Variants of the Low Oxygen Sensors EGLN1 and HIF-1AN Associated with Acute Mountain Sickness
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
| Demographic Characteristics and Physiological Parameters | AMS+ | AMS− | p Value |
|---|---|---|---|
| Age (year) | 22.7 ± 3.5 | 22.9 ± 3.8 | 0.840 |
| Height (cm) | 171.7 ± 4.9 | 171.7 ± 4.8 | 0.940 |
| Weight (kg) | 64.7 ± 10.4 | 64.7 ± 10.4 | 0.830 |
| Smoking rate (%) | 49.5 | 53.1 | 0.472 |
| Heart rate (bpm) | 86.5 ± 12.9 | 84.7 ± 12.4 | 0.156 |
| SaO2 (%) | 88.1 ± 2.90 | 88.8 ± 2.98 | 0.013 * |
2.2. Genotyping of Target Single Nucleotide Polymorphisms (SNPs)
2.3. Genetic Models of the Egl Nine Homolog 1 (EGLN1) and Hypoxia-Inducible Factor 1-α Inhibitor (HIF-1AN) Genes and the Risk of Acute Mountain Sickness (AMS)
| rs Number | Gene Name | Allele | Chromosome Position | MAF | HWE p |
|---|---|---|---|---|---|
| rs1054399 | HIF-1AN | C/T | Chr10:100552808 | 0.185 | 0.187 |
| rs11190613 | HIF-1AN | C/T | Chr10:100554240 | 0.187 | 0.188 |
| rs11292 | HIF-1AN | C/T | Chr10:100553850 | 0.187 | 0.188 |
| rs11816840 | HIF-1AN | C/G | Chr10:100549463 | 0.182 | 0.188 |
| rs3750633 | HIF-1AN | A/G | Chr10:100548457 | 0.187 | 0.188 |
| rs12406290 | EGLN1 | A/G | Chr 1:231423480 | 0.460 | 0.378 |
| rs12757362 | EGLN1 | C/G | Chr 1:231426746 | 0.060 | 1.000 |
| rs1339894 | EGLN1 | A/G | Chr 1:231424811 | 0.001 | 1.000 |
| rs1361384 | EGLN1 | A/G | Chr 1:231424487 | 0.005 | 0.970 |
| rs2009873 | EGLN1 | A/G | Chr 1:231363490 | 0.420 | 0.487 |
| rs2153364 | EGLN1 | A/G | Chr 1:231424474 | 0.530 | 0.532 |
| rs2486729 | EGLN1 | A/G | Chr 1:231399838 | 0.420 | 0.302 |
| rs2739513 | EGLN1 | A/G | Chr 1:231379455 | 0.430 | 0.541 |
| SNP Number | Model | Genotype | AMS+ [n (%)] | AMS− [n (%)] | OR (95% CI) | p-Value | AIC | BIC |
|---|---|---|---|---|---|---|---|---|
| rs12406290 | Codominant | AA | 65 (34.6) | 42 (22.7) | 1.00 | – | – | – |
| AG | 86 (45.7) | 96 (51.9) | 1.74 (1.07–2.84) | – | – | – | ||
| GG | 37 (19.7) | 47 (25.4) | 1.91 (1.06–3.41) | 0.04 | 522.9 | 554.3 | ||
| Dominant | AA | 65 (34.6) | 42 (22.7) | 1.00 | – | – | – | |
| AG + GG | 123 (65.4) | 143 (77.3) | 1.79 (1.13–2.84) | 0.012 * | 521 | 548.5 | ||
| Recessive | AA + AG | 151 (80.3) | 138 (74.6) | 1.00 | – | – | – | |
| GG | 37 (19.7) | 47 (25.4) | 1.34 (0.82–2.19) | 0.24 | 526 | 553.5 | ||
| Overdominant | AA + GG | 102 (54.3) | 89 (48.1) | 1.00 | – | – | – | |
| AG | 86 (45.7) | 96 (51.9) | 1.31 (0.87–1.97) | 0.19 | 525.7 | 553.1 | ||
| Log-additive | – | – | – | 1.40 (1.05–1.87) | 0.022 | 522.1 | 549.6 | |
| rs2153364 | Codominant | AA | 63 (33.5) | 38 (21.1) | 1.00 | – | – | – |
| AG | 88 (46.8) | 95 (52.8) | 1.85 (1.12–3.05) | – | – | – | ||
| GG | 37 (19.7) | 47 (26.1) | 2.14 (1.18–3.88) | 0.019 | 514.4 | 545.6 | ||
| Dominant | AA | 63 (33.5) | 38 (21.1) | 1.00 | – | – | – | |
| AG + GG | 125 (66.5) | 142 (78.9) | 1.94 (1.20–3.11) | 0.0057 * | 512.7 | 540 | ||
| Recessive | AA + AG | 151 (80.3) | 133 (73.9) | 1.00 | – | – | – | |
| GG | 37 (19.7) | 47 (26.1) | 1.43 (0.88–2.34) | 0.15 | 518.2 | 545.6 | ||
| Overdominant | AA + GG | 100 (53.2) | 85 (47.2) | 1.00 | – | – | – | |
| AG | 88 (46.8) | 95 (52.8) | 1.30 (0.86–1.96) | 0.22 | 518.8 | 546.1 | ||
| Log-additive | – | – | – | 1.47 (1.10–1.98) | 0.0095 | 513.6 | 540.9 |
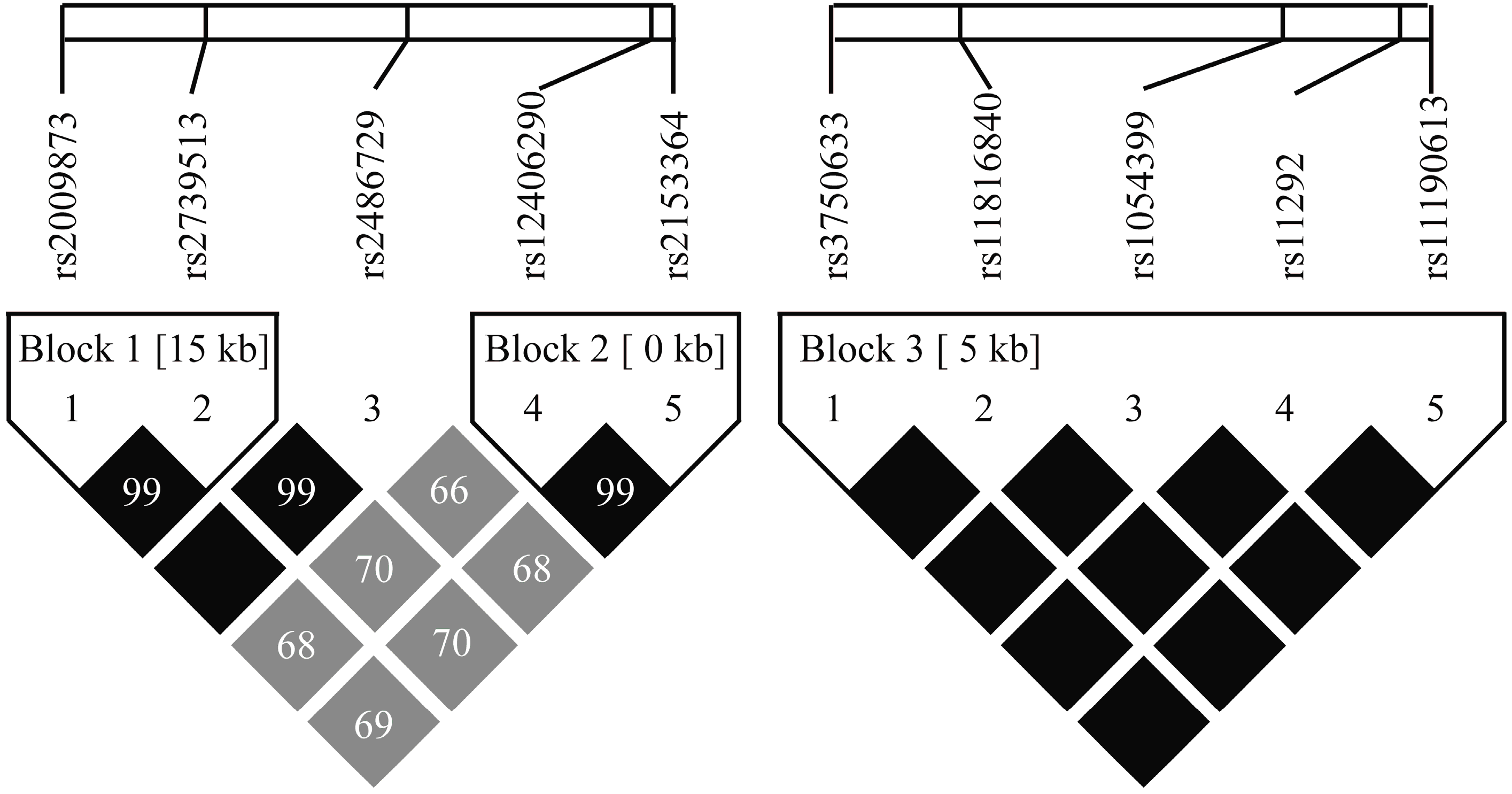
2.4. HIF-1AN and EGLN1 Haplotypes and the Risk of AMS
3. Discussion
| Gene | Blocks | Haplotype a | Frequency of AMS− (%) | Frequency of AMS+ (%) | p-Value | Odds Ratio 95% CI |
|---|---|---|---|---|---|---|
| EGLN1 | Global haplotype association test p = 0.004 c | |||||
| Block 1 | AA | 60.9 | 54.75 | – | 1.00 b | |
| GG | 39.1 | 43.63 | 0.12 | 1.25 (0.94–1.66) | ||
| Global haplotype association test p = 0.036 c | ||||||
| Block 2 | AA | 56.83 | 48.92 | – | 1.00 b | |
| GG | 42.1 | 50.79 | 0.029 * | 1.38 (1.04–1.85) | ||
| HIF-1AN | Global haplotype association test p = 0.7 c | |||||
| Block 3 | CTTGG | 90.26 | 90.63 | – | 1.00 b | |
| TCACA | 9.74 | 9.37 | 0.7 | 0.91 (0.57–1.46) | ||
4. Methods
4.1. Subjects
4.2. AMS Score and Physical Signs
4.3. SNP Selection
4.4. Genotyping
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roach, R.; Bärtsch, P.; Hackett, P.; Oelz, O. The Lake Louise AMS scoring consensus committee. The Lake Louise acute mountain sickness scoring system. In Hypoxia and Molecular Medicine; Sutton, J.R., Houston, C.S., Coates, G., Eds.; Queen City Printers: Burlington, VT, USA, 1993; pp. 272–274. [Google Scholar]
- MacInnis, M.J.; Koehle, M.S.; Rupert, J.L. Evidence for a genetic basis for altitude illness: 2010 Update. High Alt. Med. Biol. 2010, 11, 349–368. [Google Scholar] [PubMed]
- Wilson, M.H.; Newman, S.; Imray, C.H. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009, 8, 175–191. [Google Scholar] [PubMed]
- Semenza, G.L. Life with oxygen. Science 2007, 318, 62–64. [Google Scholar] [PubMed]
- Wu, T.; Li, S.; Ward, M.P. Tibetans at extreme altitude. Wilderness Environ. Med. 2005, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Ding, S.Q.; Liu, J.L.; Yu, M.T.; Jia, J.H.; Duan, J.Q.; Chai, Z.C.; Dai, R.C.; Zhang, S.L.; Liang, B.Z.; et al. Reduced incidence and severity of acute mountain sickness in Qinghai-Tibet railroad construction workers after repeated 7-month exposures despite 5-month low altitude periods. High Alt. Med. Biol. 2009, 10, 221–232. [Google Scholar] [CrossRef]
- Xiang, K.; Ouzhuluobu; Peng, Y.; Yang, Z.; Zhang, X.; Cui, C.; Zhang, H.; Li, M.; Zhang, Y.; Bianba; et al. Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation. Mol. Biol. Evol. 2013, 30, 1889–1898. [Google Scholar] [CrossRef]
- Yi, X.; Liang, Y.; Huerta-Sanchez, E.; Jin, X.; Cuo, Z.X.; Pool, J.E.; Xu, X.; Jiang, H.; Vinckenbosch, N.; Korneliussen, T.S.; et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 2010, 329, 75–78. [Google Scholar] [CrossRef]
- Ji, L.D.; Qiu, Y.Q.; Xu, J.; Irwin, D.M.; Tam, S.C.; Tang, N.L.; Zhang, Y.P. Genetic adaptation of the hypoxia-inducible factor pathway to oxygen pressure among Eurasian human populations. Mol. Biol. Evol. 2012, 29, 3359–3570. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.; Chen, Y.; Luo, Y. Dexamethasone for the prevention of acute mountain sickness: Systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, G.S.; Dickson, K.S.; Gray, N.K. Regulation of mRNA translation by 5'- and 3'-UTR-binding factors. Trends Biochem. Sci. 2003, 28, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C. Micro RNAs are complementary to 3'-UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genetics 2002, 30, 363–364. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Yang, Y.; Tan, J.; Lou, H.; Jin, W.; Yang, L.; Pan, X.; Wang, J.; Shen, Y.; et al. A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol. Biol. Evol. 2011, 28, 1003–1011. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Guo, G.; Zhu, G.; Sun, W.; Yin, C.; Ren, X.; Wang, T.; Liu, M. Association of arterial oxygen saturation and acute mountain sickness susceptibility: A meta-analysis. Cell Biochem. Biophys. 2014, 70, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Huck, G.; Stiehl, D.P.; Jelkmann, W.; Hellwig-Burgel, T. Dexamethasone impairs hypoxia-inducible factor-1 function. Biochem. Biophys. Res. Commun. 2008, 372, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Guzy, R.D.; Schumacker, P.T. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006, 91, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Pagani, L.; Ayub, Q.; MacArthur, D.G.; Xue, Y.; Baillie, J.K.; Chen, Y.; Kozarewa, I.; Turner, D.J.; Tofanelli, S.; Bulayeva, K.; et al. High altitude adaptation in Daghestani populations from the Caucasus. Hum. Genet. 2012, 131, 423–433. [Google Scholar] [CrossRef]
- Bigham, A.; Bauchet, M.; Pinto, D.; Mao, X.; Akey, J.M.; Mei, R.; Scherer, S.W.; Julian, C.G.; Wilson, M.J.; Lopez Herraez, D.; et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 2010, 6, e1001116. [Google Scholar] [CrossRef]
- Song, D.; Li, L.S.; Arsenault, P.R.; Tan, Q.; Bigham, A.W.; Heaton-Johnson, K.J.; Master, S.R.; Lee, F.S. Defective Tibetan PHD2 binding to p23 links high altitude adaptation to altered oxygen sensing. J. Biol. Chem. 2014, 289, 14656–14665. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, F.R.; Huff, C.; Myllymaki, M.; Olenchock, B.; Swierczek, S.; Tashi, T.; Gordeuk, V.; Wuren, T.; Rili, G.; McClain, D.A.; et al. A genetic mechanism for Tibetan high-altitude adaptation. Nat. Genet. 2014, 46, 951–956. [Google Scholar] [CrossRef]
- Zhang, N.; Fu, Z.; Linke, S.; Chicher, J.; Gorman, J.J.; Visk, D.; Haddad, G.G.; Poellinger, L.; Peet, D.J.; Powell, F.; et al. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 2010, 11, 364–378. [Google Scholar] [CrossRef]
- Jacobs, E.J.; Hsing, A.W.; Bain, E.B.; Stevens, V.L.; Wang, Y.; Chen, J.; Chanock, S.J.; Zheng, S.L.; Xu, J.; Thun, M.J.; et al. Polymorphisms in angiogenesis-related genes and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 972–977. [Google Scholar] [CrossRef]
- Peng, H.; Hamanaka, R.B.; Katsnelson, J.; Hao, L.L.; Yang, W.; Chandel, N.S.; Lavker, R.M. MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J. 2012, 26, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.Z.; Zhang, J.H.; Gao, X.B.; Li, M.; Yu, J.; Liu, X.; Dong, J.Q.; Chen, G.Z.; Huang, L. Risk factors for high-altitude headache upon acute high-altitude exposure at 3700 m in young Chinese men: A cohort study. J. Headache Pain 2013, 14, 35. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Sole, X.; Guino, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liang, K.Y. Sample size calculations for studies with correlated observations. Biometrics 1997, 53, 937–947. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, E.; Zhang, J.; Jin, J.; Qin, J.; Li, H.; Huang, L. Variants of the Low Oxygen Sensors EGLN1 and HIF-1AN Associated with Acute Mountain Sickness. Int. J. Mol. Sci. 2014, 15, 21777-21787. https://doi.org/10.3390/ijms151221777
Zhang E, Zhang J, Jin J, Qin J, Li H, Huang L. Variants of the Low Oxygen Sensors EGLN1 and HIF-1AN Associated with Acute Mountain Sickness. International Journal of Molecular Sciences. 2014; 15(12):21777-21787. https://doi.org/10.3390/ijms151221777
Chicago/Turabian StyleZhang, Enhao, Jihang Zhang, Jun Jin, Jun Qin, Huijie Li, and Lan Huang. 2014. "Variants of the Low Oxygen Sensors EGLN1 and HIF-1AN Associated with Acute Mountain Sickness" International Journal of Molecular Sciences 15, no. 12: 21777-21787. https://doi.org/10.3390/ijms151221777




