2.1. Chitosan Promoted Gentamicin-Induced Dispersal of Listeria Monocytogenes Biofilms
Listeria monocytogenes is one of the most important causative agents of the serious foodborne disease, listeriosis in a wide range of mammalians [
9,
10,
11].
L. monocytogenes may produce multicellular biofilms most frequently in food-related environments where bacterial cells have been shown to be much more resistant to stress and to sanitizing agents than planktonic cells [
12]. To see whether chitosan and antibiotics had a synergistic effect on dispersal of
L. monocytogenes biofilms, four classes of antibiotics that were widely used and represented different antibiotic families were initially chosen. They included gentamicin (belonging to the aminoglycoside family), rifampicin (belonging to the naphthalenic ansamycin family), tetracycline (belonging to the tetracycline family) and carbenicillin (belonging to the penicillin family).
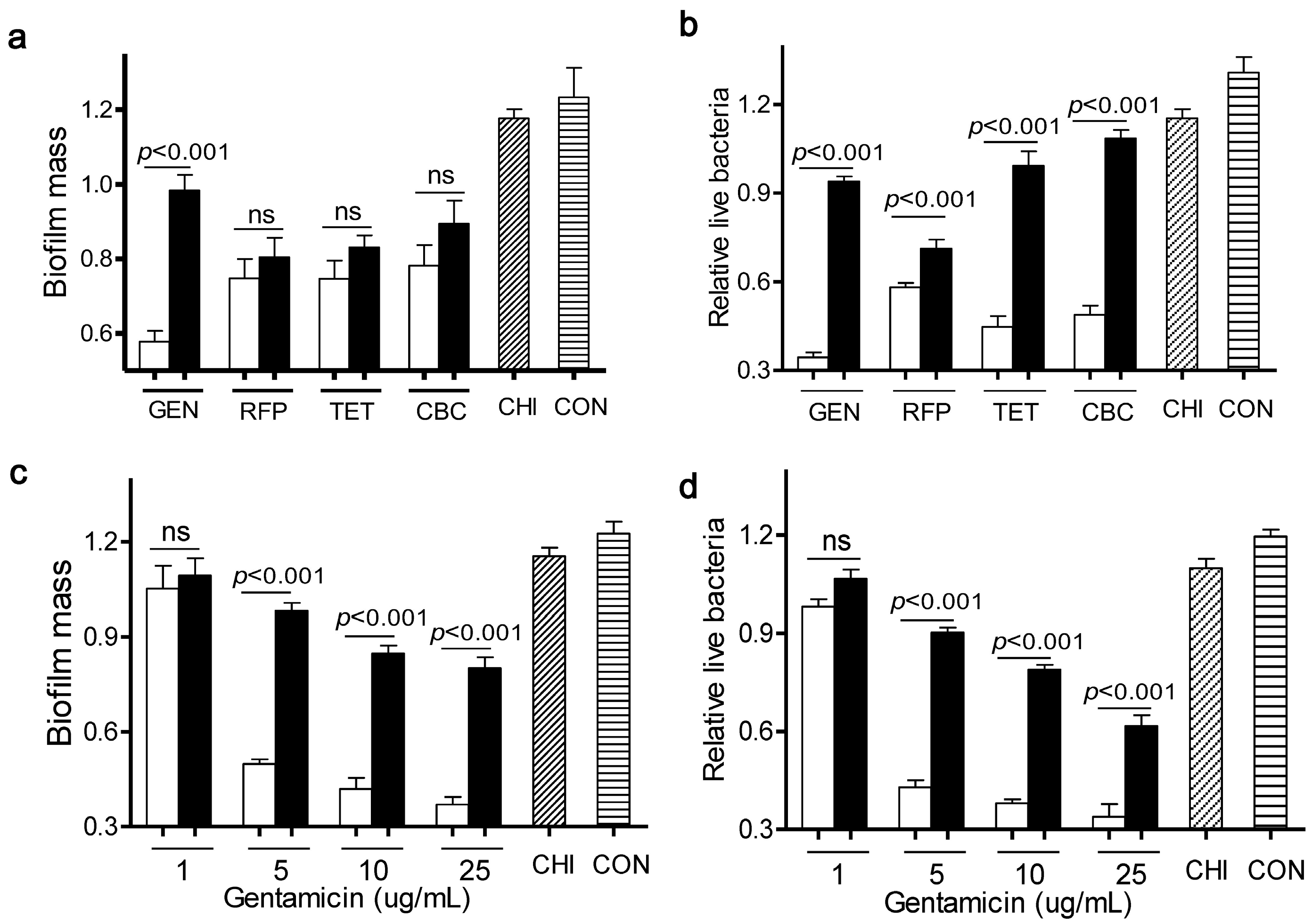
As shown in
Figure 1a,b, four antibiotics at 5 µg/mL elicited a very mild capacity in dispersal of
L. monocytogenes biofilms and killing of live bacteria, compared to the control. Meanwhile, chitosan alone at 200 µg/mL had little effect on both bioflim mass and viable cells. In contrast, when
L. monocytogenes biofilms were exposed to a mixture of chitosan and individual antibiotic, there were much fewer viable cells than with the antibiotic alone. This observation was consistent with the concept that the chitosan combination could enhance the antimicrobial activity of antibiotics [
13,
14]. Interestingly, only a combination of gentamicin and chitosan significantly dispersed
L. monocytogenes biofilms, when compared to the individual antibiotic alone. These data indicated that chitosan selectively increased the anti-biofilm efficacy of particular antibiotics such as gentamicin.
Figure 1.
Different effects of antibiotics in combination with chitosan against L. monocytogenes biofilms. (a,b) Biofilms were exposed to chitosan (CHI, 200 µg/mL), different antibiotics (gentamicin (GEN), rifampicin (RFP), tetracycline (TET) and carbenicillin (CBC), 5 µg/mL) or the respective mixtures for 24 h; (c,d) Biofilms were exposed to chitosan (200 µg/mL), different concentrations of gentamicin (1, 5, 10, 25 µg/mL) or the respective mixtures for 24 h. Biofilms incubated with tryptic soy broth (TSB) were used as control. Biofilm mass and viable cells were quantified. White column: chitosan-antibiotic combination; Black column: antibiotic alone; Column with italic lines: Chitosan alone; Column with horizontal lines: control. Error bars represent SD. ns: no significant difference.
Figure 1.
Different effects of antibiotics in combination with chitosan against L. monocytogenes biofilms. (a,b) Biofilms were exposed to chitosan (CHI, 200 µg/mL), different antibiotics (gentamicin (GEN), rifampicin (RFP), tetracycline (TET) and carbenicillin (CBC), 5 µg/mL) or the respective mixtures for 24 h; (c,d) Biofilms were exposed to chitosan (200 µg/mL), different concentrations of gentamicin (1, 5, 10, 25 µg/mL) or the respective mixtures for 24 h. Biofilms incubated with tryptic soy broth (TSB) were used as control. Biofilm mass and viable cells were quantified. White column: chitosan-antibiotic combination; Black column: antibiotic alone; Column with italic lines: Chitosan alone; Column with horizontal lines: control. Error bars represent SD. ns: no significant difference.
To further explore the synergism between chitosan and gentamicin in biofilm dispersal, we treated
L. monocytogenes biofilms for 24 h with chitosan (200 µg/mL) in the presence of various concentrations of gentamicin (1, 5, 10, 25 µg/mL). Results showed that a sufficient amount of gentamicin was required for this combination to remain the anti-biofilm and bactericidal activity towards
L. monocytogenes biofilms (
Figure 1c,d). Then, we determined the synergistic interactions between gentamicin and chitosan using Check board titration [
15]. As shown in
Table 1, gentamicin showed a clear synergistic action with chitosan against
L. monocytogenes biofilms (Fractional inhibitory concentration index (FIC
index) <0.15).
Table 1.
Fractional inhibitory concentration index (FICindex) of combination of gentamicin with chitosan against L. monocytogenes biofilms.
Table 1.
Fractional inhibitory concentration index (FICindex) of combination of gentamicin with chitosan against L. monocytogenes biofilms.
| EC50 (µg/mL) | FICindex | Remark |
|---|
| Alone | Combination |
| GEN | CHI | GEN | CHI |
| 50 | >4000 | 5 | 200 | <0.15 | Synergy |
Next we tested whether the gentamicin/chitosan combination dispersed
L. monocytogenes biofilms in a time-dependent fashion. As shown, this combination demonstrated a more pronounced effect in the decrease of both biofilm mass (
Figure 2a) and viable cells (
Figure 2b) than gentamicin or chitosan alone after short- or long-term treatment. These observations were also evidenced by visualization of biofilms by fluorescence microscopy (
Figure 2c), confocal microscopy (
Figure 3a) and scanning electron microscopy (
Figure 3b), in which there were fewer scattered cell aggregates in
L. monocytogenes biofilms after exposure to the gentamicin/chitosan combination than that of individual reagents.
Figure 2.
The gentamicin/chitosan combination disrupted the L. monocytogenes biofilms in a time-dependent manner. Biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 6, 12 and 24 h. Biofilms incubated with TSB were used as control (CON). Biofilm mass (a) and viable cells (b) were quantified, and biofilm architectures were further examined by fluorescence microscopy (c). Scale bars were 10 µm.
Figure 2.
The gentamicin/chitosan combination disrupted the L. monocytogenes biofilms in a time-dependent manner. Biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 6, 12 and 24 h. Biofilms incubated with TSB were used as control (CON). Biofilm mass (a) and viable cells (b) were quantified, and biofilm architectures were further examined by fluorescence microscopy (c). Scale bars were 10 µm.
Figure 3.
Disruption of biofilm architectures by the gentamicin/chitosan combination. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 24 h. Biofilms incubated with TSB were used as control. (a) Confocal microscopy, three-dimensional reconstructions were shown in the bottom views, scale bars were 50 µm; (b) Scanning electron microscopy, scale bars were 1 µm.
Figure 3.
Disruption of biofilm architectures by the gentamicin/chitosan combination. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 24 h. Biofilms incubated with TSB were used as control. (a) Confocal microscopy, three-dimensional reconstructions were shown in the bottom views, scale bars were 50 µm; (b) Scanning electron microscopy, scale bars were 1 µm.
2.2. Factors Involved in Anti-Biofilm Capacity of the Gentamicin/Chitosan Combination
It is generally accepted that the anti-biofilm effect of chitosan is largely dependent on
N-deacetylation degrees [
6] and molecular weights [
16]. To examine whether the molecular mass of chitosan played a role in the anti-biofilm capacity of the gentamicin/chitosan combination,
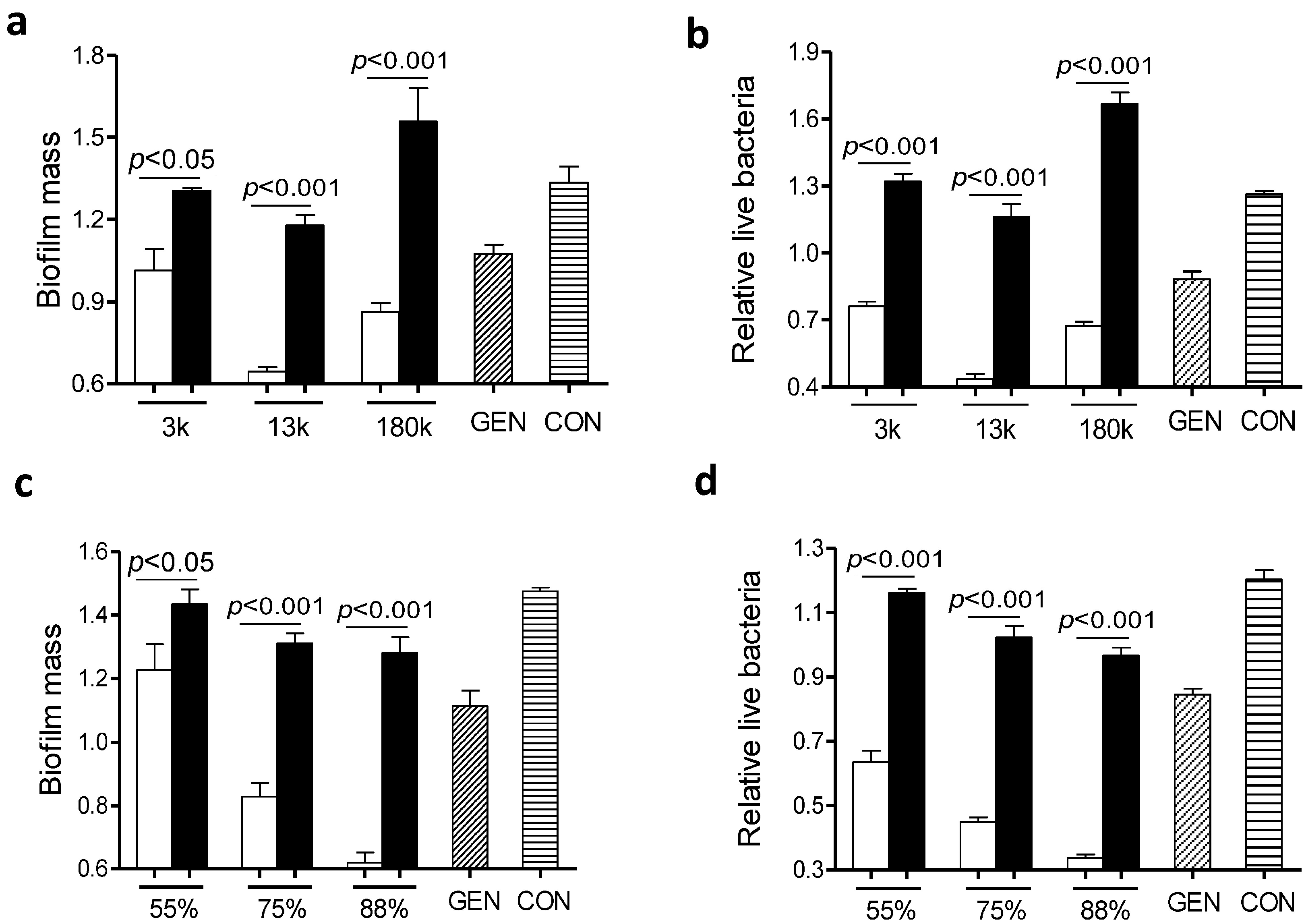
L. monocytogenes biofilms were exposed to gentamicin in the presence of chitosan with different molecular mass of ~3000, ~13,000 or 180,000 Da. All three chitosan-gentamicin mixtures tested reduced more biofilm mass than the respective chitosan alone did (
Figure 4a,b). In addition, a combination of gentamicin and chitosan with a molecular mass of ~13,000 Da elicited the highest anti-biofilm activity of all experimental groups.
Next we evaluated the impact of positive charges of chitosan on the anti-biofilm capacity of the gentamicin/chitosan combinations. Chitosan with different
N-deacetylation degrees (DD: 50%, 75%, 88%) alone was very limited in disruption of biofilms (
Figure 4c,d). Although all three chitosan-gentamicin mixtures tested reduced more biofilm mass than the respective chitosan alone did, it was obvious that gentamicin combined with chitosan possessing the highest
N-deacetylation degree displayed a stronger anti-biofilm activity. This raised the question whether other antibacterial polycationic biopolymers such as poly-
l-lysine [
17], instead of chitosan, also worked in a similar fashion. Results showed that the combination of poly-
l-lysine and gentamicin indeed had stronger anti-biofilm and bactericidal activities than the individual agent alone did (
Figure 5). This finding suggests that polycationic properties enabled higher anti-biofilm and bactericidal capacities for biopolymer-antibiotic combinations.
Figure 4.
Chitosan with specific molecular mass and N-deacetylation degree was essential in retaining the high anti-biofilm capacity of the gentamicin/chitosan combination. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 24 h. (a,b) Chitosan with different molecular mass (3000, 13,000 and 180,000 Da); (c,d) Chitosan with different N-deacetylation degrees (DD: 50%, 75% and 88%). White column: chitosan-antibiotic combination; Black column: chitosan alone; Column with italic lines: antibiotic alone; Column with horizontal lines: control. Error bars represent SD.
Figure 4.
Chitosan with specific molecular mass and N-deacetylation degree was essential in retaining the high anti-biofilm capacity of the gentamicin/chitosan combination. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 24 h. (a,b) Chitosan with different molecular mass (3000, 13,000 and 180,000 Da); (c,d) Chitosan with different N-deacetylation degrees (DD: 50%, 75% and 88%). White column: chitosan-antibiotic combination; Black column: chitosan alone; Column with italic lines: antibiotic alone; Column with horizontal lines: control. Error bars represent SD.
Figure 5.
Effects of the poly-l-lysine/gentamicin combination on disruption of L. monocytogenes biofilms. L. monocytogenes biofilms were exposed to poly-l-lysine (PLY, 200 µg/mL), gentamicin (5 µg/mL) or the respective mixture for 24 h. Biofilm mass (a) and viable cells (b) were quantified. Error bars represent SD.
Figure 5.
Effects of the poly-l-lysine/gentamicin combination on disruption of L. monocytogenes biofilms. L. monocytogenes biofilms were exposed to poly-l-lysine (PLY, 200 µg/mL), gentamicin (5 µg/mL) or the respective mixture for 24 h. Biofilm mass (a) and viable cells (b) were quantified. Error bars represent SD.
2.3. Effects of the Gentamicin/Chitosan Combination on Different Stages of L. monocytogenes Biofilms
To ask whether the anti-biofilm of the gentamicin/chitosan combination depended on different stages of biofilm formation, planktonic
L. monocytogenes were seeded in 96-well plates for 6, 12, 24, 48 or 72 h and then treated for 24 h with individual reagents or their combination. At all time-points tested, the gentamicin/chitosan combination displayed a stronger anti-biofilm and bactericidal activity than individual reagent alone did (
Figure 6a,b). A similar finding was also observed in the case of planktonic
L. monocytogenes exposed to reagents at the beginning (
Figure 6c,d). These data clearly indicate that the gentamicin-chitosan combination was effective in the inhibition of biofilm formation and disruption of immature or mature biofilms at different stages.
Figure 6.
Effects of the gentamicin/chitosan combination on different stages of L. monocytogenes biofilms. (a,b) Biofilms were preformed for 6, 12, 24, 48 and 72 h, and then exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 24 h; (c,d) L. monocytogenes were seeded in 96-well plates in the presence of chitosan (200 µg/mL), gentamicin (5 µg/mL) or the respective mixture for 24 h. Biofilm mass and viable cells were quantified. Error bars represent SD.
Figure 6.
Effects of the gentamicin/chitosan combination on different stages of L. monocytogenes biofilms. (a,b) Biofilms were preformed for 6, 12, 24, 48 and 72 h, and then exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 24 h; (c,d) L. monocytogenes were seeded in 96-well plates in the presence of chitosan (200 µg/mL), gentamicin (5 µg/mL) or the respective mixture for 24 h. Biofilm mass and viable cells were quantified. Error bars represent SD.
2.4. Mechanistic Insights into the Anti-Biofilm Capability of the Gentamicin/Chitosan Combination
It has been suggested that positive charges make aminoglycoside antibiotics bind to negatively charged polymers in the biofilm matrix, which might be responsible for the slow penetration of antibiotics [
18]. Since chitosan has been shown to penetrate biofilms due to the ability of cationic chitosan to disrupt negatively charged cell membranes as microbes settle on the surface [
6,
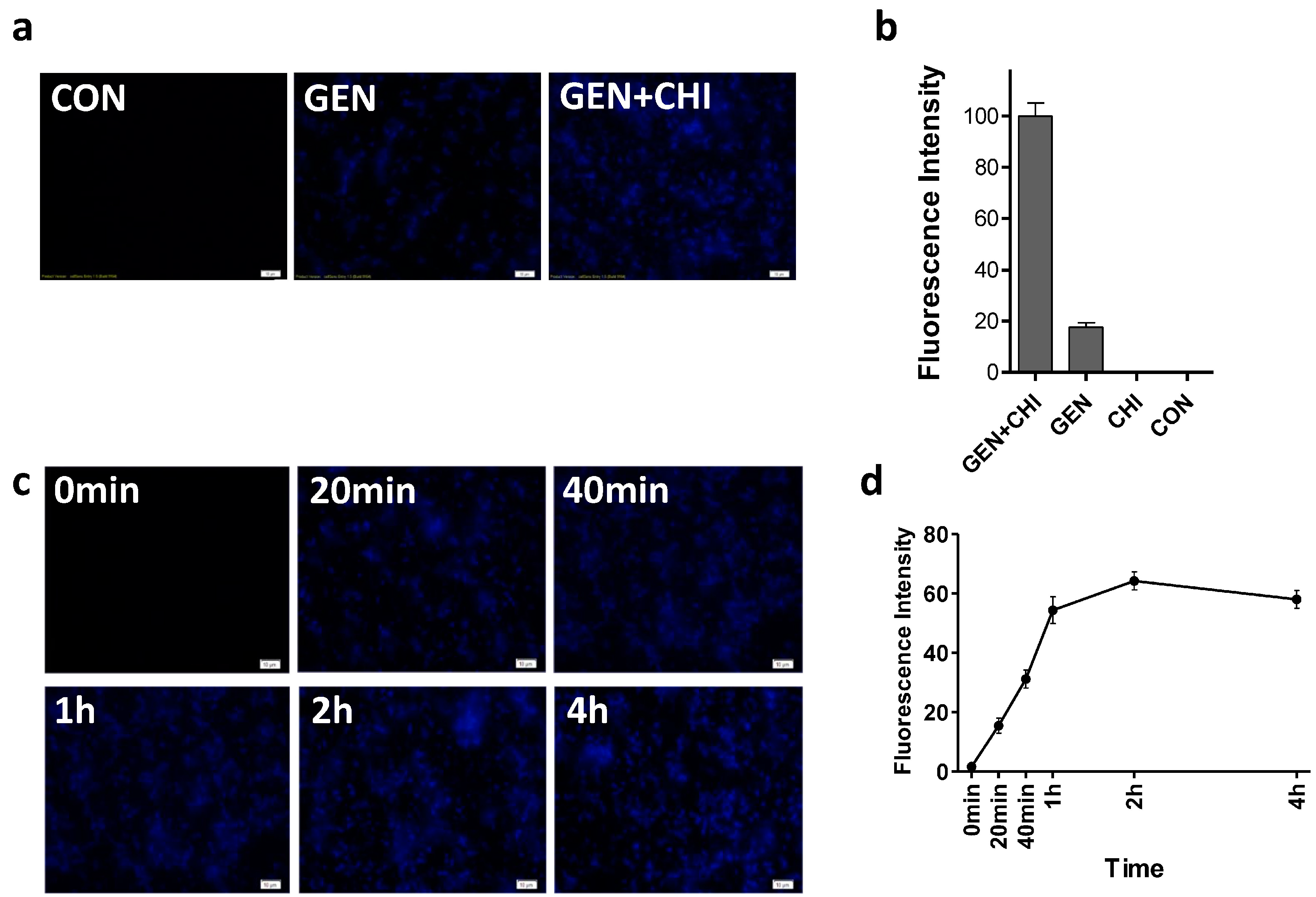
19], we attempted to see whether chitosan could make more gentamicin accessible into biofilms. Using a primary polyclonal antibody to gentamicin produced in rabbit and a second Dylight 405-conjugated goat anti-rabbit IgG, gentamicin residing in
L. monocytogenes biofilms was visualized. Biofilms exposed to gentamicin alone exhibited a weak blue fluorescence (
Figure 7a,b). In contrast, the intense blue fluorescence was observed in
L. monocytogenes biofilms treated with the gentamicin/chitosan combination. The time-course of immunofluorescence indicated that gentamicin residing in
L. monocytogenes biofilms increased very quickly in 1 h and then remained almost constant (
Figure 7c,d).
Figure 7.
Chitosan-facilitated gentamicin accessibility into L. monocytogenes biofilms. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) and the mixture for 1 h (a,b) or different periods (c,d). Biofilms incubated with TSB were used as control. Gentamicin residing in biofilms was examined by Immunofluorescence. Immunoreactivity was quantified by using Image Pro Plus. Scale bars = 10 µm.
Figure 7.
Chitosan-facilitated gentamicin accessibility into L. monocytogenes biofilms. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) and the mixture for 1 h (a,b) or different periods (c,d). Biofilms incubated with TSB were used as control. Gentamicin residing in biofilms was examined by Immunofluorescence. Immunoreactivity was quantified by using Image Pro Plus. Scale bars = 10 µm.
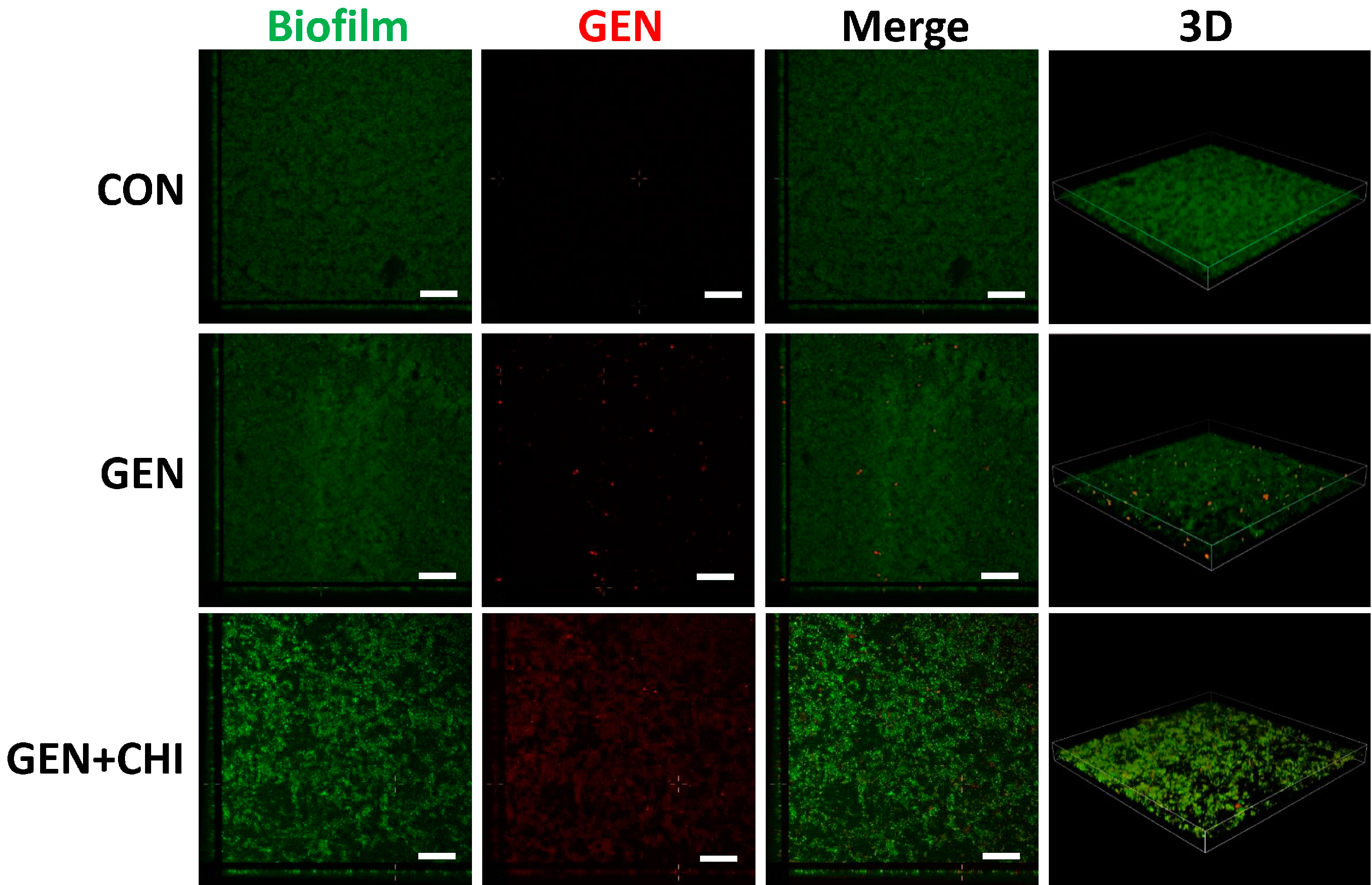
To evaluate whether chitosan made gentamicin penetration into or attachment to
L. monocytogenes biofilms, 5-(4,6-dichlorotriazinyl) aminofluorescein (5-DTAF, green fluorescence) was used to label bacteria and gentamicin was visualized with a primary polyclonal antibody followed by a second Dylight 594-conjugated goat anti-rabbit IgG (red fluorescence). 3D reconstruction from confocal microscopy indicated that chitosan enabled gentamicin penetration into
L. monocytogenes biofilms, instead of simple attachment to the surface (
Figure 8).
Figure 8.
CLSM analysis of gentamicin penetration into biofilms. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 1 h. The biofilm is depicted in green, while gentamicin is depicted in red. Scale bars indicates 50 µm.
Figure 8.
CLSM analysis of gentamicin penetration into biofilms. L. monocytogenes biofilms were exposed to chitosan (200 µg/mL), gentamicin (5 µg/mL) or the mixture for 1 h. The biofilm is depicted in green, while gentamicin is depicted in red. Scale bars indicates 50 µm.