Serum Calreticulin Is a Negative Biomarker in Patients with Alzheimer’s Disease
Abstract
:1. Introduction
2. Results and Discussion
2.1. Demographic Characters
| AD Patients (n = 128) | Healthy Subjects (n = 130) | Statistical Value | p-Value | |
|---|---|---|---|---|
| Gender (male/female) | 58/70 | 60/70 | 7.3 a | 0.825 |
| Age (years) | 65.4 ± 21.0 | 65.1 ± 21.0 | 0.21 b | 0.933 |
| BMI (kg/m2) | 24.9 ± 4.8 | 25.6 ± 5.1 | 0.34 b | 0.900 |
| Diabetes/Non-diabetes | 12/116 | 12/118 | 91.9 a | 0.898 |
| High blood pressure/Normal blood pressure | 28/100 | 30/100 | 84.0 a | 0.825 |
| Coronary heart disease/Non-coronary heart disease | 26/102 | 27/103 | 89.8 a | 0.875 |
| Smoker/Non-smoker | 48/80 | 50/80 | 82.8 a | 0.923 |
| Drinker/Non-drinker | 40/88 | 43/87 | 64.9 a | 0.881 |
| Educational years | 11.6 ± 3.2 | 10.9 ± 4.1 | 1.2 b | 0.266 |
| Left-handed/Right handed | 12/116 | 14/116 | 119.0 a | 0.872 |
| During of illness (years) | 9.9 ± 6.6 | - | - | - |
| Pathological MRI (%) | 77 (60%) | - | - | - |
| Neuropsychological impairment (2 T-scores 35) (%) | 90 (70%) | - | - | - |
| MMSE scores | 19.2 ± 4.8 | 29.6 ± 2.6 | 7.71 b | 0.001 |
| CDR scale | - | - | ||
| CDR 0 | 0 | 130 | - | |
| CDR 0.5 | 60 | 0 | - | |
| CDR 1 | 38 | 0 | - | |
| CDR 2+ | 30 | 0 | - |
2.2. The mRNA Levels of Serum Calreticulin in AD Patients and Healthy Subjects
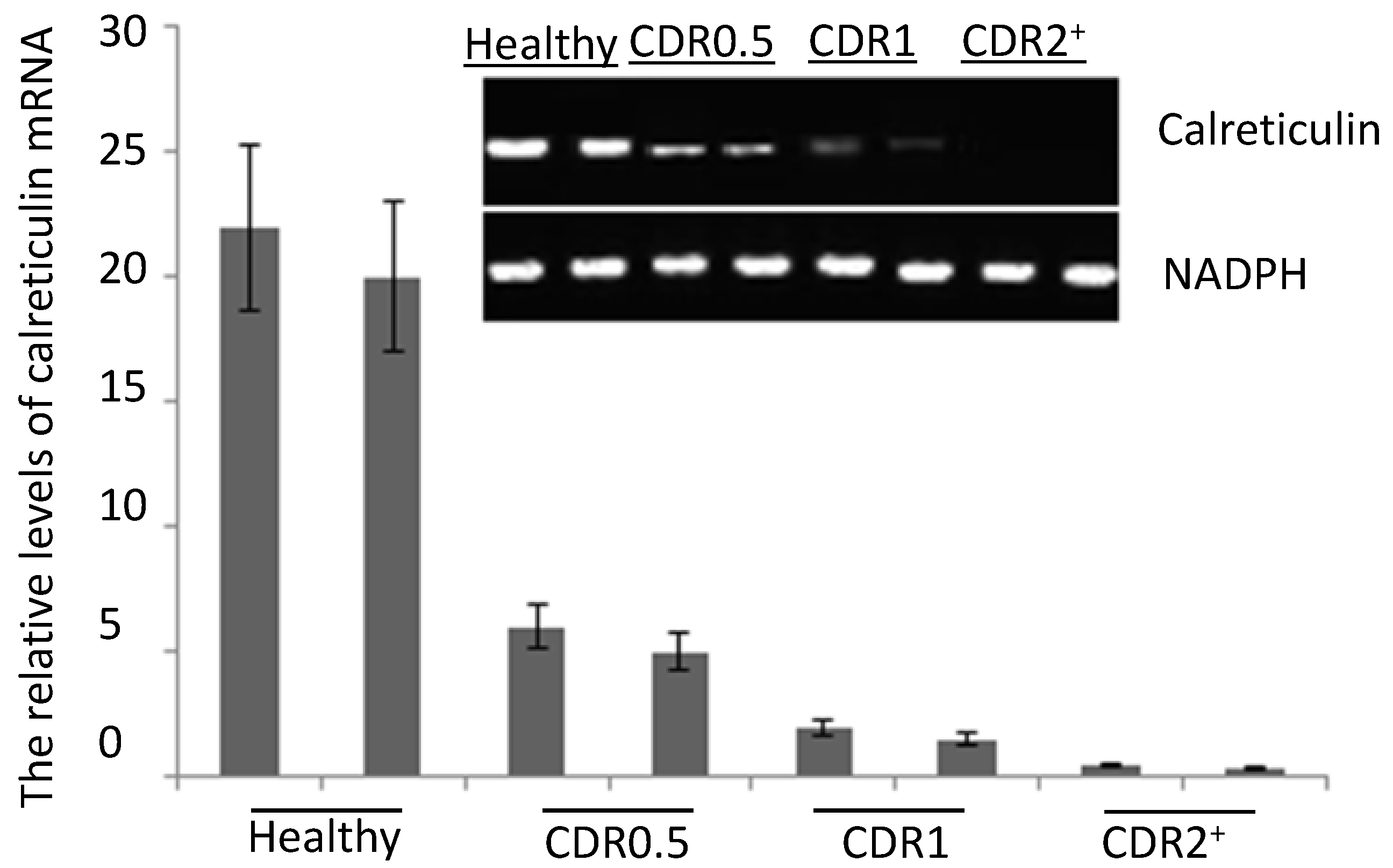
2.3. ELISA Analysis

2.4. Western Blot Analysis

3. Experimental Section
3.1. Participants
3.2. mRNA Extraction
3.3. Real-Time qRT-PCR
3.4. ELISA
3.5. Western Blot
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Guzman-Velez, E.; Feinstein, J.S.; Tranel, D. Feelings without memory in Alzheimer disease. Cogn. Behav. Neurol. 2014, 27, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Sato, N.; Morishita, R. Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: Relevance to pathogenesis and therapy. Front. Aging Neurosci. 2014, 6, 171. [Google Scholar] [PubMed]
- Malkki, H. Alzheimer disease: Effects of the APOE epsilon4 allele on brain development. Nat. Rev. Neurol. 2014, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Rassas, A.A.; Mrabet Khiari, H.; Hadj Fredj, S.; Sahnoun, S.; Batti, H.; Zakraoui, N.O.; Cherif, A.; Anane, N.; Ben Ali, N.; Messaoud, T.; et al. High APOE epsilon 4 allele frequencies associated with Alzheimer disease in a Tunisian population. Neurol. Sci. 2012, 33, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Redwine, L.; Mills, P.J.; Sada, M.; Dimsdale, J.; Patterson, T.; Grant, I. Differential immune cell chemotaxis responses to acute psychological stress in Alzheimer caregivers compared to non-caregiver controls. Psychosom. Med. 2004, 66, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Anesthesia-Alzheimer disease link probed. JAMA 2007, 297, 1760. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.M.; Chen, K.; Kaszniak, A.W.; Lee, W.; Alexander, G.E.; Bandy, D.; Fleisher, A.S.; Caselli, R.J.; Reiman, E.M. Higher serum glucose levels are associated with cerebral hypometabolism in Alzheimer regions. Neurology 2013, 80, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, M.N.; Chen, Z.; Zhong, C. Environmental factors in the development and progression of late-onset Alzheimer’s disease. Neurosci. Bull. 2014, 30, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Qian, J.; Monsell, S.E.; Frosch, M.P.; Betensky, R.A.; Hyman, B.T. Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. J. Neuropathol. Exp. Neurol. 2013, 72, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Hampel, H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003, 2, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Dekosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delocourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Afshari, F.M.; Rajput, A. How to interpret a blood contaminated traumatic spinal tap? Neurology 2010, 74, A211–A211. [Google Scholar]
- Watabe-Rudolph, M.; Song, Z.; Lausser, L.; Schnack, C.; Begus-Nahrmann, Y.; Scheithauer, M.O.; Rettinger, G.; Otto, M.; Tumani, H.; Thal, D.R.; et al. Chitinase enzyme activity in CSF is a powerful biomarker of Alzheimer disease. Neurology 2012, 78, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Trueba-Saiz, A.; Cavada, C.; Fernandez, A.M.; Leon, T.; Gonzalez, D.A.; Fortea Ormaechea, J.; Lleo, A.; del Ser, T.; Nunez, A.; Torres-Aleman, I. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl. Psychiatry 2013, 3, e330. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Yang, Y.H.; Lu, C.Y.; Jong, S.B.; Chen, L.J.; Lin, Y.F.; Wu, S.J.; Chu, P.Y.; Chung, T.W.; Tyan, Y.C. Activity-dependent neuroprotector homeobox protein: A candidate protein identified in serum as diagnostic biomarker for Alzheimer’s disease. J. Proteomics 2012, 75, 3617–3629. [Google Scholar] [CrossRef] [PubMed]
- Ranford, J.C.; Coates, A.R.; Henderson, B. Chaperonins are cell-signalling proteins: The unfolding biology of molecular chaperones. Expert Rev. Mol. Med. 2000, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Matsunami, H. Calreticulin chaperones regulate functional expression of vomeronasal type 2 pheromone receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 16651–16656. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, J.; Fujii, A.; Fujino, Y.; Tsujioka, Y.; Takahashi, M.; Tsuboi, Y.; Wada, I.; Yamada, T. Different expression of calreticulin and immunoglobulin binding protein in Alzheimer’s disease brain. Acta Neuropathol. 2000, 100, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Duus, K.; Hansen, P.R.; Houen, G. Interaction of calreticulin with amyloid beta peptide 1–42. Protein Pept. Lett. 2008, 15, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hanenberg, M.; McAfoose, J.; Kulic, L.; Welt, T.; Wirth, F.; Parizek, P.; Strobel, L.; Cattepoel, S.; Spani, C.; Derungs, R.; et al. Amyloid-beta peptide-specific DARPins as a novel class of potential therapeutics for Alzheimer disease. J. Biol. Chem. 2014, 289, 27080–27089. [Google Scholar] [CrossRef] [PubMed]
- Besser, L.M.; Gill, D.P.; Monsell, S.E.; Brenowitz, W.; Meranus, D.H.; Kukull, W.; Gustafson, D.R. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2014, 28, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, E.D.; Townley, R.A.; Honea, R.A.; Burns, J.M.; Alzheimer’s Disease Neuroimaging, I. Alzheimer disease biomarkers are associated with body mass index. Neurology 2011, 77, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Sebastiao, I.; Candeias, E.; Santos, M.S.; de Oliveira, C.R.; Moreira, P.I.; Duarte, A.I. Insulin as a bridge between type 2 diabetes and alzheimer disease—How anti-diabetics could be a solution for dementia. Front. Endocrinol. (Lausanne) 2014, 5, 110. [Google Scholar]
- Butterfield, D.A.; di Domenico, F.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta 2014, 1842, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Helkala, E.L.; Laakso, M.P.; Hanninen, T.; Hallikainen, M.; Alhainen, K.; Iivonen, S.; Mannermaa, A.; Tuomilehto, J.; Nissinen, A.; et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 2002, 137, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Arlt, S.; Kontush, A.; Muller-Thomsen, T.; Beisiegel, U. Lipid peroxidation as a common pathomechanism in coronary heart disease and Alzheimer disease. Z. Gerontol .Geriatr. 2001, 34, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Garrido, G.J.; Lautenschlager, N.T.; Hulse, G.K.; Jamrozik, K.; Flicker, L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am. J. Geriatr. Psychiatry 2008, 16, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wiscott, R.; Kopera-Frye, K.; Seifert, L. Possible consequences of social drinking in the early stages of Alzheimer disease. Geriatr. Nurs. 2001, 22, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.; Laks, J.; Engelhardt, E.; Conn, D. Alcohol abuse in an elderly woman taking donepezil for Alzheimer disease. J. Clin. Psychopharmacol. 2006, 26, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Pradier, C.; Sakarovitch, C.; Le Duff, F.; Layese, R.; Metelkina, A.; Anthony, S.; Tifratene, K.; Robert, P. The mini mental state examination at the time of Alzheimer’s disease and related disorders diagnosis, according to age, education, gender and place of residence: A cross-sectional study among the French National Alzheimer database. PLoS One 2014, 9, e103630. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.M.; Mintun, M.A.; D’Angelo, G.; Xiong, C.; Grant, E.A.; Morris, J.C. Alzheimer disease and cognitive reserve: Variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch. Neurol. 2008, 65, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- De Leon, M.J.; la Regina, M.E.; Ferris, S.H.; Gentes, C.I.; Miller, J.D. Reduced incidence of left-handedness in clinically diagnosed dementia of the Alzheimer type. Neurobiol. Aging 1986, 7, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, W.H. Presbyophrenia (Alzheimer’s disease). Proc. R. Soc. Med. 1913, 6, 13–14. [Google Scholar] [PubMed]
- Kemnitz, J.W. Calorie restriction and aging in nonhuman primates. ILAR J. 2011, 52, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Higashino, A.; Kageyama, T.; Kantha, S.S.; Terao, K. Detection of elevated antibody against calreticulin by ELISA in aged cynomolgus monkey plasma. Zool. Sci. 2011, 28, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Warmlander, S.K.; Graslund, A.; Abrahams, J.P. Non-chaperone proteins can inhibit aggregation and cytotoxicity of Alzheimer amyloid beta peptide. J. Biol. Chem. 2014, 289, 27766–27775. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A. Elevated serum DDE and risk for Alzheimer disease. JAMA Neurol. 2014, 71, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Squitti, R.; Ghidoni, R.; Siotto, M.; Ventriglia, M.; Benussi, L.; Paterlini, A.; Magri, M.; Binetti, G.; Cassetta, E.; Caprara, D.; et al. Value of serum nonceruloplasmin copper for prediction of mild cognitive impairment conversion to Alzheimer disease. Ann. Neurol. 2014, 75, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Bible, E. Alzheimer disease: High serum levels of the pesticide metabolite DDE—A potential environmental risk factor for Alzheimer disease. Nat. Rev. Neurol. 2014, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Roy, A.; Shalat, S.L.; von Stein, R.T.; Hossain, M.M.; Buckley, B.; Gearing, M.; Levey, A.I.; German, D.C. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 2014, 71, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.A.; Graham, L.V.; Pallero, M.A.; Murphy-Ullrich, J.E. Calreticulin regulates transforming growth factor-beta-stimulated extracellular matrix production. J. Biol. Chem. 2013, 288, 14584–14598. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.P.; Williams, D.B. Delineation of the lectin site of the molecular chaperone calreticulin. Cell Stress Chaperones 2005, 10, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Bosco, P.; Ferri, R.; Salluzzo, M.G.; Castellano, S.; Signorelli, M.; Nicoletti, F.; Nuovo, S.D.; Drago, F.; Caraci, F. Role of the transforming-growth-factor-beta1 gene in late-onset Alzheimer’s disease: Implications for the treatment. Curr. Genomics 2013, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Yu, J.T.; Jiang, T.; Tan, L. Endoplasmic reticulum dysfunction in Alzheimer’s disease. Mol. Neurobiol. 2014. [Google Scholar] [CrossRef]
- Schworer, R.; Zubkova, O.V.; Turnbull, J.E.; Tyler, P.C. Synthesis of a targeted library of heparan sulfate hexa- to dodecasaccharides as inhibitors of beta-secretase: Potential therapeutics for Alzheimer’s disease. Chemistry 2013, 19, 6817–6823. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.R. Aluminum disruption of calcium homeostasis and signal transduction resembles change that occurs in aging and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 255–273. [Google Scholar] [PubMed]
- Bibi, A.; Agarwal, N.K.; Dihazi, G.H.; Eltoweissy, M.; van Nguyen, P.; Mueller, G.A.; Dihazi, H. Calreticulin is crucial for calcium homeostasis mediated adaptation and survival of thick ascending limb of Henle’s loop cells under osmotic stress. Int. J. Biochem. Cell Biol. 2011, 43, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Dreses-Werringloer, U.; Lambert, J.C.; Vingtdeux, V.; Zhao, H.; Vais, H.; Siebert, A.; Jain, A.; Koppel, J.; Rovelet-Lecrux, A.; Hannequin, D.; et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell 2008, 133, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Marissal, N.; Moumen, A.; Sunyach, C.; Pellegrino, C.; Dudley, K.; Henderson, C.E.; Raoul, C.; Pettmann, B. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J. Neurosci. 2012, 32, 4901–4912. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Tang, D.; Lu, S.Y.; Jiang, Z.F. Endoplasmic reticulum stress as a novel neuronal mediator in Alzheimer’s disease. Neurol. Res. 2014. [Google Scholar] [CrossRef]
- Duan, L.; Bhattacharyya, B.J.; Belmadani, A.; Pan, L.; Miller, R.J.; Kessler, J.A. Stem cell derived basal forebrain cholinergic neurons from Alzheimer’s disease patients are more susceptible to cell death. Mol. Neurodegener. 2014, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Tamaoka, A. Alzheimer’s disease: Definition and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA). Nihon Rinsho 2011, 69, 240–245. [Google Scholar] [PubMed]
- Haris, M.; Singh, A.; Cai, K.; McArdle, E.; Fenty, M.; Davatzikos, C.; Trojanowski, J.Q.; Melhem, E.R.; Clark, C.M.; Borthakur, A. T(1rho) MRI in Alzheimer’s disease: Detection of pathological changes in medial temporal lobe. J. Neuroimaging 2011, 21, e86–90. [Google Scholar] [CrossRef] [PubMed]
- Reichenberg, A.; Harvey, P.D.; Bowie, C.R.; Mojtabai, R.; Rabinowitz, J.; Heaton, R.K.; Bromet, E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr. Bull. 2009, 35, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Q.; Cao, Y.; Gao, J. Serum Calreticulin Is a Negative Biomarker in Patients with Alzheimer’s Disease. Int. J. Mol. Sci. 2014, 15, 21740-21753. https://doi.org/10.3390/ijms151221740
Lin Q, Cao Y, Gao J. Serum Calreticulin Is a Negative Biomarker in Patients with Alzheimer’s Disease. International Journal of Molecular Sciences. 2014; 15(12):21740-21753. https://doi.org/10.3390/ijms151221740
Chicago/Turabian StyleLin, Qiao, Yunpeng Cao, and Jie Gao. 2014. "Serum Calreticulin Is a Negative Biomarker in Patients with Alzheimer’s Disease" International Journal of Molecular Sciences 15, no. 12: 21740-21753. https://doi.org/10.3390/ijms151221740




