; Ito, T.; Okabe, S. Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl. Environ. Microbiol 2004, 70, 1641–1650. [Google Scholar]
- Okabe, H.; Kindaichi, T.; Nakamura, K.; Ito, T. Eco-physiology of autotrophic nitrifying biofilms. Water Sci. Technol 2005, 52, 225–232. [Google Scholar]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol 1998, 50, 589–596. [Google Scholar]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng 1994, 43, 1131–1138. [Google Scholar]
- Daims, H.; Wagner, M. In situ techniques and digital image analysis methods for quantifying spatial localization patterns of nitrifiers and other microorganisms in biofilm and flocs. Method Enzymol 2011, 496, 185–215. [Google Scholar]
- Juretschko, S.; Timmermann, G.; Schmid, M.; Schleifer, K.H.; Pommerening-Roser, A.; Koops, H.P.; Wagner, M. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol 1998, 64, 3042–3051. [Google Scholar]
- Koops, H.P.; Pommerening-Roser, A. Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol. Ecol 2001, 37, 1–9. [Google Scholar]
- Bollmann, A.; Bar-Gilissen, M.J.; Laanbroek, H.J. Growth at low ammonium concentrations and starvation response as potential factors involved in niche differentiation among ammonia-oxidizing bacteria. Appl. Environ. Microbiol 2002, 68, 4751–4757. [Google Scholar]
- Park, H.; Rosenthal, A.; Jezek, R.; Ramalingam, K.; Fillos, J.; Chandran, K. Impact of inocula and growth mode on the molecular microbial ecology of anaerobic ammonia oxidation (anammox) bioreactor communities. Water Res 2010, 44, 5005–5013. [Google Scholar]
- Van der Star, W.R.; Miclea, A.I.; van Dongen, U.G.; Muyzer, G.; Picioreanu, C.; van Loosdrecht, M.C. The membrane bioreactor: A novel tool to grow anammox bacteria as free cells. Biotechnol. Bioeng 2008, 101, 286–294. [Google Scholar]
- Egli, K.; Bosshard, F.; Werlen, C.; Lais, P.; Siegrist, H.; Zehnder, A.J.B.; van der Meer, J.R. Microbial composition and structure of a rotating biological contactor biofilm treating ammonium-rich wastewater without organic carbon. Microb. Ecol 2003, 45, 419–432. [Google Scholar]
- Isaka, K.; Date, Y.; Sumino, T.; Yoshie, S.; Tsuneda, S. Growth characteristic of anaerobic ammonium-oxidizing bacteria in an anaerobic biological filtrated reactor. Appl. Microbiol. Biotechnol 2006, 70, 47–52. [Google Scholar]
- Terada, A.; Lackner, S.; Tsuneda, S.; Smets, B.F. Redox-stratification controlled biofilm (rescobi) for completely autotrophic nitrogen removal: The effect of co- versus counter-diffusion on reactor performance. Biotechnol. Bioeng 2007, 97, 40–51. [Google Scholar]
- McQuarrie, J.P.; Boltz, J.P. Moving bed biofilm reactor technology: Process applications, design, and performance. Water Environ. Res 2011, 83, 560–575. [Google Scholar]
- Almstrand, R.; Lydmark, P.; Sorensson, F.; Hermansson, M. Nitrification potential and population dynamics of nitrifying bacterial biofilms in response to controlled shifts of ammonium concentrations in wastewater trickling filters. Bioresour. Technol 2011, 102, 7685–7691. [Google Scholar]
- Lydmark, P.; Lind, M.; Sorensson, F.; Hermansson, M. Vertical distribution of nitrifying populations in bacterial biofilms from a full-scale nitrifying trickling filter. Environ. Microbiol 2006, 8, 2036–2049. [Google Scholar]
- Manz, W.; Amann, R.; Ludwig, W.; Wagner, M.; Schleifer, K.H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: Problems and solutions. Syst. Appl. Microbiol 1992, 15, 593–600. [Google Scholar]
- Almstrand, R.; Lydmark, P.; Lindgren, P.E.; Sorensson, F.; Hermansson, M. Dynamics of specific ammonia-oxidizing bacterial populations and nitrification in response to controlled shifts of ammonium concentrations in wastewater. Appl. Microbiol. Biotechnol 2013, 97, 2183–2191. [Google Scholar]
- Daims, H.; Lucker, S.; Wagner, M. Daime, a novel image analysis program for microbial ecology and biofilm research. Environ. Microbiol 2006, 8, 200–213. [Google Scholar]
- Loy, A.; Horn, M.; Wagner, M. Probebase: An online resource for rRNA-targeted oligonucleotide probes. Nucl. Acids Res 2003, 31, 514–516. [Google Scholar]
| Flow (L h−1) | HRT (h) | Temp (°C) | pH | DO (mg L−1) | NH4+ (mg N L−1) | NO2− (mg N L−1) | NO3− (mg N L−1) | N removal | |
|---|---|---|---|---|---|---|---|---|---|
| Average | 1.2 | 6.3 | 28.3 | 7.8 | 3.6 | 71 | 14 | 25 | 66% |
| S.D. | 0.2 | 0.7 | 2.9 | 0.3 | 0.5 | 40 | 13 | 12 | 14% |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Almstrand, R.; Persson, F.; Daims, H.; Ekenberg, M.; Christensson, M.; Wilén, B.-M.; Sörensson, F.; Hermansson, M. Three-Dimensional Stratification of Bacterial Biofilm Populations in a Moving Bed Biofilm Reactor for Nitritation-Anammox. Int. J. Mol. Sci. 2014, 15, 2191-2206. https://doi.org/10.3390/ijms15022191
Almstrand R, Persson F, Daims H, Ekenberg M, Christensson M, Wilén B-M, Sörensson F, Hermansson M. Three-Dimensional Stratification of Bacterial Biofilm Populations in a Moving Bed Biofilm Reactor for Nitritation-Anammox. International Journal of Molecular Sciences. 2014; 15(2):2191-2206. https://doi.org/10.3390/ijms15022191
Chicago/Turabian StyleAlmstrand, Robert, Frank Persson, Holger Daims, Maria Ekenberg, Magnus Christensson, Britt-Marie Wilén, Fred Sörensson, and Malte Hermansson. 2014. "Three-Dimensional Stratification of Bacterial Biofilm Populations in a Moving Bed Biofilm Reactor for Nitritation-Anammox" International Journal of Molecular Sciences 15, no. 2: 2191-2206. https://doi.org/10.3390/ijms15022191




