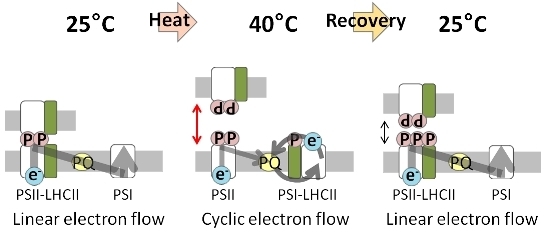
Regulation of Photochemical Energy Transfer Accompanied by Structural Changes in Thylakoid Membranes of Heat-Stressed Wheat
Abstract
:1. Introduction
2. Results and Discussion
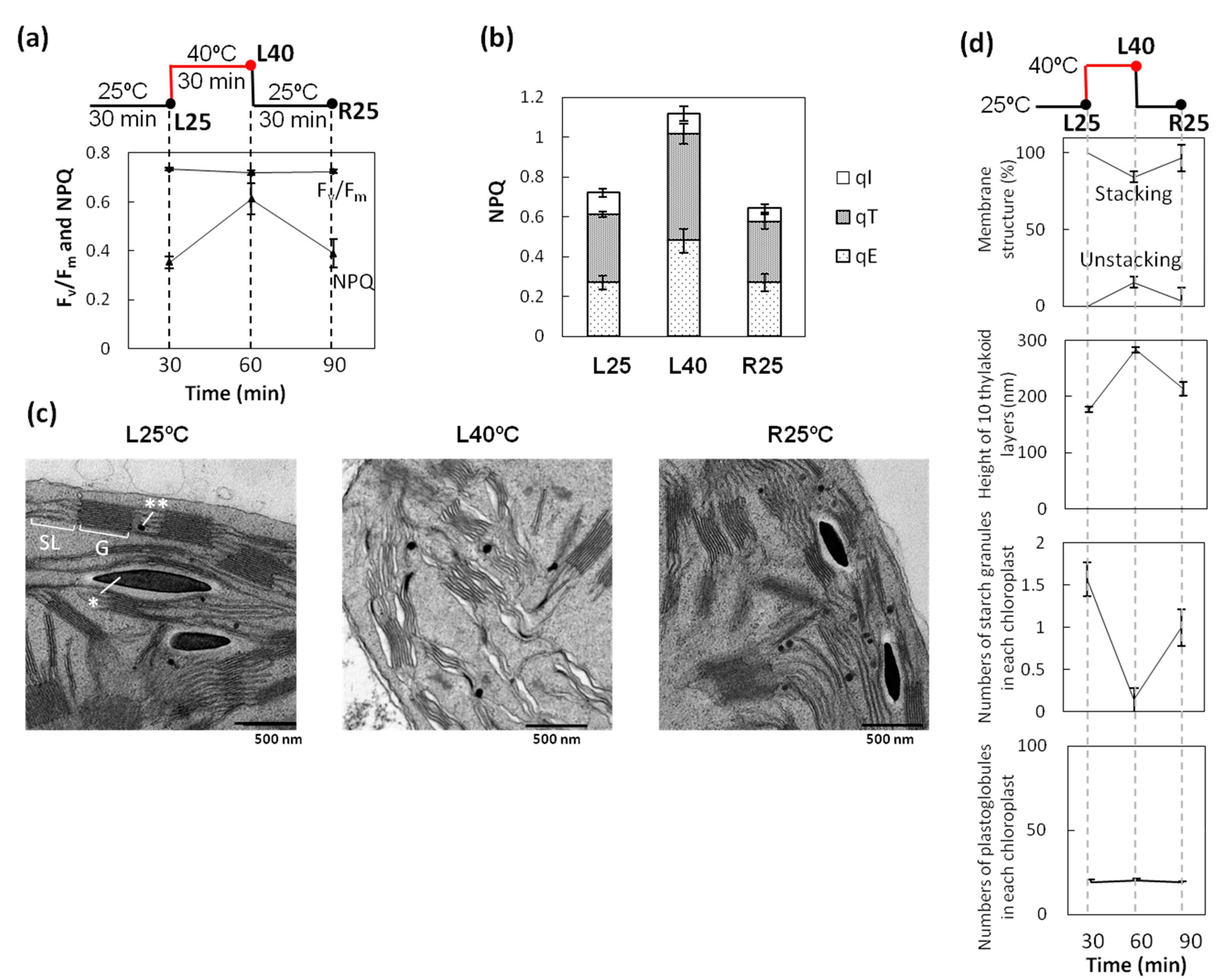
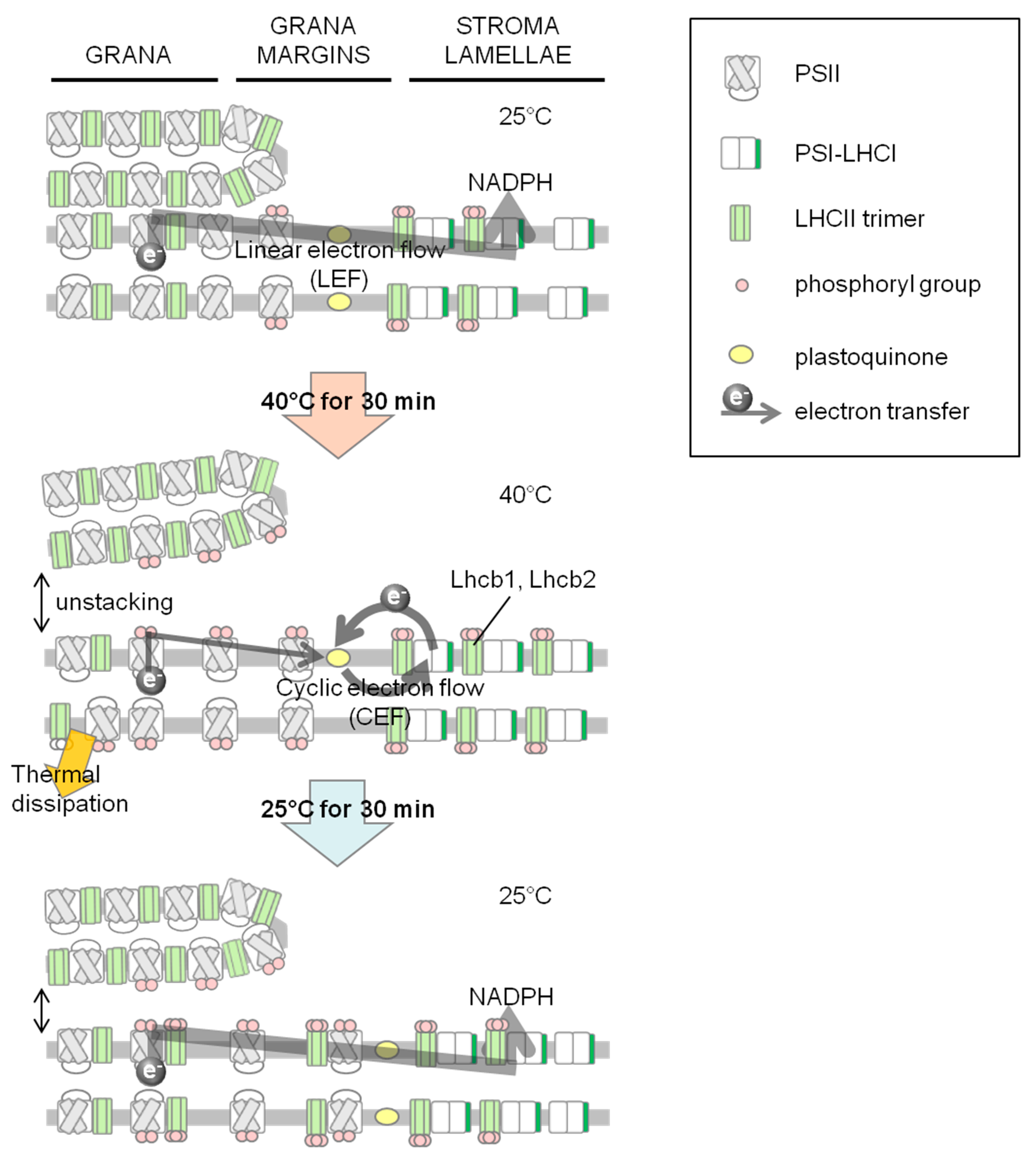
2.1. Increase in NPQ (Non-Photochemical Quenching) of Chlorophyll Fluorescence and Unstacked Region in Thylakoid Membranes under Heat Stress
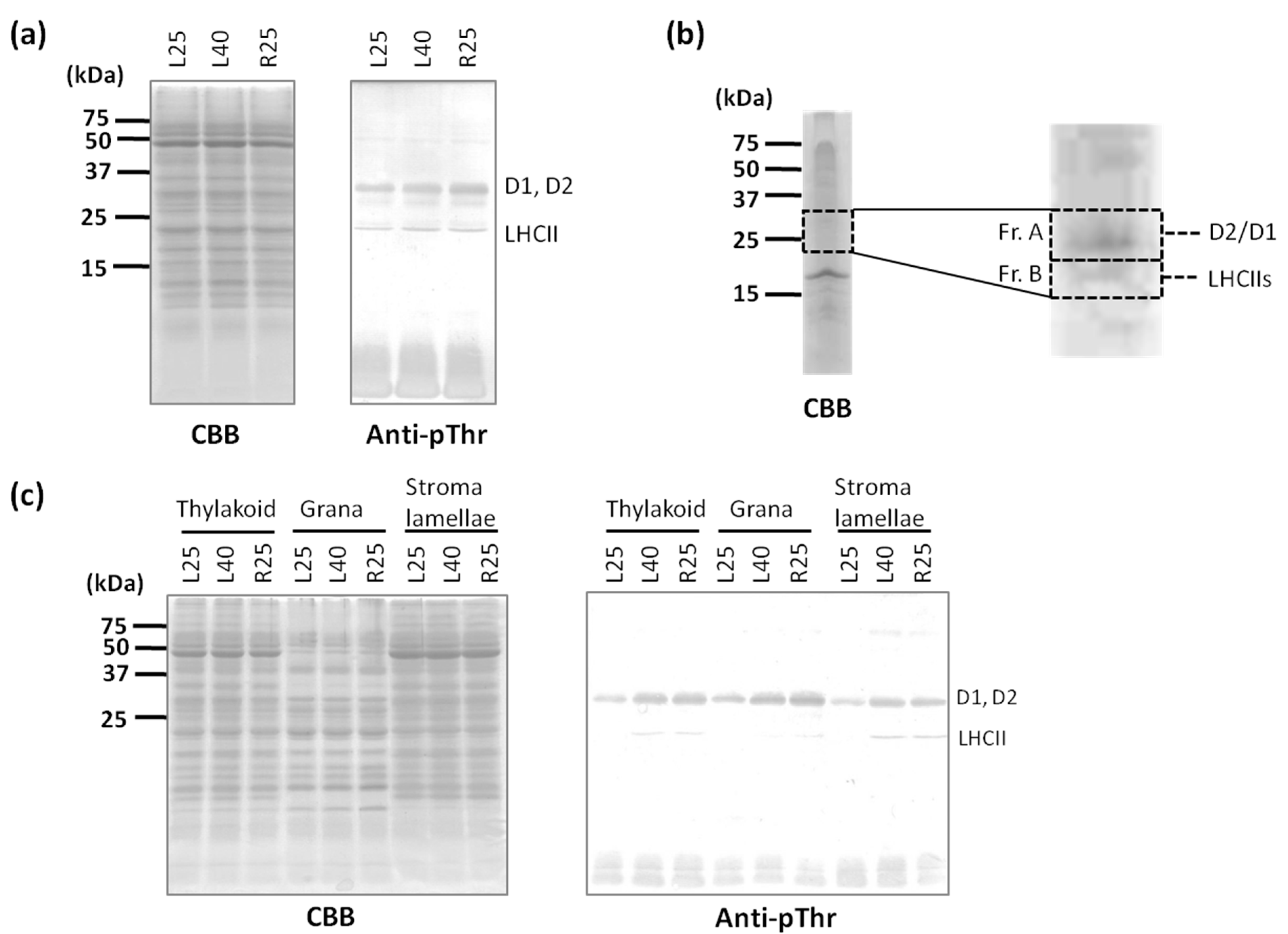
2.2. Phosphorylation Level of Thylakoid Proteins Increased by Heat Treatment
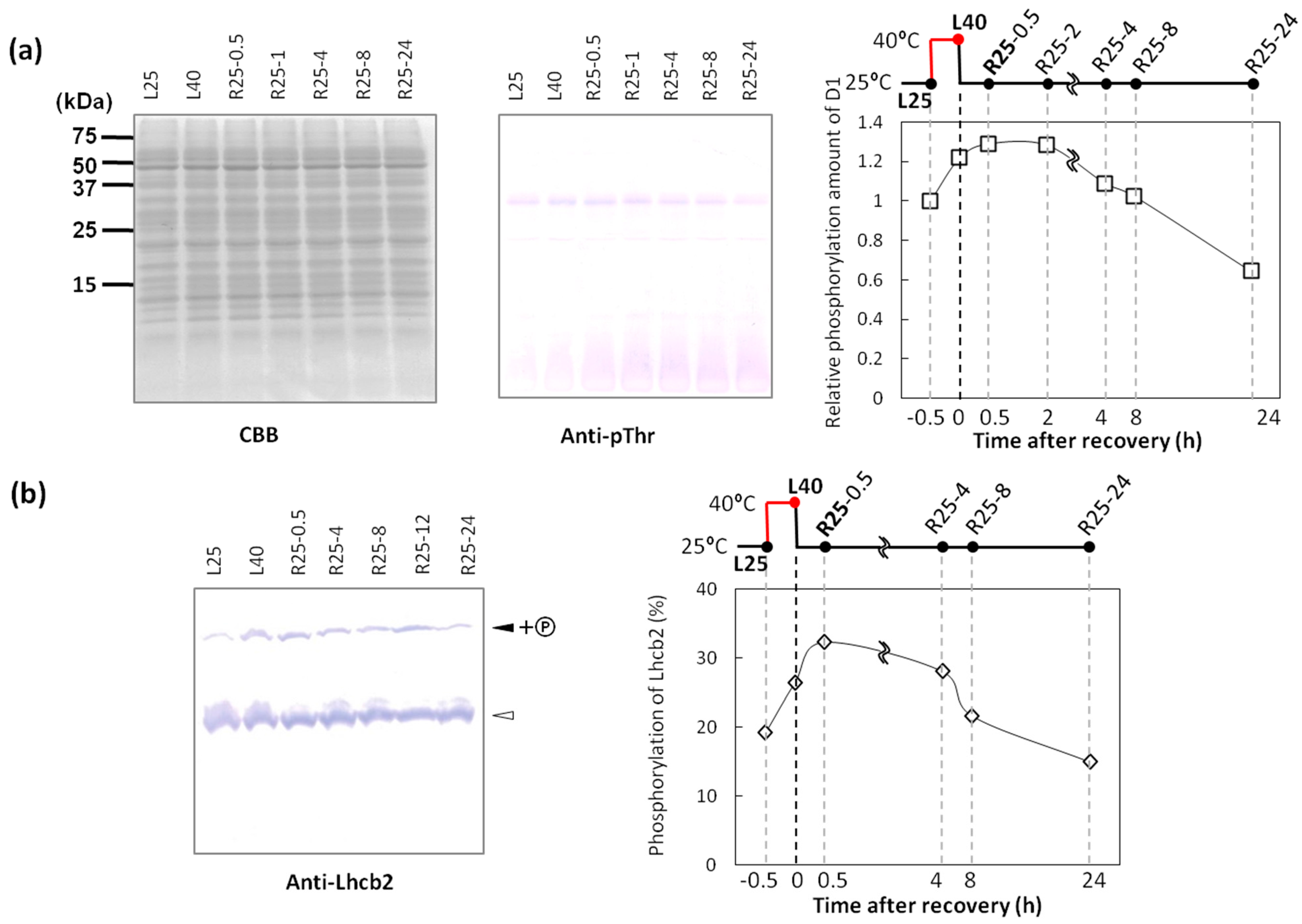
2.3. Dephosphorylation of Thylakoid Proteins Was Retarded during Recovery from Heat Stress
2.4. PSI–LHCII Supercomplex Increased Following Heat Stress in Light
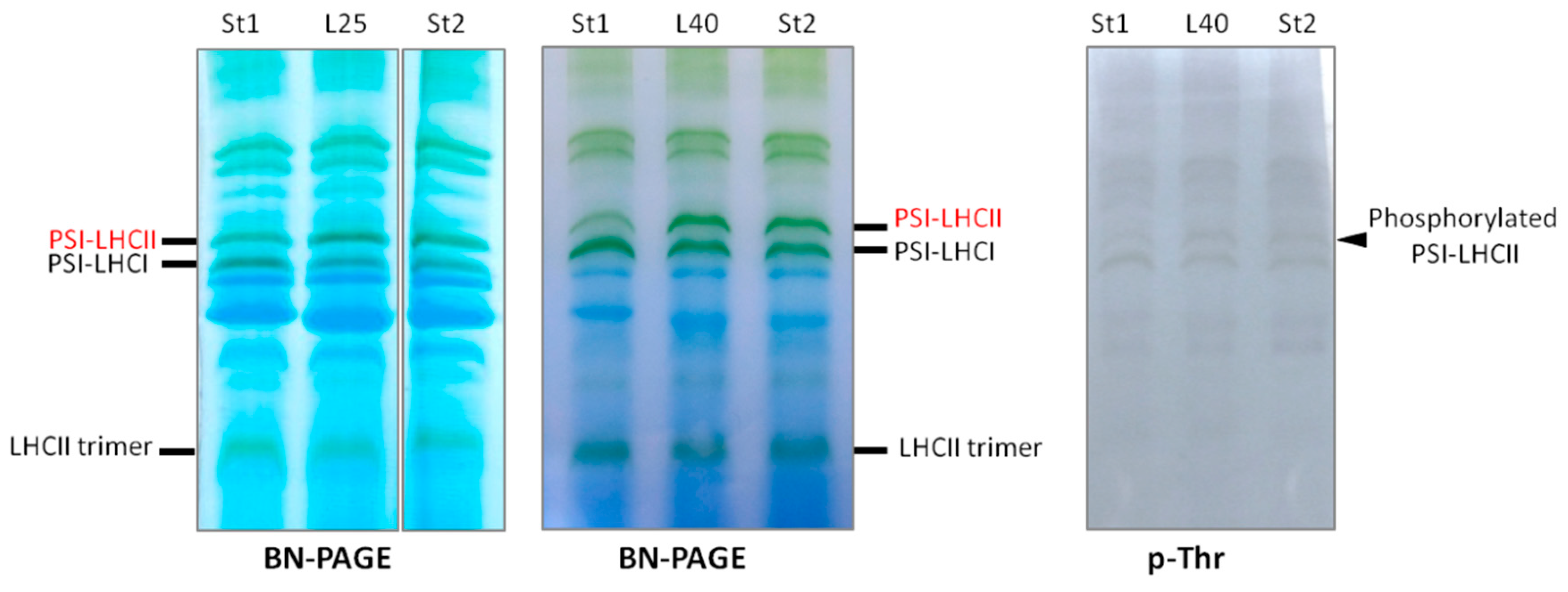
3. Experimental Section
3.1. Plant Materials and Heat Treatments
3.2. Induction of Conventional State Transition
3.3. Measurement of the Maximal Photochemical Quantum Yield of PSII and Chlorophyll Fluorescence Analysis
3.4. Preparation of Thylakoid Membranes
3.5. Digitonin Fractionation of Thylakoid Membranes
3.6. Protein Analysis
3.7. Protein Identification by Mass Spectrometry
3.8. Blue Native-PAGE
3.9. Transmission Electron Microscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharkey, T.D.; Zhang, R. High Temperature Effects on Electron and Proton Circuits of Photosynthesis. J. Integr. Plant Biol. 2010, 52, 712–722. [Google Scholar]
- Eckardt, N.A.; Portis, A.R., Jr. Heat Denaturation Profiles of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase (Rubisco) and Rubisco Activase and the Inability of Rubisco Activase to Restore Activity of Heat-Denatured Rubisco. Plant Physiol. 1997, 113, 243–248. [Google Scholar]
- Santarius, K.; Müller, M. Investigations on heat resistance of spinach leaves. Planta 1979, 146, 529–538. [Google Scholar]
- Strasser, B. Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. Photosynth. Res. 1997, 52, 147–155. [Google Scholar]
- Cao, J.; Govindjee. Chlorophyll a fluorescence transient as an indicator of active and inactive Photosystem II in thylakoid membranes. Biochim. Biophys. Acta 1990, 1015, 180–188. [Google Scholar]
- Havaux, M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993, 16, 461–467. [Google Scholar]
- Heckathorn, S.A.; Downs, C.A.; Coleman, J.S. Small Heat Shock Proteins Protect Electron Transport in Chloroplasts and Mitochondria during Stress. Am. Zool. 1999, 39, 865–876. [Google Scholar]
- Heckathorn, S.A.; Downs, C.A.; Sharkey, T.D.; Coleman, J.S. The Small, Methionine-Rich Chloroplast Heat-Shock Protein Protects Photosystem II Electron Transport during Heat Stress. Plant Physiol. 1998, 116, 439–444. [Google Scholar]
- Peñuelas, J.; Llusià, J.; Asensio, D.; MunnÉ-Bosch, S. Linking isoprene with plant thermotolerance, antioxidants and monoterpene emissions. Plant Cell Environ. 2005, 28, 278–286. [Google Scholar]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar]
- Chaitanya, K.V.; Sundar, D.; Masilamani, S.; Ramachandra Reddy, A. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002, 36, 175–180. [Google Scholar]
- Minagawa, J. State transitions—The molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 897–905. [Google Scholar]
- Wientjes, E.; van Amerongen, H.; Croce, R. LHCII is an antenna of both photosystems after long-term acclimation. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 420–426. [Google Scholar]
- Nellaepalli, S.; Mekala, N.R.; Zsiros, O.; Mohanty, P.; Subramanyam, R. Moderate heat stress induces state transitions in Arabidopsis thaliana. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 1177–1184. [Google Scholar]
- Ducruet, J.M.; Peeva, V.; Havaux, M. Chlorophyll thermofluorescence and thermoluminescence as complementary tools for the study of temperature stress in plants. Photosynth. Res. 2007, 93, 159–171. [Google Scholar]
- Mohanty, P.; Vani, B.; Prakash, J.S. Elevated temperature treatment induced alteration in thylakoid membrane organization and energy distribution between the two photosystems in Pisum sativum. Z. Naturforsch. C 2002, 57, 836–842. [Google Scholar]
- Yamauchi, Y.; Kimura, Y. Photosystem at High Temperature-Mechanisms of Adaptation and Damage. In Photochemistry: UV/VIS Spectroscopy, Photochemical Reactions and Photosynthesis, 1st ed.; Maes, K.J., Willems, J.M., Eds.; NOVA Science: Hauppauge, NY, USA, 2011; pp. 203–236. [Google Scholar]
- Marutani, Y.; Yamauchi, Y.; Kimura, Y.; Mizutani, M.; Sugimoto, Y. Damage to photosystem II due to heat stress without light-driven electron flow: Involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 2012, 236, 753–761. [Google Scholar]
- Havaux, M.; Tardy, F. Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: Possible involvement of xanthophyll-cycle pigments. Planta 1996, 198, 324–333. [Google Scholar]
- Bukhov, N.; Wiese, C.; Neimanis, S.; Heber, U. Heat sensitivity of chloroplasts and leaves: Leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth. Res. 1999, 59, 81–93. [Google Scholar]
- Allen, J.F.; Forsberg, J. Molecular recognition in thylakoid structure and function. Trends Plant Sci. 2001, 6, 317–326. [Google Scholar]
- Chuartzman, S.G.; Nevo, R.; Shimoni, E.; Charuvi, D.; Kiss, V.; Ohad, I.; Brumfeld, V.; Reich, Z. Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 2008, 20, 1029–1039. [Google Scholar]
- Dreier, W.; Schnarrenberger, C.; Börner, T. Light- and Stress-Dependent Enhancement of Amylolytic Activities in White and Green Barley Leaves: β-Amylases are Stress-Induced Proteins. J. Plant Physiol. 1995, 145, 342–348. [Google Scholar]
- Zhang, R.; Wise, R.; Struck, K.; Sharkey, T. Moderate heat stress of Arabidopsis thaliana leaves causes chloroplast swelling and plastoglobule formation. Photosynth. Res. 2010, 105, 123–134. [Google Scholar]
- Bellafiore, S.; Barneche, F.; Peltier, G.; Rochaix, J.-D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 2005, 433, 892–895. [Google Scholar]
- Fristedt, R.; Willig, A.; Granath, P.; Crevecoeur, M.; Rochaix, J.D.; Vener, A.V. Phosphorylation of photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis. Plant Cell 2009, 21, 3950–3964. [Google Scholar]
- Kinoshita, E.; Kinoshita-Kikuta, E.; Takiyama, K.; Koike, T. Phosphate-binding Tag, a New Tool to Visualize Phosphorylated Proteins. Mol. Cell. Proteomics 2006, 5, 749–757. [Google Scholar]
- Leoni, C.; Pietrzykowska, M.; Kiss, A.Z.; Suorsa, M.; Ceci, L.R.; Aro, E.-M.; Jansson, S. Very rapid phosphorylation kinetics suggest a unique role for Lhcb2 during state transitions in Arabidopsis. Plant J. 2013, 76, 236–246. [Google Scholar]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.-M.; Jansson, S. The Light-Harvesting Chlorophyll a/b Binding Proteins Lhcb1 and Lhcb2 Play Complementary Roles during State Transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar]
- Shapiguzov, A.; Ingelsson, B.; Samol, I.; Andres, C.; Kessler, F.; Rochaix, J.D.; Vener, A.V.; Goldschmidt-Clermont, M. The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 4782–4787. [Google Scholar]
- Pribil, M.; Pesaresi, P.; Hertle, A.; Barbato, R.; Leister, D. Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol. 2010, 8, e1000288. [Google Scholar]
- Samol, I.; Shapiguzov, A.; Ingelsson, B.; Fucile, G.; Crevecoeur, M.; Vener, A.V.; Rochaix, J.D.; Goldschmidt-Clermont, M. Identification of a Photosystem II Phosphatase Involved in Light Acclimation in Arabidopsis. Plant Cell 2012, 24, 2596–2609. [Google Scholar]
- Galka, P.; Santabarbara, S.; Khuong, T.T.; Degand, H.; Morsomme, P.; Jennings, R.C.; Boekema, E.J.; Caffarri, S. Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 2012, 24, 2963–78. [Google Scholar]
- Wientjes, E.; Drop, B.; Kouřil, R.; Boekema, E.J.; Croce, R. During State 1 to State 2 Transition in Arabidopsis thaliana, the Photosystem II Supercomplex Gets Phosphorylated but Does Not Disassemble. J. Biol. Chem. 2013, 288, 32821–32826. [Google Scholar]
- Demmig-Adams, B.; Adams Iii, W.W.; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar]
- Quick, W.P.; Stitt, M. An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochim. Biophys. Acta Bioenerg. 1989, 977, 287–296. [Google Scholar]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar]
- Khatoon, M.; Inagawa, K.; Pospíšil, P.; Yamashita, A.; Yoshioka, M.; Lundin, B.; Horie, J.; Morita, N.; Jajoo, A.; Yamamoto, Y.; et al. Quality Control of Photosystem II: Thylakoid unstacking is necessary to avoid further damage to the d1 protein and to facilitate d1 degradation under light stress in spinach thylakoids. J. Biol. Chem. 2009, 284, 25343–25352. [Google Scholar]
- Schilling, M.; Knapp, D.R. Enrichment of phosphopeptides using biphasic immobilized metal affinity-reversed phase microcolumns. J. Proteome Res. 2008, 7, 4164–4172. [Google Scholar]
- Hatano, N.; Hamada, T. Proteome Analysis of Pitcher Fluid of the Carnivorous Plant Nepenthes alata. J. Proteome Res. 2008, 7, 809–816. [Google Scholar]
- Järvi, S.; Suorsa, M.; Paakkarinen, V.; Aro, E.M. Optimized native gel systems for separation of thylakoid protein complexes: Novel super- and mega-complexes. Biochem. J. 2011, 439, 207–214. [Google Scholar]
- Peng, L.; Shimizu, H.; Shikanai, T. The Chloroplast NAD(P)H Dehydrogenase Complex Interacts with Photosystem I in Arabidopsis. J. Biol. Chem. 2008, 283, 34873–34879. [Google Scholar]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar]
- Sato, T. A modified method for lead staining of thin sections. J. Electron Microsc. 1968, 17, 158–159. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marutani, Y.; Yamauchi, Y.; Miyoshi, A.; Inoue, K.; Ikeda, K.-i.; Mizutani, M.; Sugimoto, Y. Regulation of Photochemical Energy Transfer Accompanied by Structural Changes in Thylakoid Membranes of Heat-Stressed Wheat. Int. J. Mol. Sci. 2014, 15, 23042-23058. https://doi.org/10.3390/ijms151223042
Marutani Y, Yamauchi Y, Miyoshi A, Inoue K, Ikeda K-i, Mizutani M, Sugimoto Y. Regulation of Photochemical Energy Transfer Accompanied by Structural Changes in Thylakoid Membranes of Heat-Stressed Wheat. International Journal of Molecular Sciences. 2014; 15(12):23042-23058. https://doi.org/10.3390/ijms151223042
Chicago/Turabian StyleMarutani, Yoko, Yasuo Yamauchi, Akihito Miyoshi, Kanako Inoue, Ken-ichi Ikeda, Masaharu Mizutani, and Yukihiro Sugimoto. 2014. "Regulation of Photochemical Energy Transfer Accompanied by Structural Changes in Thylakoid Membranes of Heat-Stressed Wheat" International Journal of Molecular Sciences 15, no. 12: 23042-23058. https://doi.org/10.3390/ijms151223042