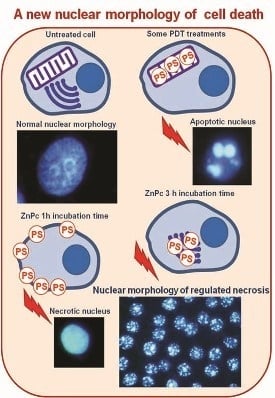
Regulated Necrosis in HeLa Cells Induced by ZnPc Photodynamic Treatment: A New Nuclear Morphology
Abstract
:1. Introduction
2. Results
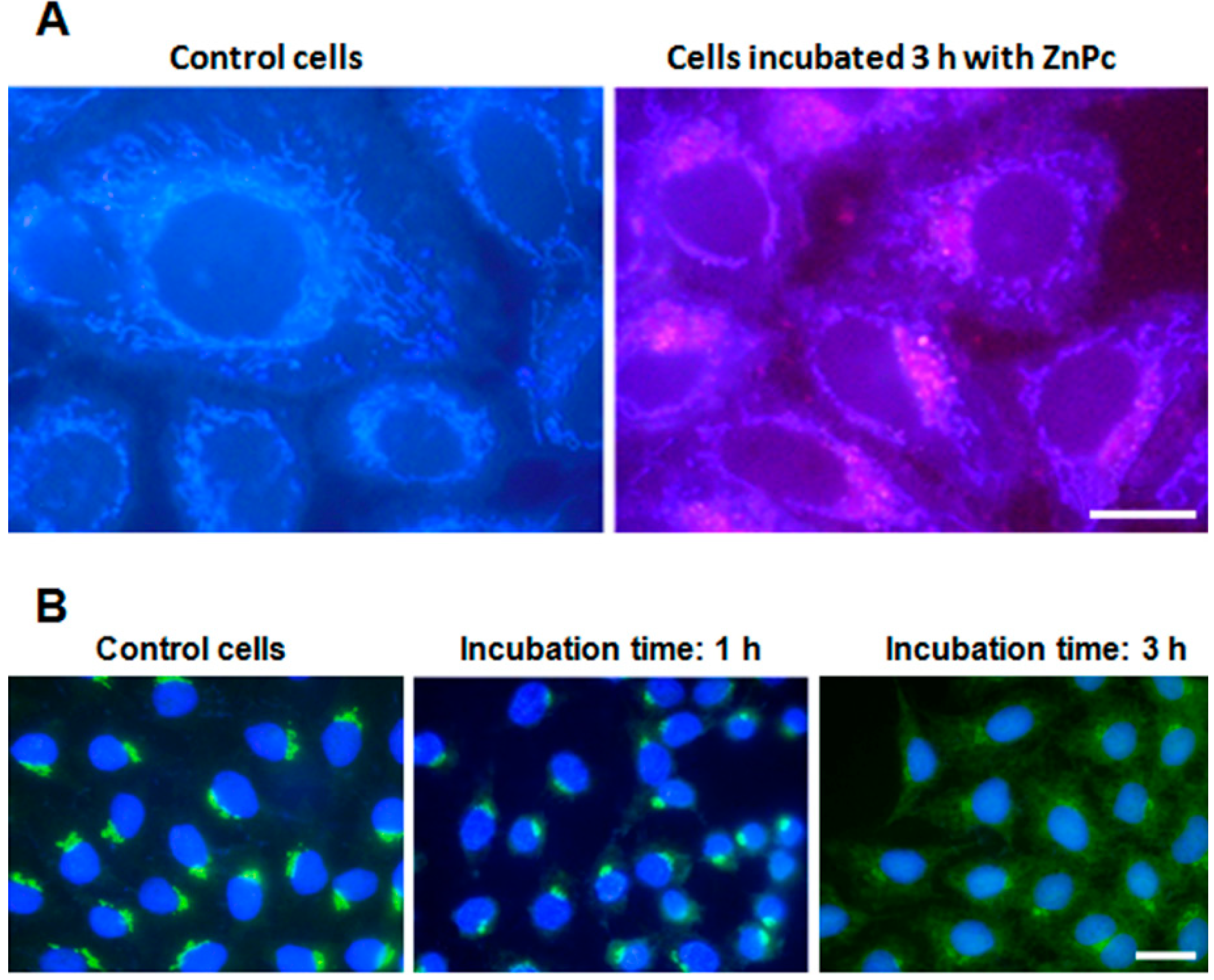
2.1. ZnPc Localization in HeLa Cells
2.2. Cell Survival
| Treatments | Dark Toxicity | 10 min Irradiation Time (2.4 J/cm2) | ||
|---|---|---|---|---|
| Surviving Fraction (%) | SD | Surviving Fraction (%) | SD | |
| Control | 100 | 3.42 | 100 | 3.72 |
| ZnPc 1 h | 99.49 | 4.65 | 8.07 | 4.64 |
| ZnPc 3 h | 98.61 | 2.91 | 4.28 | 4.37 |
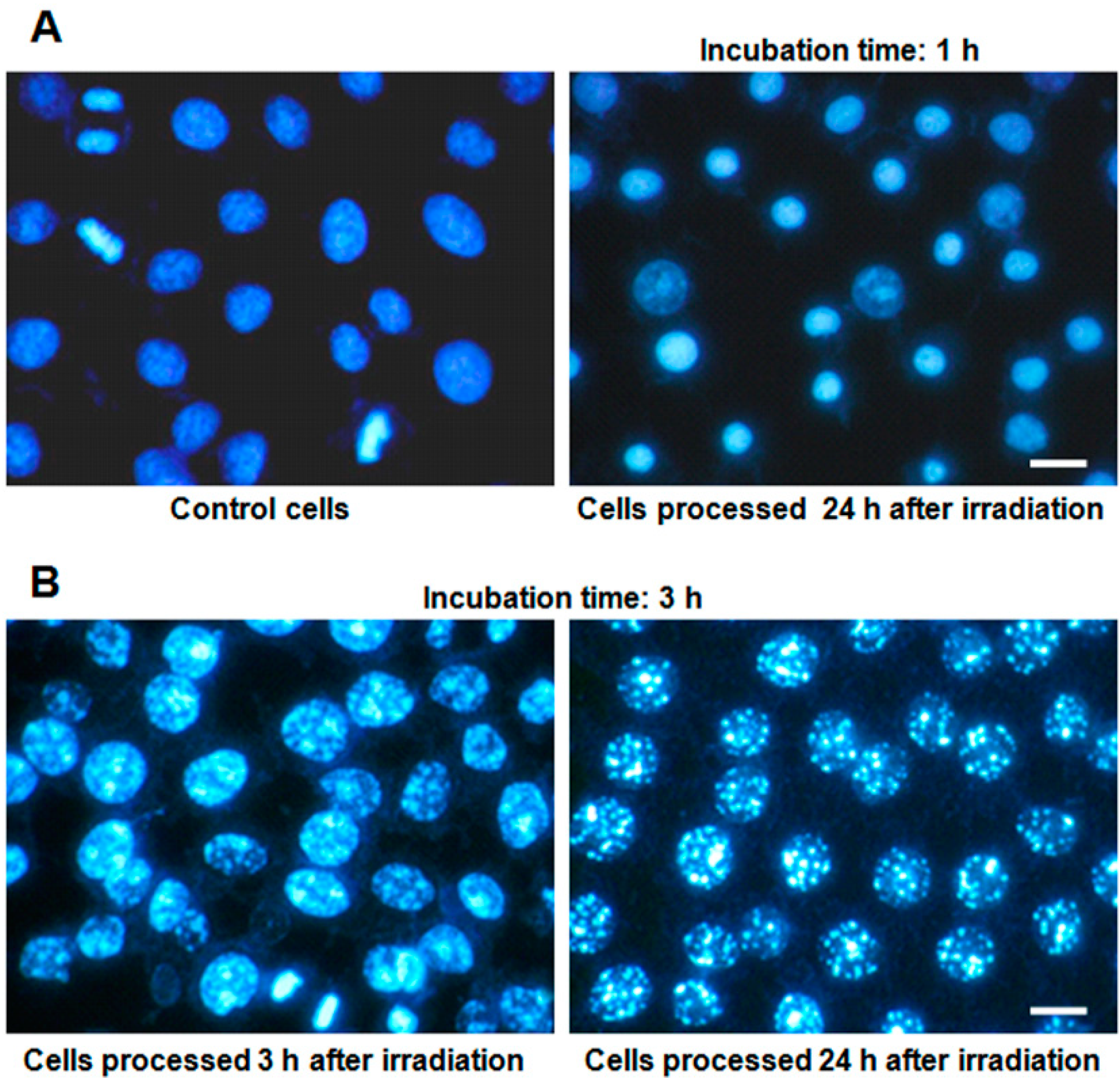
2.3. Nuclear Morphology
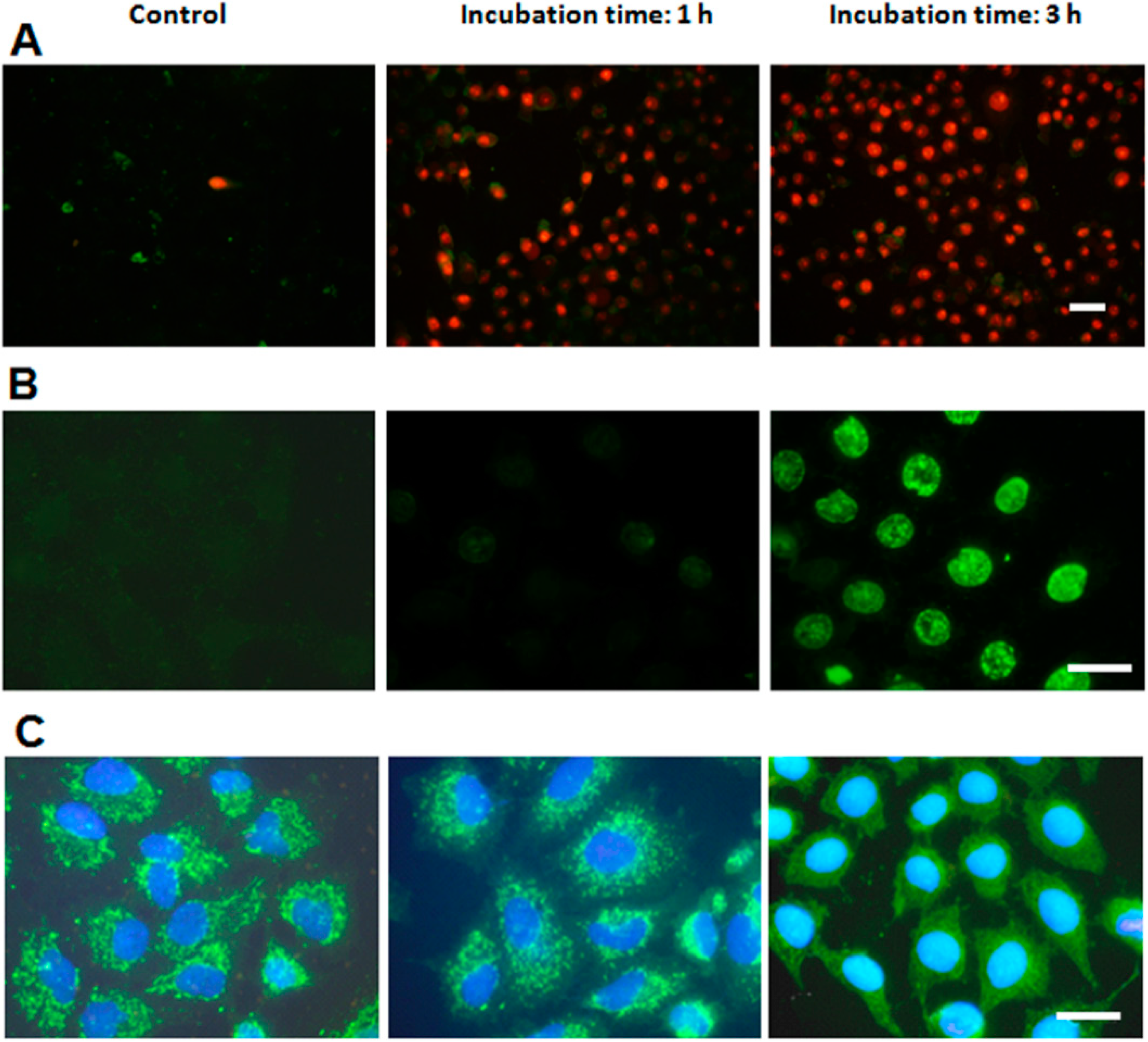
2.4. Characterization of Cell Death Type
2.5. Treatments in Presence of Necrostatin-1
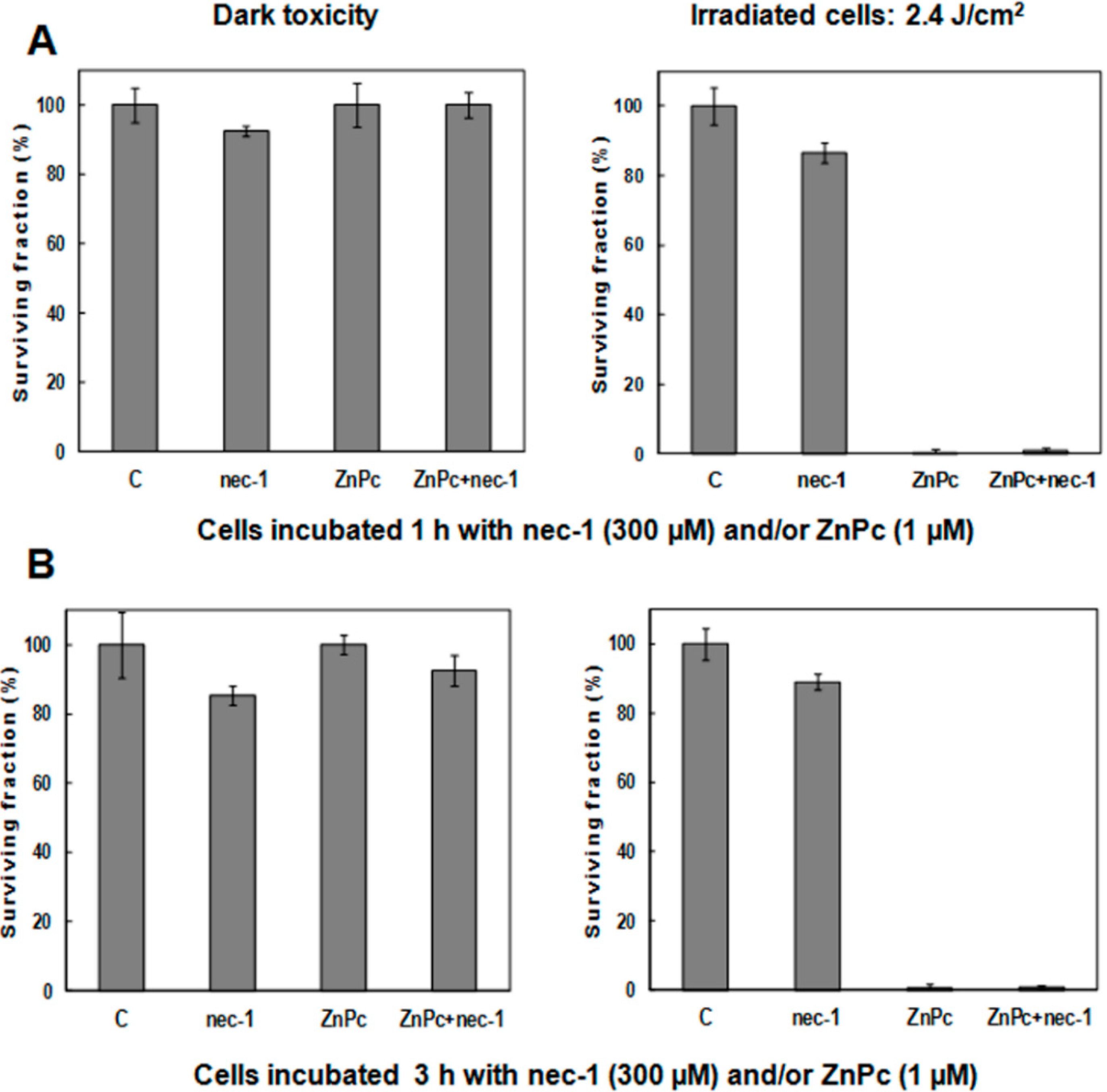
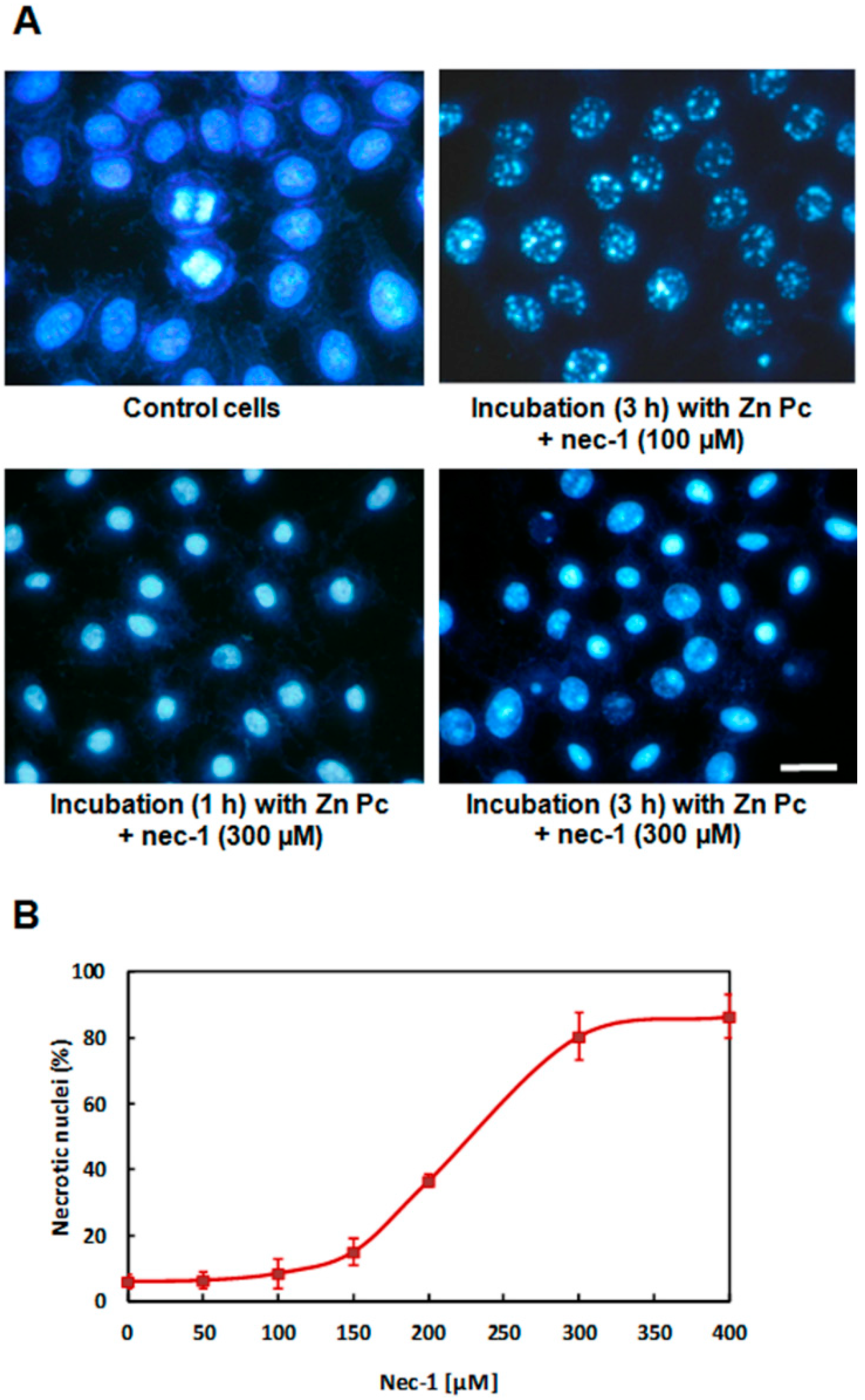
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. ZnPc Liposomes Preparation
4.3. Subcellular Localization
4.4. Photodynamic Treatments.
4.5. Cell Viability
4.6. Golgin-130 Immunostaining
4.7. Hoechst-33258 Staining
4.8. Annexin V/Propidium Iodide Assay
4.9. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labeling (TUNEL) Assay
4.10. Cytochrome c (Cyt-c) Immunostaining
4.11. Necrostatin-1 (Nec-1) Treatments
4.12. Microscopy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Baron, E.D. Photodynamic therapy: Current evidence and applications in dermatology. Semin. Cutan. Med. Surg. 2011, 30, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–287. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, J.; Vasquez, L.; Iyo, F.; Abengoechea, S. Photodynamic therapy in the management of circumscribed choroidal hemangioma. Can. J. Ophthalmol. 2012, 47, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- St Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar]
- Rodriguez, M.E.; Zhang, P.; Azizuddin, K.; Delos Santos, G.B.; Chiu, S.M.; Xue, L.Y.; Berlin, J.C.; Peng, X.; Wu, H.; Lam, M.; et al. Structural factors and mechanisms underlying the improved photodynamic cell killing with silicon phthalocyanine photosensitizers directed to lysosomes versus mitochondria. Photochem. Photobiol. 2009, 85, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Reiners, J.J., Jr.; Agostinis, P.; Berg, K.; Oleinick, N.L.; Kessel, D. Assessing autophagy in the context of photodynamic therapy. Autophagy 2010, 6, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Krammer, B.; Verwanger, T. Molecular response to hypericin-induced photodamage. Curr. Med. Chem. 2012, 19, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Piette, J.; Volanti, C.; Vantieghem, A.; Matroule, J.Y.; Habraken, Y.; Agostinis, P. Cell death and growth arrest in response to photodynamic therapy with membrane-bound photosensitizers. Biochem. Pharmacol. 2003, 66, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Fabris, C.; Valduga, G.; Miotto, G.; Borsetto, L.; Jori, G.; Garbisa, S.; Reddi, E. Photosensitization with zinc (II) phthalocyanine as a switch in the decision between apoptosis and necrosis. Cancer Res. 2001, 61, 7495–7500. [Google Scholar] [PubMed]
- Yoo, J.O.; Lim, Y.C.; Kim, Y.M.; Ha, K.S. Differential cytotoxic responses to low- and high-dose photodynamic therapy in human gastric and bladder cancer cells. J. Cell. Biochem. 2011, 112, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Hail, N.; Carter, B.Z.; Konopleva, M.; Andreeff, M. Apoptosis effector mechanisms: A requiem performed in different keys. Apoptosis 2006, 11, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Formigli, L.; Papucci, L.; Tani, A.; Schiavone, N.; Tempestini, A.; Orlandini, G.E.; Capaccioli, S.; Orlandini, S.Z. Aponecrosis: Morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J. Cell. Physiol. 2000, 182, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Asare, N.; Låg, M.; Lagadic-Gossmann, D.; Rissel, M.; Schwarze, P.; Holme, J.A. 3-Nitrofluoranthene (3-NF) but not 3-aminofluoranthene (3-AF) elicits apoptosis as well as programmed necrosis in Hepa1c1c7 cells. Toxicology 2009, 255, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Baritaud, M.; Boujrad, H.; Lorenzo, H.K.; Krantic, S.; Susin, S.A. Histone H2AX: The missing link in AIF-mediated caspase independent programmed necrosis. Cell Cycle 2010, 9, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death. Cell Death. Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Vanlangenakker, N.; Berghe, T.V.; Vandenabeele, P. Many stimuli pull the necrotic trigger, an overview. Cell Death. Differ. 2012, 19, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Fettweis, G.; Piette, J. RIP3 expression induces a death profile change in U2OS osteosarcoma cells after 5-ALA-PDT. Lasers Surg. Med. 2011, 43, 557–564. [Google Scholar] [PubMed]
- Coupienne, I.; Fettweis, G.; Rubio, N.; Agostinis, P.; Piette, J. 5-ALA-PDT induces RIP3-dependent necrosis in glioblastoma. Photochem. Photobiol. Sci. 2011, 10, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, J.; Stockert, J.C.; Villanueva, A.; Rello-Varona, S.; Juarranz, A.; Cañete, M. Caspase-2: A possible trigger of apoptosis induced in A-549 tumor cells by ZnPc photodynamic treatment. Int. J. Oncol. 2006, 28, 1057–1063. [Google Scholar] [PubMed]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Cañete, M.; Stockert, J.C.; Villanueva, A. Preclinical photodynamic therapy research in Spain. 3. Localization of photosensitizers and mechanisms of cell death in vitro. J. Porphyr. Phthalocyanines 2009, 13, 544–551. [Google Scholar] [CrossRef]
- Rello-Varona, S.; Stockert, J.C.; Cañete, M.; Acedo, P.; Villanueva, A. Mitotic catastrophe induced in HeLa cells by photodynamic treatment with Zn(II)-phthalocyanine. Int. J. Oncol. 2008, 32, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Mace, P.D.; Riedl, S.J. Molecular cell death platforms and assemblies. Curr. Opin. Cell Biol. 2010, 22, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E. Noncaspase proteases in apoptosis. Leukemia 2000, 14, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Martin, S.J. Caspase-independent cell death. Nat. Med. 2005, 11, 725–730. [Google Scholar] [PubMed]
- Moubarak, R.S.; Yuste, V.J.; Artus, C.; Bouharrour, A.; Greer, P.A.; Menissier-de Murcia, J.; Susin, S.A. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Mol. Cell. Biol. 2007, 27, 4844–4862. [Google Scholar] [PubMed]
- Rello, S.; Stockert, J.C.; Moreno, V.; Gámez, A.; Pacheco, M.; Juarranz, A.; Cañete, M.; Villanueva, A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005, 10, 201–208. [Google Scholar] [PubMed]
- Thapa, R.J.; Nogusa, S.; Chen, P.; Maki, J.L.; Lerro, A.; Andrake, M.; Rall, G.F.; Degterev, A.; Balachandran, S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. PNAS 2013, 13, 1103–1109. [Google Scholar]
- Xie, T.; Peng, W.; Liu, Y.; Yan, C.; Maki, J.; Degterev, A.; Yuan, J.; Shi, Y. Structural basis of RIP1 inhibition by necrostatins. Structure 2013, 21, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Choksi, S.; Shen, H.M. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J. Biol. Chem. 2004, 279, 10822–10828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; . Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Karch, J.; Kwong, J.Q.; Burr, A.R.; Sargent, M.A.; Elrod, J.W.; Peixoto, P.M.; Martinez-Caballero, S.; Osinska, H.; Cheng, E.H.; Robbins, J.; et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife 2013, 27, e00772. [Google Scholar]
- Ginevra, F.; Biffanti, S.; Pagnan, A.; Biolo, R.; Reddi, E.; Jori, G. Delivery of the tumour photosensitizer zinc(II)-phthalocyanine to serum proteins by different liposomes: Studies in vitro and in vivo. Cancer Lett. 1990, 49, 59–65. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, J.; Villanueva, A.; Stockert, J.C.; Cañete, M. Regulated Necrosis in HeLa Cells Induced by ZnPc Photodynamic Treatment: A New Nuclear Morphology. Int. J. Mol. Sci. 2014, 15, 22772-22785. https://doi.org/10.3390/ijms151222772
Soriano J, Villanueva A, Stockert JC, Cañete M. Regulated Necrosis in HeLa Cells Induced by ZnPc Photodynamic Treatment: A New Nuclear Morphology. International Journal of Molecular Sciences. 2014; 15(12):22772-22785. https://doi.org/10.3390/ijms151222772
Chicago/Turabian StyleSoriano, Jorge, Angeles Villanueva, Juan Carlos Stockert, and Magdalena Cañete. 2014. "Regulated Necrosis in HeLa Cells Induced by ZnPc Photodynamic Treatment: A New Nuclear Morphology" International Journal of Molecular Sciences 15, no. 12: 22772-22785. https://doi.org/10.3390/ijms151222772