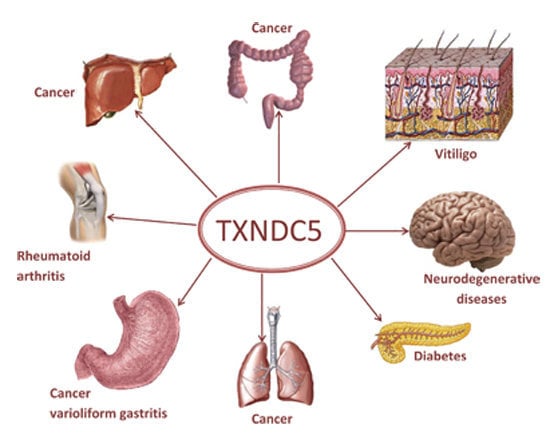
TXNDC5, a Newly Discovered Disulfide Isomerase with a Key Role in Cell Physiology and Pathology
Abstract
:1. Introduction
2. TXNDC5 Gene
2.1. Orthologous Genes
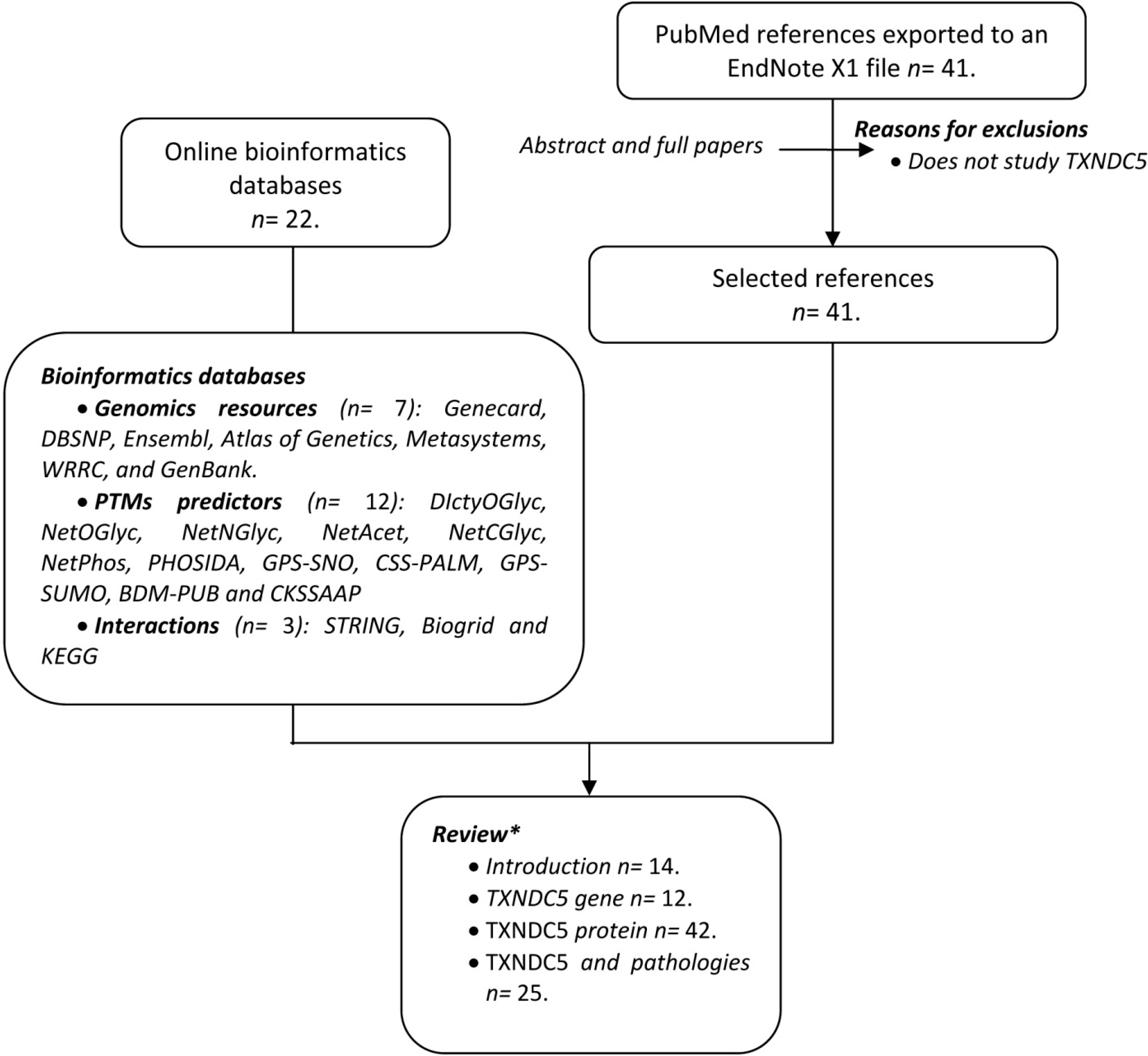
2.2. Paralogous Genes
| Organism | Taxonomic Classification | Gene | Human Similarity |
|---|---|---|---|
| Chimpanzee (Pan troglodytes) | Mammalia | TXNDC5 | 99.44 (n) 98.98 (a) |
| Mouse (Mus musculus) | Mammalia | TXNDC5 | 86.81 (n) 89.57 (a) |
| Rat (Rattus norvegicus) | Mammalia | TXNDC5 | 85.83 (n) 89.84 (a) |
| Cow (Bos taurus) | Mammalia | TXNDC5 | 85.42 (n) 89.33 (a) |
| Dog (Canis familiaris) | Mammalia | TXNDC5 | 85.91 (n) 87.76 (a) |
| Opossum (Monodelphis domestica) | Mammalia | TXNDC5 | 78 (a) |
| Platypus (Ornithorhynchus anatinus) | Mammalia | TXNDC5 | 69 (a) |
| Chicken (Gallus gallus) | Aves | TXNDC5 | 74.53 (n) 74.53 (a) |
| Lizard (Anolis carolinensis) | Reptilia | TXNDC5 | 66 (a) |
| African clawed frog (Xenopus laevis) | Amphibia | BC045245.1 | 76.01 (n) |
| Tropical clawed frog (Xenopus tropicalis) | Amphibia | MGC75894 | 76.45 (n) |
| Zebrafish (Danio rerio) | Actinopterygii | Dr.25420 | 76.05 (n) |
| Rainbow trout (Oncorhynchus mykiss) | Actinopterygii | BX307368.1 | 75.21 (n) |
| Sea squirt (Ciona intestinalis) | Ascidiacea | Cin.2468 | 73.28 (n) |
| Sea squirt (Ciona savignyi) | Ascidiacea | − | 44 (a) |
| Fruit fly (Drosophila melanogaster) | Insecta | Prtp | 54.83 (n) 47.16 (a) |
| Mosquito (Anopheles gambiae) | Insecta | AgaP_AGAP000044 | 53.96 (n) 43.51 (a) |
2.3. Gene Polymorphisms
2.4. TXNDC5 Transcripts
2.5. TXNDC5 Transcriptional Regulation
| Transcription Factor | Biology Process | Tissue Expression |
|---|---|---|
| HTF or HER-2 | Growth factor receptor | Epithelial tissue |
| ATF6 | Leucine zipper in response to misfolded proteins via cAMP | Ubiquitous expression |
| XBP1 | Plasma cell differentiation in response to ER stress | Ubiquitous expression |
| Pax6 | Brain and eye morphogenesis | Developing sensory organs, central nervous system and endocrine system |
| ATF | Differentiation, proliferation and apoptosis | Ubiquitous expression |
| cMyb | Pro-oncogene | Ubiquitous expression |
| Max1 | Proliferation and apoptosis through H3 Lys-9 methyl-transferase complex | Ubiquitous expression |
| Arnt | PAH pro-carcinogen activator in response to hypoxia | Ubiquitous expression |
| USF1 | Cell differentiation and proliferation | Ubiquitous expression |
| FCHL (Familiar combined hyperlipidemia) transcription factor | Muscle and fat tissue |
2.6. TXNDC5 Post-Transcriptional Regulation
3. TXNDC5 Protein
3.1. Protein Structure

3.2. Post-Translational Modifications (PTMs)
| Modification | Modified Amino Acids | Prediction Server | References |
|---|---|---|---|
| Glycosylation | Thr (174, 304, 306), Ser (308) | DictyOGlyc | [27] |
| Thr (174, 299, 302, 304, 306), Ser (183, 308) | NetOGlyc | [28] | |
| No reports (*) | NetNGlyc | [29] | |
| Acetylation | No reports (**) | NetAcet | [30] |
| Mannosylation | No reports (***) | NetCGlyc | [31] |
| Phosphorylation | Thr (138, 167, 174, 306, 335), Ser (62, 108, 125, 183, 285, 364, 392, 409, 412, 419), Tyr (106, 151,192, 289) | NetPhos | [32] |
| Thr (174, 304, 306), Ser (62, 108, 125, 129, 183, 197, 238, 292, 308, 392, 409, 412, 419) | Phosida | [33,34] | |
| S-nitrosylation | Cys (89, 92, 217, 220, 350, 353) | GPS-SNO | [35] |
| Palmitoylation | Cys (220) | CSS-Palm | [36] |
| Sumoylation | Lys (150, 241, 429) | GPS-SUMO | [37,38] |
| Ubiquitination | Lys (33,68,71,78,116,169,206,208,149,303, 318,334,335, 337), Lys (41,68,71,78) | BDM-PUB | [39] |
| CKSSAAP | [40,41] |
3.3. Protein Functions
3.4. Protein Interactions
| Biological Process | Proteins |
|---|---|
| Histone deacetylation | HdAFX, HDAC10, HDAC2 and RPD |
| Transcription factors | ATF, BZW1,GNAI3, ZNF207, ZNF706 and ZNHIT2 |
| Splicing | PRPF4 |
| Cell cycle control | CDK5 and VCP |
| Cell signaling | NENF (MAPK1/ERK2, MAPK3/ERK1 and ATK1/ATK phosphorylase), PPP1R2 (PP1 regulatory subunit (inhibitor)), Adipo-R1, AdipoR2, YWHANG and YWHAQ |
| Cell transport | YecS (amino acid and cys ABC transport) |
| Cell movement | CSE1L, DBN1 and WF1 |
| Ubiquitination | YOD1, HMG-20, CUL3, UBC, UBE2V1, UBXN1 and NPLOC4 |
| Neddylation | DCUN1D1 |
| Chaperones | HSP90AA1, TBCB, TGM2, TRMT1 and UNC45A |
| Carbohydrate metabolism | ALDOC, LDH and PGD |
| ATP | ATP6V1A |
| Amyloids | APP |
| Retrovirus | ENV |
| Type of Agents | Compounds |
|---|---|
| Carcinogens | 2,3,7,8-Tetrachlorodibenzodioxine (herbicide), 4'-Diaminodiphenylmethane, Benzopyrene |
| Toxins | Aflatoxin B1 |
| Immunosuppressor | Cyclosporine A |
| K+ channel activator | Diazoside |
| Adrenaline analogous | Isoprenaline |
| ROS | Nitric oxide |
| Oxocarbon | Trimellitic anhydride |
| PPARα | Pirinixic acid |
| Non-steroid anti-inflammatory | Paracetamol |
| Antibiotics | Quinolones |
| Anti-epilepsy drug | Valproic acid |
| Metals | Zinc |
| Vitamins | Ascorbic acid |
4. TXNDC5 in Physiology and Pathology
4.1. Diabetes

4.2. TXNDC5 and Liver Diseases
4.3. Rheumatoid Arthritis
4.4. TXNDC5 and Cancer
4.5. TXNDC5 and Neurodegenerative Diseases
4.6. TXNDC5 and Vitiligo
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thioredoxin Domain Containing 5. Crown Human Genome Center Website. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=TXNDC5&search=d9b50614b9aa4ce1d5c88c4e96de902e (accessed on 11 July 2014).
- Sullivan, D.C.; Huminiecki, L.; Moore, J.W.; Boyle, J.J.; Poulsom, R.; Creamer, D.; Barker, J.; Bicknell, R. Endo-PDI, a novel protein-disulfide isomerase-like protein that is preferentially expressed in endothelial cells acts as a stress survival factor. J. Biol. Chem. 2003, 278, 47079–47088. [Google Scholar] [CrossRef] [PubMed]
- Nissom, P.M.; Lo, S.L.; Lo, J.C.; Ong, P.F.; Lim, J.W.; Ou, K.; Liang, R.C.; Seow, T.K.; Chung, M.C. Hcc-2, a novel mammalian ER thioredoxin that is differentially expressed in hepatocellular carcinoma. FEBS Lett. 2006, 580, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Xu, B.; Wang, L.; Wang, Y.; Yan, S. Investigating a pathogenic role for TXNDC5 in tumors. Int. J. Oncol. 2013, 43, 1871–1884. [Google Scholar] [PubMed]
- Zhang, L.; Hou, Y.; Li, N.; Wu, K.; Zhai, J. The influence of TXNDC5 gene on gastric cancer cell. J. Cancer Res. Clin. Oncol. 2010, 136, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Y.; Lu, B.; Xu, E.; Huang, Q.; Lai, M. Differential expression of mimecan and thioredoxin domain-containing protein 5 in colorectal adenoma and cancer: a proteomic study. Exp. Biol. Med. (Maywood) 2007, 232, 1152–1159. [Google Scholar] [CrossRef]
- Vincent, E.E.; Elder, D.J.; Phillips, L.; Heesom, K.J.; Pawade, J.; Luckett, M.; Sohail, M.; May, M.T.; Hetzel, M.R.; Tavare, J.M. Overexpression of the TXNDC5 protein in non-small cell lung carcinoma. Anticancer Res. 2011, 31, 1577–1582. [Google Scholar] [PubMed]
- Duivenvoorden, W.C.; Paschos, A.; Hopmans, S.N.; Austin, R.C.; Pinthus, J.H. Endoplasmic reticulum protein ERp46 in renal cell carcinoma. PLoS One 2014, 9, e90389. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.L.; Xiang, J.N.; Yang, L.Y. Role of ERp46 in β-cell lipoapoptosis through endoplasmic reticulum stress pathway as well as the protective effect of exendin-4. Biochem. Biophys. Res. Commun. 2012, 426, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Cui, Y.; Zong, M.; Zhao, Y.; Yan, X.; Chen, Y.; Han, J. Identification of proteins with increased expression in rheumatoid arthritis synovial tissues. J. Rheumatol. 2009, 36, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhao, Y.; Yan, X.; Pan, J.; Fang, K.; Wang, L. Investigating a pathogenic role for TXNDC5 in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R124. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, Y.; Xu, H.; Yan, X.; Chang, X. Investigate pathogenic mechanism of TXNDC5 in rheumatoid arthritis. PLoS One 2013, 8, e53301. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Liu, C.M.; Liu, Y.L.; Shen-Jang Fann, C.; Hsiao, P.C.; Wu, J.Y.; Hung, S.I.; Chen, C.H.; Wu, H.M.; Jou, Y.S.; et al. Clustering by neurocognition for fine mapping of the schizophrenia susceptibility loci on chromosome 6p. Genes Brain Behav. 2009, 8, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Shin, M.K.; Uhm, Y.K.; Kim, H.J.; Chung, J.H.; Lee, M.H. Association of TXNDC5 gene polymorphisms and susceptibility to nonsegmental vitiligo in the Korean population. Br. J. Dermatol. 2010, 162, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- dbSNP Short Genetic Variations Website. Available online: http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=81567 (accessed on 11 July 2014).
- Ensembl Website. Available online: http://www.ensembl.org/Homo_sapiens/Gene/Idhistory?db=core;g=ENSG00000239264;r=6:7881483-8026646 (accessed on 11 July 2014).
- Atlas of Genetics and Cytogenetics in Oncology and Haematology Home Page. Available online: http://atlasgeneticsoncology.org/ (accessed on 13 July 2014).
- Karolchik, D.; Barber, G.P.; Casper, J.; Clawson, H.; Cline, M.S.; Diekhans, M.; Dreszer, T.R.; Fujita, P.A.; Guruvadoo, L.; Haeussler, M.; et al. The UCSC genome browser database: 2014 update. Nucleic Acids Res. 2014, 42, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Database of Conjoined Genes Home Page. Available online: http://metasystems.riken.jp/conjoing/ (accessed on 13 July 2014).
- Morand, J.P.; Macri, J.; Adeli, K. Proteomic profiling of hepatic endoplasmic reticulum-associated proteins in an animal model of insulin resistance and metabolic dyslipidemia. J. Biol. Chem. 2005, 280, 17626–17633. [Google Scholar] [CrossRef] [PubMed]
- Knoblach, B.; Keller, B.O.; Groenendyk, J.; Aldred, S.; Zheng, J.; Lemire, B.D.; Li, L.; Michalak, M. ERp19 and ERp46, new members of the thioredoxin family of endoplasmic reticulum proteins. Mol. Cell Proteomics 2003, 2, 1104–1119. [Google Scholar] [CrossRef]
- Gulerez, I.E.; Kozlov, G.; Rosenauer, A.; Gehring, K. Structure of the third catalytic domain of the protein disulfide isomerase ERp46. Acta Crystallogr. F Struct. Biol. Cryst. Commun. 2012, 68, 378–381. [Google Scholar] [CrossRef]
- Funkner, A.; Parthier, C.; Schutkowski, M.; Zerweck, J.; Lilie, H.; Gyrych, N.; Fischer, G.; Stubbs, M.T.; Ferrari, D.M. Peptide binding by catalytic domains of the protein disulfide isomerase-related protein ERp46. J. Mol. Biol. 2013, 425, 1340–1362. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. The Pfam protein families database. Nucleic Acids Res. 2009. [Google Scholar] [CrossRef]
- Gupta, R.; Jung, E.; Gooley, A.A.; Williams, K.L.; Brunak, S.; Hansen, J. Scanning the available Dictyostelium discoideum proteome for O-linked GlcNAc glycosylation sites using neural networks. Glycobiology 1999, 9, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Jung, E.; Brunak, S. Prediction of N-glycosylation sites in human proteins, 2004. NetAcet 1.0 Server Website. Available online: http://www.cbs.dtu.dk/services/NetNGlyc/ (accessed on 30 July 2014).
- Kiemer, L.; Bendtsen, J.D.; Blom, N. NetAcet: Prediction of N-terminal acetylation sites. Bioinformatics 2005, 21, 1269–1270. [Google Scholar] [CrossRef] [PubMed]
- Julenius, K. NetCGlyc 1.0: Prediction of mammalian C-mannosylation sites. Glycobiology 2007, 17, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Boil. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Gnad, F.; Ren, S.; Cox, J.; Olsen, J.V.; Macek, B.; Oroshi, M.; Mann, M. PHOSIDA (phosphorylation site database): Management, structural and evolutionary investigation, and prediction of phosphosites. Genome Biol. 2007, 8, R250. [Google Scholar] [CrossRef] [PubMed]
- Gnad, F.; Gunawardena, J.; Mann, M. PHOSIDA 2011: The posttranslational modification database. Nucleic Acids Res. 2011, 39 (Suppl. 1), D253–D260. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, Z.; Gao, X.; Jin, C.; Wen, L.; Yao, X.; Ren, J. GPS-SNO 1.0 Website. Available online: http://sno.biocuckoo.org/faq.php (accessed on 30 July 2014).
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. CSS-Palm 2.0: An updated software for palmitoylation sites prediction. Protein Eng. Des. Sel. 2008, 21, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xie, Y.; Zheng, Y.; Jiang, S.; Liu, W.; Mu, W.; Zhao, Y.; Xue, Y.; Ren, J. GPS-SUMO: A tool for the prediction of sumoylation sites and SUMO-interaction motifs. Nucleic Acids Res. 2014, 42, 325–330. [Google Scholar] [CrossRef]
- Ren, J.; Gao, X.; Jin, C.; Zhu, M.; Wang, X.; Shaw, A.; Wen, L.; Yao, X.; Xue, Y. Systematic study of protein sumoylation: Development of a site-specific predictor of SUMOsp 2.0. Proteomics 2009, 9, 3409–3412. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, X.; Ren, J.; Jin, C.; Xue, Y. BDM-PUB: Computational prediction of protein ubiquitination sites with a bayesian discriminant method, 2009. BDM-PUB: Prediction of Ubiquitination Website. Available online: http://bdmpub.biocuckoo.org/prediction.php (accessed on 4 November 2014).
- Chen, Z.; Chen, Y.Z.; Wang, X.F.; Wang, C.; Yan, R.X.; Zhang, Z. Prediction of ubiquitination sites by using the composition of k-spaced amino acid pairs. PLoS One 2011, 6, e22930. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, Y.; Song, J.; Zhang, Z. hCKSAAP_UbSite: Improved prediction of human ubiquitination site by exploiting amino acid pattern and properties. Biochim. Biophys. Acta 2013, 1834, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Okumura, M.; Masui, S.; Kanemura, S.; Inoue, M.; Saiki, M.; Yamaguchi, H.; Hikima, T.; Suzuki, M.; Akiyama, S.; et al. Radically different thioredoxin domain arrangement of ERp46, an efficient disulfide bond introducer of the mammalian PDI family. Structure 2013, 22, 431–443. [Google Scholar] [CrossRef]
- Araki, K.; Iemura, S.; Kamiya, Y.; Ron, D.; Kato, K.; Natsume, T.; Nagata, K. Ero1-α and PDIs constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases. J. Cell Biol. 2013, 202, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.; Oka, O.B.; Bulleid, N.J. Inactivation of mammalian Ero1-α is catalysed by specific protein disulfide-isomerases. Biochem. J. 2014, 461, 107–113. [Google Scholar] [PubMed]
- Sato, Y.; Kojima, R.; Okumura, M.; Hagiwara, M.; Masui, S.; Maegawa, K.; Saiki, M.; Horibe, T.; Suzuki, M.; Inaba, K. Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding. Sci. Rep. 2013, 3, 2456. [Google Scholar] [PubMed]
- Pace, P.E.; Peskin, A.V.; Han, M.H.; Hampton, M.B.; Winterbourn, C.C. Hyperoxidized peroxiredoxin 2 interacts with the protein disulfide-isomerase ERp46. Biochem. J. 2013, 453, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.X.; Fu, Y.; Sun, X.L.; Ding, Y.Z.; Li, C.H.; Pang, W.; Pan, S.; Zhu, Y. Proteomic analysis of endothelial lipid rafts reveals a novel role of statins in antioxidation. J. Proteome Res. 2012, 11, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.L.; Babelova, A.; Mieth, A.; Weigert, A.; Mooz, J.; Rajalingam, K.; Heide, H.; Wittig, I.; Lopes, L.R.; Brandes, R.P. Endo-PDI is required for TNF-α-induced angiogenesis. Free Radic. Biol. Med. 2013, 65, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Charlton, H.K.; Webster, J.; Kruger, S.; Simpson, F.; Richards, A.A.; Whitehead, J.P. ERp46 binds to Adipo-R1, but not Adipo-R2, and modulates adiponectin signalling. Biochem. Biophys. Res. Commun. 2010, 392, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Heiker, J.T.; Kosel, D.; Beck-Sickinger, A.G. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol. Chem. 2010, 391, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- STRING—Known and Predicted Protein—Protein Interactions Website. Available online: http://string905.embl.de/newstring_cgi/show_input_page.pl?UserId=PmDmpCSX1WyX&sessionId=GY6ARW_A5G24&info_box_type_input_page=general (accessed on 30 July 2014).
- Biological General Repository for Interaction Datasets Home Page. Available online: http://thebiogrid.org/ (accessed on 13 July 2014).
- KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: http://www.genome.jp/kegg/ (accessed on 30 July 2014).
- Gene Editing Rat Resource Center Home Page. Available online: http://rgd.mcw.edu/ (accessed on 30 July 2014).
- NCBI GenBank Home page. Available online: http://www.ncbi.nlm.nih.gov/genbank (accessed on 7 July 2014).
- Nagappan, A.; Park, H.S.; Park, K.I.; Kim, J.A.; Hong, G.E.; Kang, S.R.; Zhang, J.; Kim, E.H.; Lee, W.S.; Won, C.K.; et al. Proteomic analysis of differentially expressed proteins in vitamin C-treated AGS cells. BMC Biochem. 2013, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Karamessinis, P.; Peroulis, M.; Kypreou, K.; Kavvadas, P.; Pagakis, S.; Politis, P.K.; Charonis, A. ERp46 is reduced by high glucose and regulates insulin content in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E812–E821. [Google Scholar] [CrossRef]
- Sabri, A.; Lai, D.; D’Silva, A.; Seeho, S.; Kaur, J.; Ng, C.; Hyett, J. Differential placental gene expression in term pregnancies affected by fetal growth restriction and macrosomia. Fetal Diagn. Ther. 2014, 36, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, 270–277. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. Fast docking using the CHARMM force field with EADock DSS. J. Comput. Chem. 2011, 32, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Torres, A.; Barcelo-Batllori, S.; Martinez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Arnal, C.; Guillen, N.; Acin, S.; Osada, J. Proteomics and gene expression analyses of squalene-supplemented mice identify microsomal thioredoxin domain-containing protein 5 changes associated with hepatic steatosis. J. Proteomics 2012, 77, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Real, C.I.; Megger, D.A.; Sitek, B.; Jahn-Hofmann, K.; Ickenstein, L.M.; John, M.J.; Walker, A.; Timm, J.; Kuhlmann, K.; Eisenacher, M.; et al. Identification of proteins that mediate the pro-viral functions of the interferon stimulated gene 15 in hepatitis C virus replication. Antivir. Res. 2013, 100, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, S.K.; Shin, H.P.; Lee, S.M.; Chung, J.H. TXNDC5 gene polymorphism contributes to increased risk of hepatocellular carcinoma in the Korean male population. Anticancer Res. 2013, 33, 3983–3987. [Google Scholar] [PubMed]
- Zhang, L.; Hou, Y.H.; Wu, K.; Zhai, J.S.; Lin, N. Proteomic analysis reveals molecular biological details in varioliform gastritis without Helicobacter pylori infection. World J Gastroenterol. 2010, 16, 3664–3673. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Jin, U.H.; Kang, J.H.; Kim, S.B.; Guthrie, A.S.; Sreevalsan, S.; Lee, J.S.; Safe, S. The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Mol. Cancer Res. 2014, 12, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Affer, M.; Chesi, M.; Chen, W.D.; Keats, J.J.; Demchenko, Y.N.; Tamizhmani, K.; Garbitt, V.M.; Riggs, D.L.; Brents, L.A.; Roschke, A.V.; et al. Promiscuous MYC locus rearrangements hijack enhancers but mostly super-enhancers to dysregulate MYC expression in multiple myeloma. Leukemia 2014, 28, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Oh, S.H.; Lee, J.H.; Kwon, Y.S.; Ryu, D.J.; Lee, K.H. Can blood components with age-related changes influence the ageing of endothelial cells? Exp. Dermatol. 2010, 19, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Komatsubara, A.T.; Asano, T.; Tsumoto, H.; Shimizu, K.; Nishiuchi, T.; Yoshizumi, M.; Ozawa, K. Proteomic analysis of S-nitrosylation induced by 1-methyl-4-phenylpyridinium (MPP+). Proteome Sci. 2013, 10, 74. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horna-Terrón, E.; Pradilla-Dieste, A.; Sánchez-de-Diego, C.; Osada, J. TXNDC5, a Newly Discovered Disulfide Isomerase with a Key Role in Cell Physiology and Pathology. Int. J. Mol. Sci. 2014, 15, 23501-23518. https://doi.org/10.3390/ijms151223501
Horna-Terrón E, Pradilla-Dieste A, Sánchez-de-Diego C, Osada J. TXNDC5, a Newly Discovered Disulfide Isomerase with a Key Role in Cell Physiology and Pathology. International Journal of Molecular Sciences. 2014; 15(12):23501-23518. https://doi.org/10.3390/ijms151223501
Chicago/Turabian StyleHorna-Terrón, Elena, Alberto Pradilla-Dieste, Cristina Sánchez-de-Diego, and Jesús Osada. 2014. "TXNDC5, a Newly Discovered Disulfide Isomerase with a Key Role in Cell Physiology and Pathology" International Journal of Molecular Sciences 15, no. 12: 23501-23518. https://doi.org/10.3390/ijms151223501