Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review
Abstract
:1. Introduction
2. Exogenous Contrast Agents
2.1. Contrast Agents for PAI
2.1.1. Dyes
| Photoacoustic Contrast Agent | Type | Absorption Peak (nm) | Size (nm) | Modification Application | Application | Ref. |
|---|---|---|---|---|---|---|
| Indocyanine-green | NIR Fluorescent Dye | 810 | <2 | CarbonNanotube, PEG, PEBBLEs | PAT, in tissue phantoms and in vivo | [7,16,17,18,19] |
| Methylene blue | NIR Fluorescent Dye | 650–700 | <2 | PAT, in tissue phantoms | [15] | |
| Alexa Fluor 750 | NIR Fluorescent Dye | 750 | <2 | Multispectral PAI, in vivo | [8,9] | |
| IRDye800CW | NIR Fluorescent Dye | 750–800 | <2 | NPR-1 | PAS, in vivo | [13] |
| IRDye800-c(KRGDf) | NIR Fluorescent Dye | 750–790 | <2 | Integral proteinαvβ3 | PAS, in vivo | [20] |
| Evans Blue | NIR Fluorescent Dye | 550 | <2 | PAT, in vivo | [10] | |
| PPCy-C8 | NIR Fluorescent Dye | 754–789 | <2 | Perfluorocarbon | In vivo, dual-modality PAI-FI | [21] |
| Cypate-C18 | NIR Fluorescent Dye | 754–790 | <2 | Perfluorocarbon | In vivo, dual-modality PAI-FI | [21] |
| Caspase-9 Probe | NIR Fluorescent Dye | 640 | <2 | PAI, in vivo | [11] | |
| MMPSence™ 680 | NIR Fluorescent Dye | 620, 680 | <2 | PAI, in tissue phantoms | [14] | |
| BHQ3 | Quencher | 672 | <2 | PAI, in vitro | [12] | |
| QXL680 | Quencher | 680 | <2 | PAI, in vitro | [12] | |
| Au Nanospheres | Plasmonic Noble Metal Nanoparticle | 520–550 | 20–80 | PEG | PAT, in vivo | [22,23] |
| Au Nanoshells | Plasmonic Noble Metal Nanoparticle | 700–1100 | 50–500 | PEG | PAT, in vivo | [24,25] |
| Au Nanorods | Plasmonic Noble Metal Nanoparticle | 550–1550 | a few to hundreds of | HER2, EGFR | PAI, in vitro | [26,27,28] |
| Au Nanocages | Plasmonic Noble Metal Nanoparticle/Theranostic Contrast Agent | 820 | 25 | PAT, in vivo, photothermal therapy | [29,30,31] | |
| Au Nanoclusters | Plasmonic Noble Metal Nanoparticle | 500–550 | 100 | PAI, in vitro | [32,33] | |
| Au Nanostars | Plasmonic Noble Metal Nanoparticle | 767 | 120 | PAT, in vivo | [34,35] | |
| Au Nanobeacons | Plasmonic Noble Metal Nanoparticle | 520 | 150 | αvβ3 | PAT, in vivo | [36,37] |
| Ag Nanoplates | Plasmonic Noble Metal Nanoparticle | 550–1080 | 25–218 | a-EGFR, PEG | PAI, in vivo | [38] |
| Ag Nanosystems | Plasmonic Noble Metal Nanoparticle/Theranostic Contrast Agent | 400–500 | 180–520 | PAI, ex vivo; image-guided therapy | [39] | |
| Quantum dots | Nanoparticles Based On Other Principles | 400–750 | <10 | PAT, in vivo: Triple-modality PA-PT-Fluorescent | [40] | |
| Nanodiamond | Nanoparticles Based On Other Principles | 820 | 68.7 | PAI, in vivo | [41] | |
| Polypyrrole Nanoparticles | Nanoparticles Based On Other Principles | 700–900 | 46 | PAI, in vivo | [42] | |
| Copper Sulfide | Nanoparticles Based On Other Principles | 900 | 11 ± 3 | PAI, in vivo | [43] | |
| Graphene Nanosheets | Nanoparticles Based On Other Principles | 200–900 | 10 | PAI, in vitro | [44] | |
| Iron Oxide-gold Core-shell | Multimodality Contrast Agent | 660–900 | 1–5 | Triple-modality MRI-PAI-mmPA | [45] | |
| Gd2O3 | Multimodality Contrast Agent | 100 | DEG, gelatin | In vivo, dual-modality PAT-MRI | [46] | |
| Single-walled Carbon Nanotubes (SWNT) | Multimodality Contrast Agent | 785 | 5–8 | Protamine, PEG | In vivo, Triple-modality Raman- MRI-PAI | [47] |
| Dye-loaded Perfluorocarbon-based Nanoparticles | Multimodality Contrast Agent | 750–800 | 220 ± 11 | cypate-C18, PPCy-C8,PEG2000, phosphatidylethanolamine | In vivo, dual-modality PAI-FI | [21] |
| AuMBs | Multimodality Contrast Agent | 760 | 100–1000 | HAS | Dual-modality PAI-UI | [48] |
| Triggered Nanodroplets | Multimodality Contrast Agent | 750–800 | 300 | Perfluorocarbon | In tissue phantoms and in vivo, dual-modality PAT-UI | [49] |
| Cobalt Nanowontons | Multimodality Contrast Agent | 700 | 30–90 | Dual-modality MRI-PAT | [50] | |
| Nanoroses | Multimodality Contrast Agent | 700–850 | 30 | PAI, in vitro | [51] | |
| MPRs | Theranostic/Multimodality Contrast Agent | 532 | 120 | maleimide-DOTA-Gd | In vivo, triple-modality MRI-API-Raman; image-guided surgery | [52] |
| Goldsilica Core shell Nanorods | Theranostic Contrast Agent | 780 | 10.3 ± 1.1 | PEG | PAI, in vitro | [53,54] |
| Superparamagnetic Iron Oxide (SPIO) | Theranostic Contrast Agent | 500–780 | 80–150 | PAI, ex vivo | [55] |
2.1.2. Plasmonic Noble Metal Nanoparticles
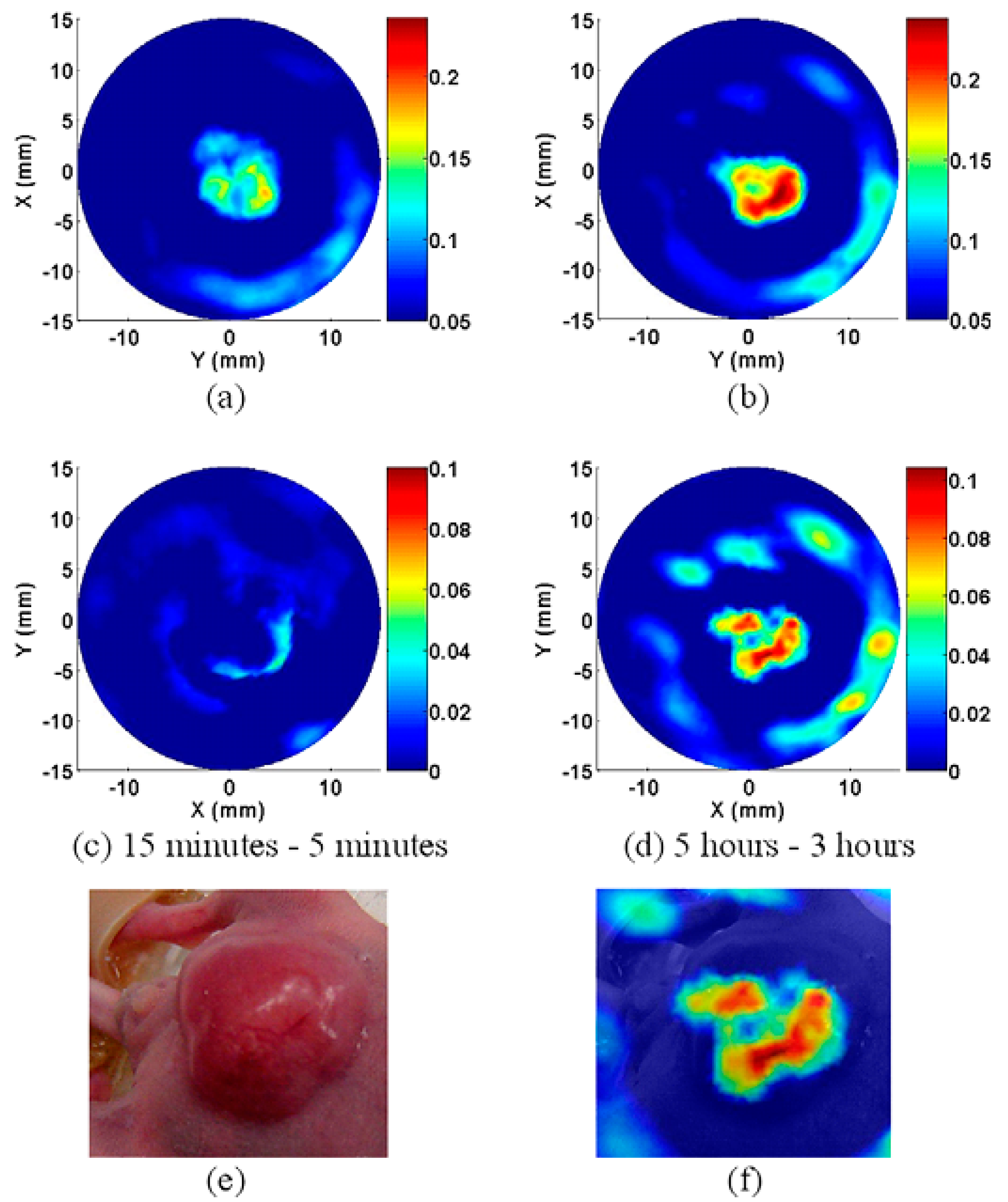
2.1.3. Nanoparticles Based on Other Principles
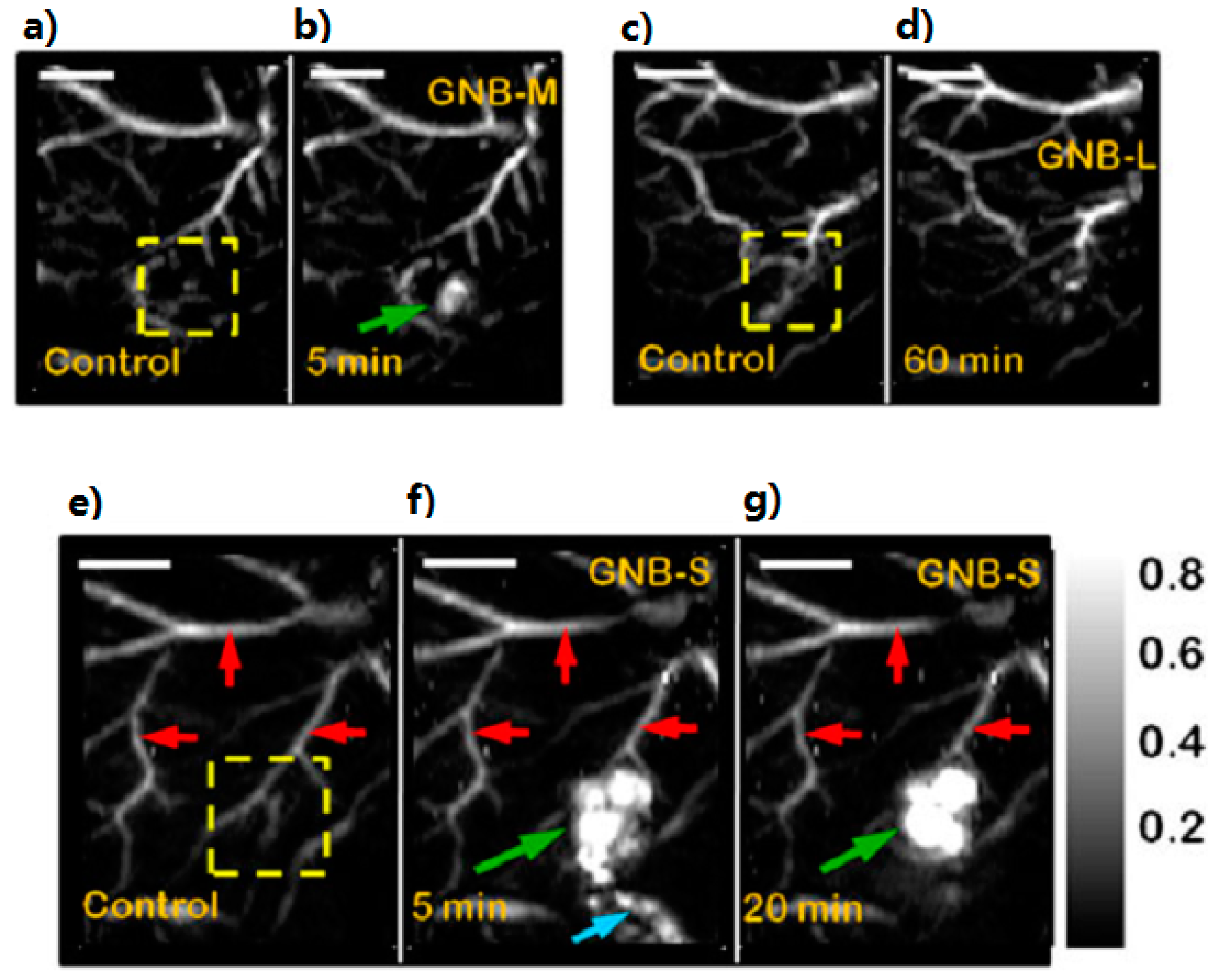

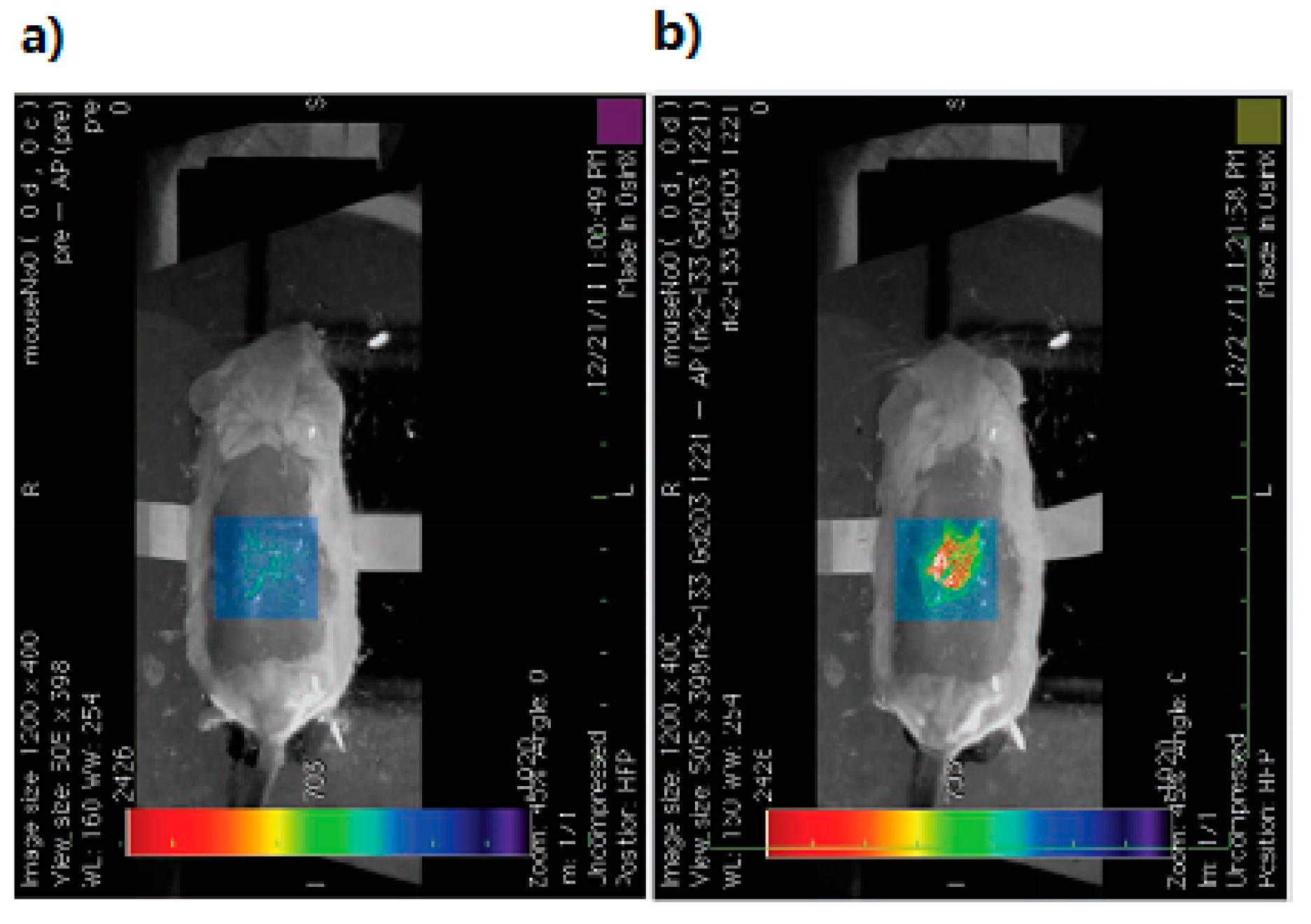
2.1.4. Multimodality Contrast Agents
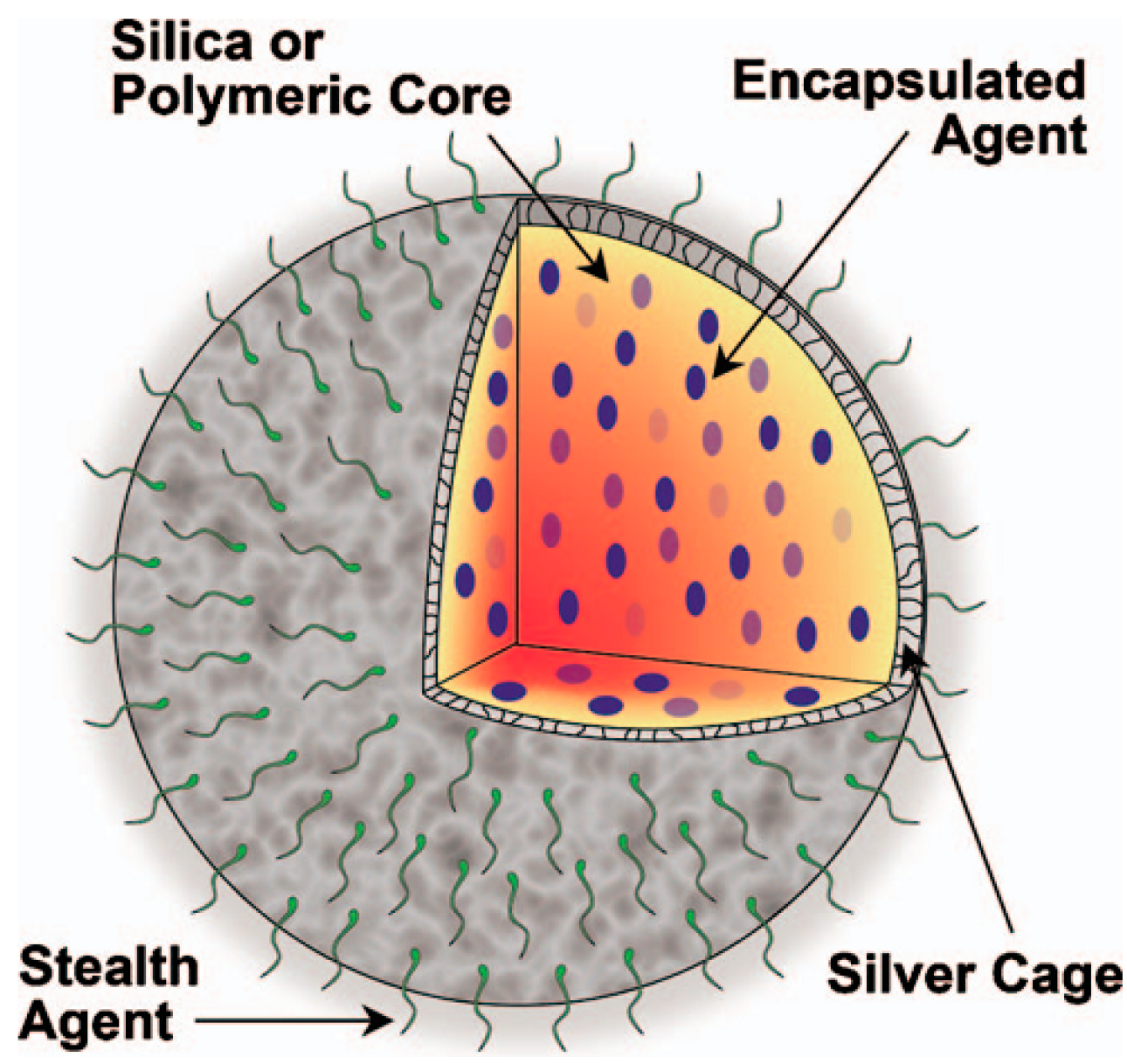
2.1.5. Theranostic Contrast Agents

2.2. Contrast Agents for TAI
| Thermoacoustic Contrast Agent | Type | Excitation Source Frequency (GHz) | Size(nm) | Modification Application | Application | Ref. |
|---|---|---|---|---|---|---|
| Carbonyl Iron | Magnetic nanoparticles | 1.2 | 2000 | TAI, in tissue phantoms | [63] | |
| Dextran-coated Fe3O4 Nanoparticles | Magnetic nanoparticles | 6 | 30–50 | Dextran | TAI, in tissue phantoms | [64] |
| NMG2[Gd(DTPA)] | Paramagnetic ionic compound | 6 | TAI, in vitro and in vivo tumor-bearing mouse | [4] | ||
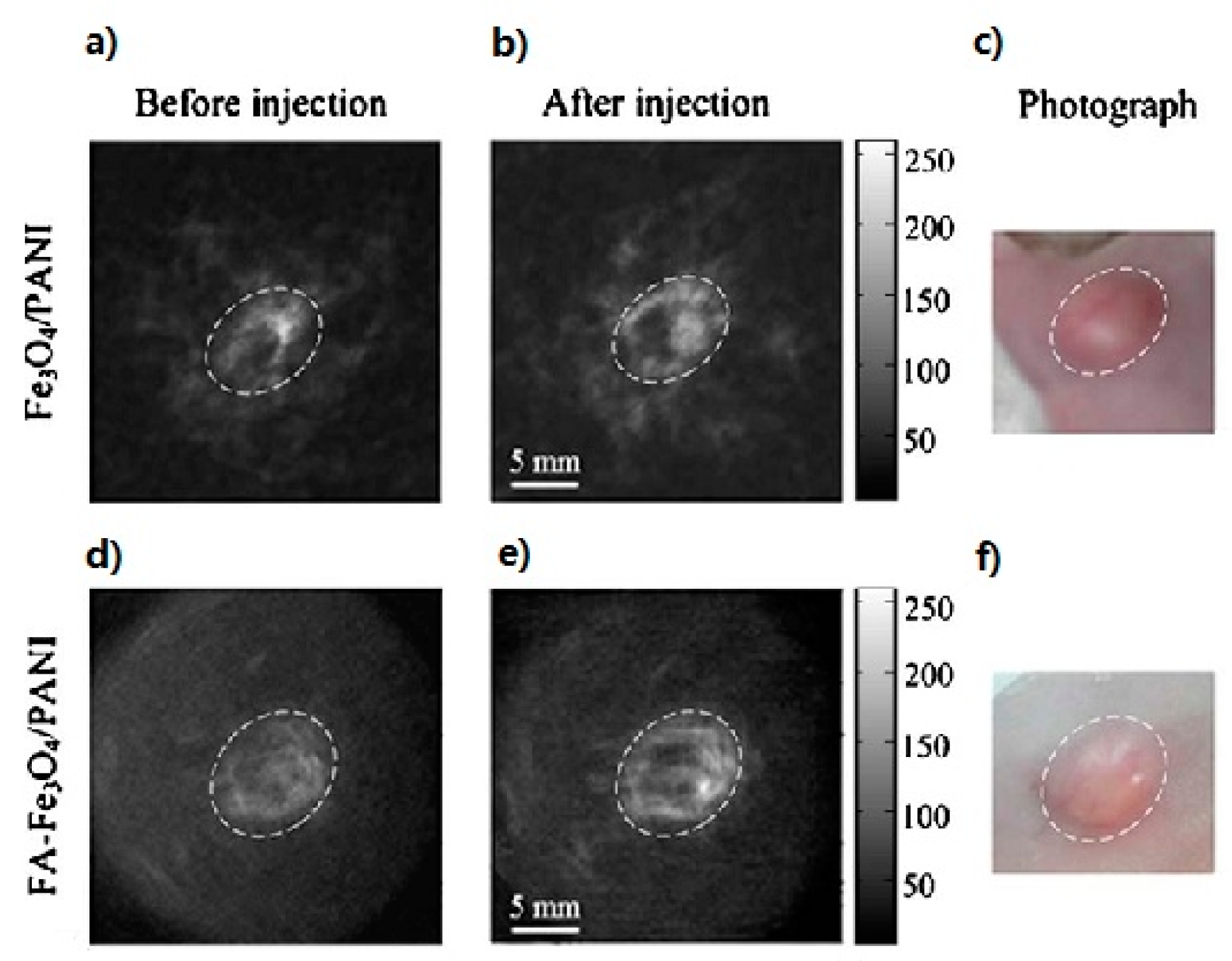
| Fe3O4/Polyaniline (PANI) | Superparamagnetic nanoparticles | 6 | 30–50 | Folic Acid (FA) | Ex vivo TAI in human blood and in vivo TAT in mouse tail, in vivo TAI of tumors | [65] |
| Fe3O4 /Au Nanoparticles | Fe3O4 core/Au shell Nanoparticles | 6 | 30–50 | FITC-labeled integrinαvβ3mAb | Triple-modality MRI-TAI-PAI | [66] |
| Single-walled Carbon Nanotubes(SWNT) | Multimodality Contrast Agent | 3 | Diameter: 1.2–2.2; length: 500–1000 | In vitro, dual-modality PAI-TAI | [67] | |
| Microbubbles | Multimodality Contrast Agent | 3 | 18,000 | UI, and in vitro TAI | [68] |
3. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 20, 305–324. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.V. Photoacoustic tomography and sensing in biomedicine. Phys. Med. Biol. 2009, 54, 59–97. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Qin, H.; Yang, S.; Xing, D. Microwave-induced thermoacoustic computed tomography with a clinical contrast agent of NMG2[Gd(DTPA)]. Appl. Phys. Lett. 2012, 100, 033701. [Google Scholar] [CrossRef]
- Huang, L.; Yao, L.; Liu, L.X.; Rong, J.; Jiang, H. Quantitative thermoacoustic tomography: Recovery of conductivity maps of heterogeneous media. Appl. Phys. Lett. 2012, 101, 244106. [Google Scholar] [CrossRef]
- Xi, L.; Grobmyer, S.R.; Zhou, G.; Qian, W.; Yang, L.; Jiang, H. Molecular photoacoustic tomography of breast cancer using receptor targeted magnetic iron oxide nanoparticles as contrast agents. J. Biophoton. 2012, 6, 401–409. [Google Scholar]
- Wang, B.; Zhao, Q.; Barkey, N.M.; Morse, D.L.; Jiang, H. Photoacoustic tomography and fluorescence molecular tomography: A comparative study based on indocyanine green. Med. Phys. 2012, 39, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Razansky, D.; Vinegoni, C.; Ntziachristos, V. Multispectral photoacoustic imaging of fluorochromes in small animals. Opt. Lett. 2007, 32, 2891–2893. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Wang, S.; Reinecke, D.; Kiser, W.; Kruger, R.A.; deGrado, T.R. Synthesis and evaluation of near-infrared (NIR) dye-herceptin conjugates as photoacoustic computed tomography (PCT) probes for HER2 expression in breast cancer. Bioconjug. Chem. 2008, 19, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Aguirre, A.; Gamelin, J.; Maurudis, A.; Zhu, Q.; Wang, L.V. Real-timephotoacoustic tomography of cortical hemodynamics in small animals. J. Biomed. Opt. 2010, 15, 010509. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cui, H.; Cai, S.; Yang, X.; Forrest, M.L. In vivo photoacoustic imaging of chemotherapy-induced apoptosis in squamous cell carcinoma using a near-infrared caspase-9 probe. J. Biomed. Opt. 2011, 16, 116026. [Google Scholar] [CrossRef] [PubMed]
- Levi, J.; Kothapalli, S.R.; Ma, T.J.; Hartman, K.; Khuri-Yakub, B.T.; Gambhir, S.S. Design, synthesis, and imaging of an activatable photoacoustic probe. J. Am. Chem. Soc. 2010, 132, 11264–11269. [Google Scholar] [CrossRef] [PubMed]
- Stantz, K.M.; Cao, M.; Liu, B.; Miller, K.D.; Guo, L. Molecular imaging of neutropilin-1 receptor using photoacoustic spectroscopy in breast tumors. BiOS Int. Soc. Opt. Photon. 2010, 7564, 75641O. [Google Scholar]
- Razansky, D.; Harlaar, N.J.; Hillebrands, J.L.; Taruttis, A.; Herzog, E.; Zeebregts, C.J.; van Dam, G.M.; Ntziachristos, V. Multispectral optoacoustic tomography of matrix metalloproteinase activity in vulnerable human carotid plaques. Mol. Imaging Biol. 2012, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Morgounova, E.; Shao, Q.; Hackel, B.J.; Thomas, D.D.; Ashkenazi, S. Photoacoustic lifetime contrast between methylene blue monomers and self-quenched dimers as a model for dual-labeled activatable probes. J. Biomed. Opt. 2013, 18, 056004. [Google Scholar] [CrossRef]
- Kim, G.; Huang, S.W.; Day, K.C.; O’Donnell, M.; Agayan, R.R.; Day, M.A.; Kopelman, R.; Ashkenazi, S. Indocyanine-green-embedded PEBBLEs as a contrast agent for photoacoustic imaging. J. Biomed. Opt. 2007, 12, 044020. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ku, G.; Wegiel, M.A.; Bornhop, D.J.; Stoica, G.; Wang, L.V. Noninvasive photoacoustic angiography of animal brains in vivo with near-infrared light and an optical contrast agent. Opt. Lett. 2004, 29, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Jeon, M.; Oh, Y.; Kang, H.W.; Kim, J.; Kim, C.; Oh, J. In vivo non-ionizing photoacoustic mapping of sentinel lymph nodes and bladders with ICG-enhanced carbon nanotubes. Phys. Med. Biol. 2012, 57, 7853–7862. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chatni, M.R.; Rao, A.L.; Vullev, V.I.; Wang, L.V.; Anvari, B. Virus-mimicking nano-constructs as a contrast agent for near infrared photoacoustic imaging. Nanoscale 2013, 5, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Oh, J.T.; Xie, X.Y.; Ku, G.; Wang, W.; Li, C.; Lungu, G.; Stoica, G.; Wang, L.V. Simultaneous molecular and hypoxia imaging of brain tumors in vivo using spectroscopic photoacoustic tomography. Proc. IEEE 2008, 96, 481–489. [Google Scholar] [CrossRef]
- Akers, W.J.; Kim, C.; Berezin, M.; Guo, K.; Fuhrhop, R.; Lanza, G.M.; Fischer, G.M.; Daltrozzo, E.; Zumbusch, A.; Cai, X.; et al. Noninvasive photoacoustic and fluorescence sentinel lymph node identification using dye-loaded perfluorocarbon nanoparticles. ACS Nano 2010, 5, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Iwakuma, N.; Sharma, P.; Moudgil, B.M.; Wu, C.; McNeill, J.; Jiang, H.; Grobmyer, S.R. Gold nanoparticles as a contrast agent for in vivo tumor imaging with photoacoustic tomography. Nanotechnology 2009, 20, 395102. [Google Scholar] [PubMed]
- Yuan, Z.; Wu, C.; Zhao, H.; Jiang, H. Imaging of small nanoparticle-containing objects by finite-element-based photoacoustic tomography. Opt. Lett. 2005, 30, 3054–3056. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Wang, X.; Ku, G.; Gill, K.L.; O’Neal, D.P.; Stoica, G.; Wang, L.V. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Lett. 2004, 4, 1689–1692. [Google Scholar] [CrossRef]
- Xiang, L.; Xing, D.; Gu, H.; Yang, D.; Zeng, L.; Yang, S. Gold nanoshell-based photoacoustic imaging application in biomedicine. In Proceedings of the IEEE International Symposium onBiophotonics, Nanophotonics and Metamaterials, Hangzhou, China, 16–18 October 2006; pp. 76–79.
- Kim, K.; Huang, S.W.; Ashkenazi, S.; O’Donnell, M.; Agarwal, A.; Kotov, N.A.; Denny, M.F.; Kaplan, M.J. Photoacoustic imaging of early inflammatory response using gold nanorods. Appl. Phys. Lett. 2007, 90, 223901. [Google Scholar] [CrossRef]
- Yang, H.W.; Liu, H.L.; Li, M.L.; His, I.W.; Fan, C.T.; Huang, C.Y.; Lu, Y.J.; Hua, M.Y.; Chou, H.Y.; Liaw, J.W.; et al. Magnetic gold-nanorod/PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials 2013, 34, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Shieh, D.B.; Wang, C.R.C.; Wei, C.W.; Liao, C.K.; Ding, A.A.; Wu, Y.N.; Poe, C.; Jhan, S. In vivo photoacoustic molecular imaging with simultaneous multiple selective targeting using antibody-conjugated gold nanorods. Opt. Express 2008, 16, 18605–18615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Skrabalak, S.E.; Li, Z.Y.; Xia, Y.; Wang, L.V. Photoacoustic tomography of a rat cerebral cortex in vivo with Au nanocages as an optical contrast agent. Nano Lett. 2007, 7, 3798–3802. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y.; et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold nanocages: From synthesis to theranostic applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Mallidi, S.; Tam, J.M.; Tam, J.O.; Murthy, A.; Johnston, K.P.; Sokolov, K.V.; Emelianov, S.Y. Utility of biodegradable plasmonic nanoclusters in photoacoustic imaging. Opt. Lett. 2010, 35, 3751–3753. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Murthy, A.; Johnston, K.P.; Sokolov, K.V.; Emelianov, S.Y. Thermal stability of biodegradable plasmonic nanoclusters in photoacoustic imaging. Opt. Express 2012, 20, 29479–29487. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Song, H.M.; Cai, X.; Yao, J.; Wei, A.; Wang, L.V. In vivo photoacoustic mapping of lymphatic systems with plasmon-resonant nanostars. J. Mater. Chem. 2011, 21, 2841–2844. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Pramanik, M.; Senpan, A.; Allen, J.S.; Zhang, H.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Molecular photoacoustic imaging of angiogenesis with integrin-targeted gold nanobeacons. FASEB J. 2011, 25, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Pramanik, M.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Recent advances in colloidal gold nanobeacons for molecular photoacoustic imaging. Contrast Media Mol. Imaging 2011, 6, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Souza, M.; Truby, R.; Luke, G.P.; Green, C.; Vreeland, E.; Emelianov, S. Silver nanoplate contrast agents for in vivo molecular photoacoustic imaging. ACS Nano 2012, 6, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.; Brannon-Peppas, L.; Emelianov, S.; Shah, J.; Gomez, S.; Gensler, H.; Karpiouk, A. Silver nanosystems for photoacoustic imaging and image-guided therapy. J. Biomed. Opt. 2010, 15, 021316. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, E.V.; Everts, M.; Galanzha, E.I.; Zharov, V.P. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008, 8, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cui, H.; Fang, C.Y.; Su, L.J.; Ren, S.; Chang, H.C.; Yang, X.; Forrest, M.L. Photoacoustic contrast imaging of biological tissues with nanodiamonds fabricated for high near-infrared absorbance. J. Biomed. Opt. 2013, 18, 026018. [Google Scholar] [CrossRef]
- Zha, Z.; Deng, Z.; Li, Y.; Li, C.; Wang, J.; Wang, S.; Qu, E.; Dai, Z. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 2013, 5, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Zhou, M.; Song, S.; Huang, Q.; Hazle, J.; Li, C. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. ACS Nano 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Yang, H.; Chiu, P.L.; Mastrogiovanni, D.D.; Flach, C.R.; Savaram, K.; Gomez, L.; Hemnarine, A.; Mendelsohn, R.; Garfunkel, E.; et al. Direct production of graphene nanosheets for near infrared photoacoustic imaging. ACS Nano 2013, 7, 8147–8157. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jia, C.; Huang, S.W.; O’Donnell, M.; Gao, X. Multifunctional nanoparticles as coupled contrast agents. Nat. Commun. 2010, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kamisugi, R.; Narazaki, M.; Matsuda, T.; Tabata, Y.; Toshimitsu, A.; Kondo, T. Size-controlled and biocompatible Gd2O3 nanoparticles for dual photoacoustic and MR imaging. Adv. Healthc. Mater. 2012, 1, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, X.; Ye, S.; Cheng, L.; Yang, K.; Guo, L.; Li, C.; Li, Y.; Liu, Z. Protamine functionalized single-walled carbon nanotubes for stem cell labeling and in vivo raman/magnetic resonance/photoacoustic triple-modal imaging. Adv. Funct. Mater. 2012, 22, 2363–2375. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liao, A.H.; Chen, J.H.; Wang, C.R.C.; Li, P.C. Photoacoustic/ultrasound dual-modality contrast agent and its application to thermotherapy. J. Biomed. Opt. 2012, 17, 0450011–0450018. [Google Scholar]
- Wilson, K.; Homan, K.; Emelianov, S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nat. Commun. 2012, 3, 618. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, L.S.; Anwar, M.S.; Liu, G.L.; Hann, B.; Xie, Z.H.; Gray, J.W.; Wang, X.; Pines, A.; Chen, F.F. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proc. Natl. Acad. Sci. USA. 2009, 106, 4085–4089. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Feldman, M.D.; Tam, J.M.; Paranjape, A.S.; Cheruku, K.K.; Larson, T.A.; Tam, J.O.; Ingram, D.R.; Paramita, V.; Villard, J.W.; et al. Small multifunctional nanoclusters (nanoroses) for targeted cellular imaging and therapy. ACS Nano 2009, 3, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
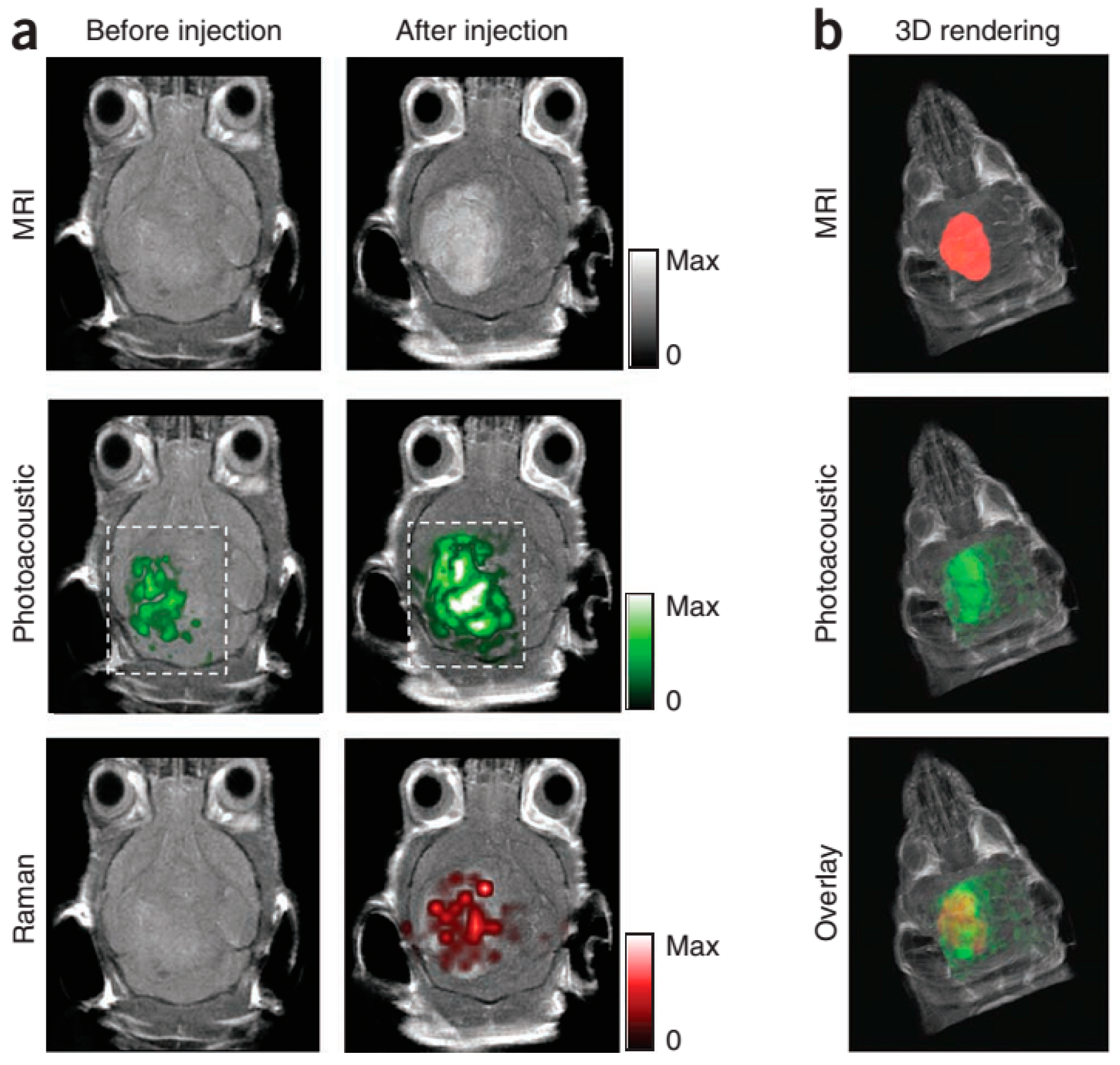
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Frey, W.; Kim, S.; Homan, K.; Kruizinga, P.; Sokolov, K.; Emelianov, S. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt. Express 2010, 18, 8867–8878. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; el-Sayed, I.H.; el-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, D.J.; Fratila, R.M.; Visscher, M.; Haken, B.T.; van Wezel, R.J.; Rottenberg, S.; Steenbergen, W.; Manohar, S.; Ruers, T.J. Intra-operative ex vivo photoacoustic nodal staging in a rat model using a clinical superparamagnetic iron oxide nanoparticle dispersion. J. Biophoton. 2013, 6, 493–404. [Google Scholar] [CrossRef]
- Millstone, J.E.; Park, S.; Shuford, K.L.; Qin, L.; Schatz, G.C.; Mirkin, C.A. Observation of a quadrupole plasmon mode for a colloidal solution of gold nanoprisms. J. Am. Chem. Soc. 2005, 127, 5312–5313. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; el-Sayed, I.H.; el-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control. Release 2006, 114, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Huang, Q.; Ku, G.; Wen, X.; Zhou, M.; Guzatov, D.; Brecht, P.; Su, R.; Oraevsky, A.; Wang, L.V.; et al. Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials 2010, 31, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, D.J.; Jose, J.; Fratila, R.M.; Visscher, M.; Velders, A.H.; ten Haken, B.; van Leeuwen, T.G.; Steenbergen, W.; Manohar, S.; Ruers, T.J. Evaluation of superparamagnetic iron oxide nanoparticles (Endorem®) as a photoacoustic contrast agent for intra-operative nodal staging. Contrast Media Mol. Imaging 2013, 8, 83–91. [Google Scholar] [PubMed]
- Wang, C.; Chen, J.; Talavage, T.; Irudayaraj, J. Gold nanorod/Fe3O4 nanoparticle “nano-pearl-necklaces” for simultaneous targeting, dual-mode imaging, and photothermal ablation of cancer cells. Angew. Chem. 2009, 121, 2797–2801. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.Y.; Zhang, H.; Xia, Y.; Li, X. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancercells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Xing, D.; Yang, D.; Zeng, L. Microwave-induced thermoacoustic imaging enhanced with a microwave contrast agent. In Proceedings of the IEEE/ICME International Conference on Complex Medical Engineering, Beijing, China, 23–27 May 2007.
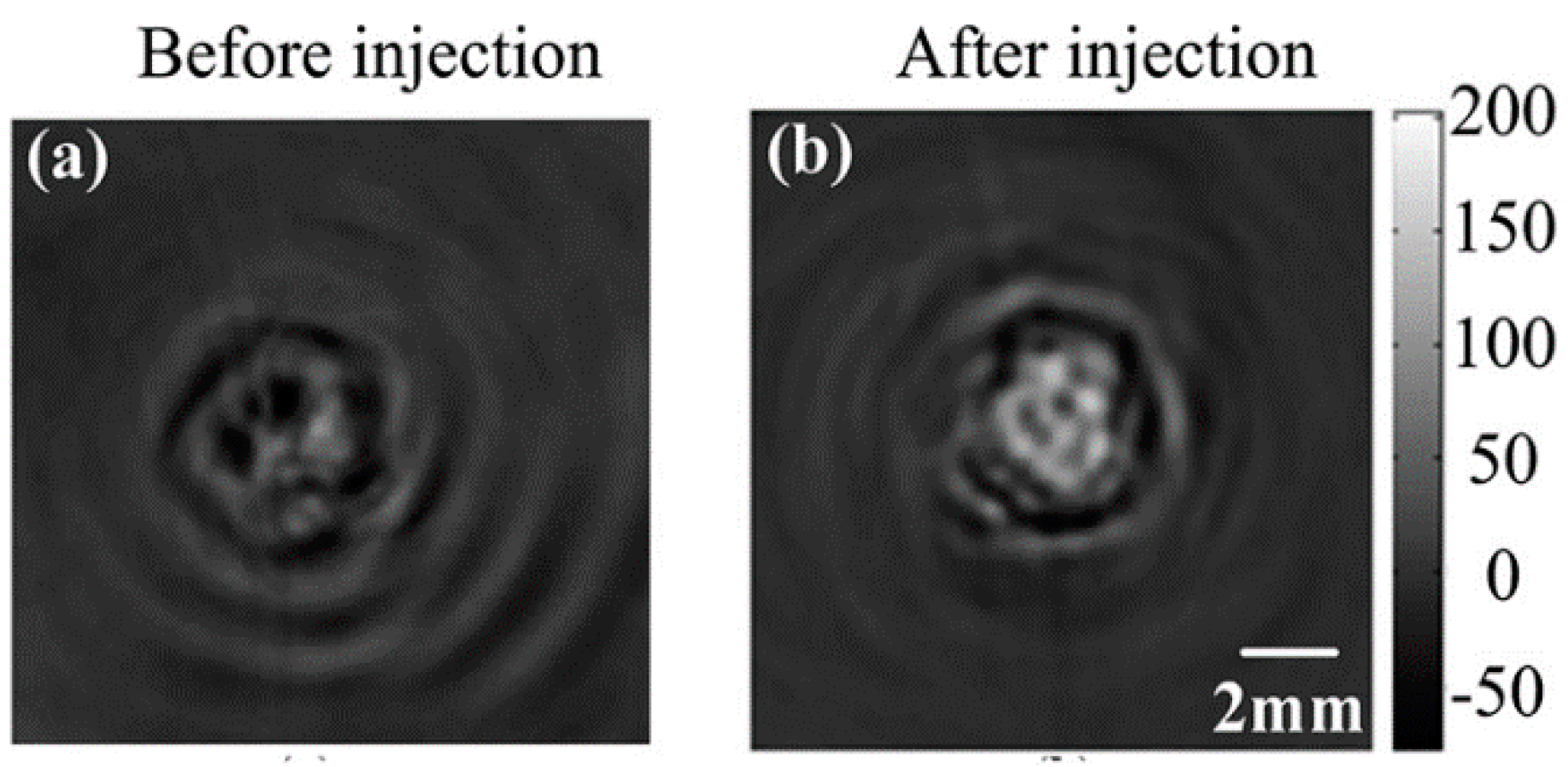
- Qin, H.; Xu, D.; Yang, S. Dextran-coated Fe3O4 magnetic nanoparticles as a contrast agent in thermoacoustic tomography for hepatocellular carcinoma detection. J. Phys. 2011, 277, 12028–12034. [Google Scholar]
- Nie, L.; Ou, Z.; Yang, S.; Xing, D. Thermoacoustic molecular tomography with magnetic nanoparticle contrast agents for targeted tumor detection. Med. Phys. 2010, 37, 4193–4200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wu, B.; Xing, D. Bio-modified Fe3O4 core/Au shell nanoparticles for targeting and multimodal imaging of cancer cells. J. Mater. Chem. 2012, 22, 470–477. [Google Scholar] [CrossRef]
- Pramanik, M.; Swierczewska, M.; Wang, L.V.; Green, D.; Sitharaman, B. Single-walled carbon nanotubes as a multimodal-thermoacoustic and photoacoustic-contrast agent. J. Biomed. Opt. 2009, 14, 034018. [Google Scholar] [CrossRef] [PubMed]
- Mashal, A.; Booske, J.H.; Hagness, S.C. Toward contrast-enhanced microwave-induced thermoacoustic imaging of breast cancer: An experimental study of the effects of microbubbles on simple thermoacoustic targets. Phys. Med. Biol. 2009, 54, 64–650. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Huang, L.; Jiang, M.S.; Jiang, H. Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review. Int. J. Mol. Sci. 2014, 15, 23616-23639. https://doi.org/10.3390/ijms151223616
Wu D, Huang L, Jiang MS, Jiang H. Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review. International Journal of Molecular Sciences. 2014; 15(12):23616-23639. https://doi.org/10.3390/ijms151223616
Chicago/Turabian StyleWu, Dan, Lin Huang, Max S. Jiang, and Huabei Jiang. 2014. "Contrast Agents for Photoacoustic and Thermoacoustic Imaging: A Review" International Journal of Molecular Sciences 15, no. 12: 23616-23639. https://doi.org/10.3390/ijms151223616




