Potential Application of Curcumin and Its Analogues in the Treatment Strategy of Patients with Primary Epithelial Ovarian Cancer
Abstract
:1. Introduction
Biological Response Modifiers in Systemic Therapy of Ovarian Cancer
2. Curcumin—General Description and Pharmacokinetics
2.1. Anticancer Properties of Curcumin

| Curcumin Concentration | Cancer Cell Line | Assessed Parameter | Outcome Measures | Reference |
|---|---|---|---|---|
| 50 μM | SKOV3 | ↓MMP-9, ↓CD44, ↓osteopontin | ↓Invasion of SKOV3 cells | [50] |
| 40 μM | HO-8910 | ↓Bcl-2, ↓Bcl-xL, ↓pro caspase-3, ↑ p53, ↑Bax | ↓Cell growth, ↑apoptosis | [51] |
| 10–50 μM | A2780 | ↓Bcl-2, ↓p53, no changes in MDM2, ↓NFκB, ↑caspase-3 | ↓Cell growth, ↑apoptosis | [52,53] |
| 10–50 μM | CaOV3 | ↑AMPK, ↑p38, ↑p53 phosphorylation | ↓Proliferation, ↑apoptosis | [54] |
| 0.1–100 μM | 2008, C13 | ↑ROS, ↓glutathione | ↓Cell proliferation, ↑apoptosis with curcumin alone, synergistic effect with cisplatin or oxaliplatin, ↓cell cycle via synergistic effect with cisplatin or oxaliplatin, ↑sensitivity to cisplatin in resistant C13 cells | [55] |
| 40 μM | CaOV3 | ↓AQP-3 | ↓EGF-induced cell migration | [56] |
| 3.12–50 μM | OVCA420, OVCA429 | ↑Caspase-3, ↓IL-6, ↓STAT-3 phosphorylation, ↓p-JAK-1 and p-JAK-2, ↓PIAS-3, SOCS-3 | ↓Cells growth, ↑apoptosis | [57] |
| 2–80 μM | HEY, OVCA429, OCC1, SKOV3 | ↓Procaspase-3, ↑active caspase-3, ↓PARP-1 substrate, ↑cytochrome c, ↓Bcl-2, surviving, ↓PI3K/Akt pathway, ↑p38 MAPK pathway | ↑Apoptosis: ↓Cell densities, marked cell rounding, long cytoplasmic projections, membrane blebs, DNA fragmentation | [58] |
| 10–60 μM | SKOV3 | ↑miR-9, ↓phosphorylation of AKT and FOXO1 | ↓Cell proliferation, ↑apoptosis, ↓ cells growth | [59] |
| 5–10 μM | PA-1 OVCAR-3 | ↓LPA-induced STAT3 phosphorylation | ↓LPA-induced IL-6 and IL-8 production, ↓cell motility | [60] |
2.2. Curcumin Analogues in Ovarian Cancer Target Treatment
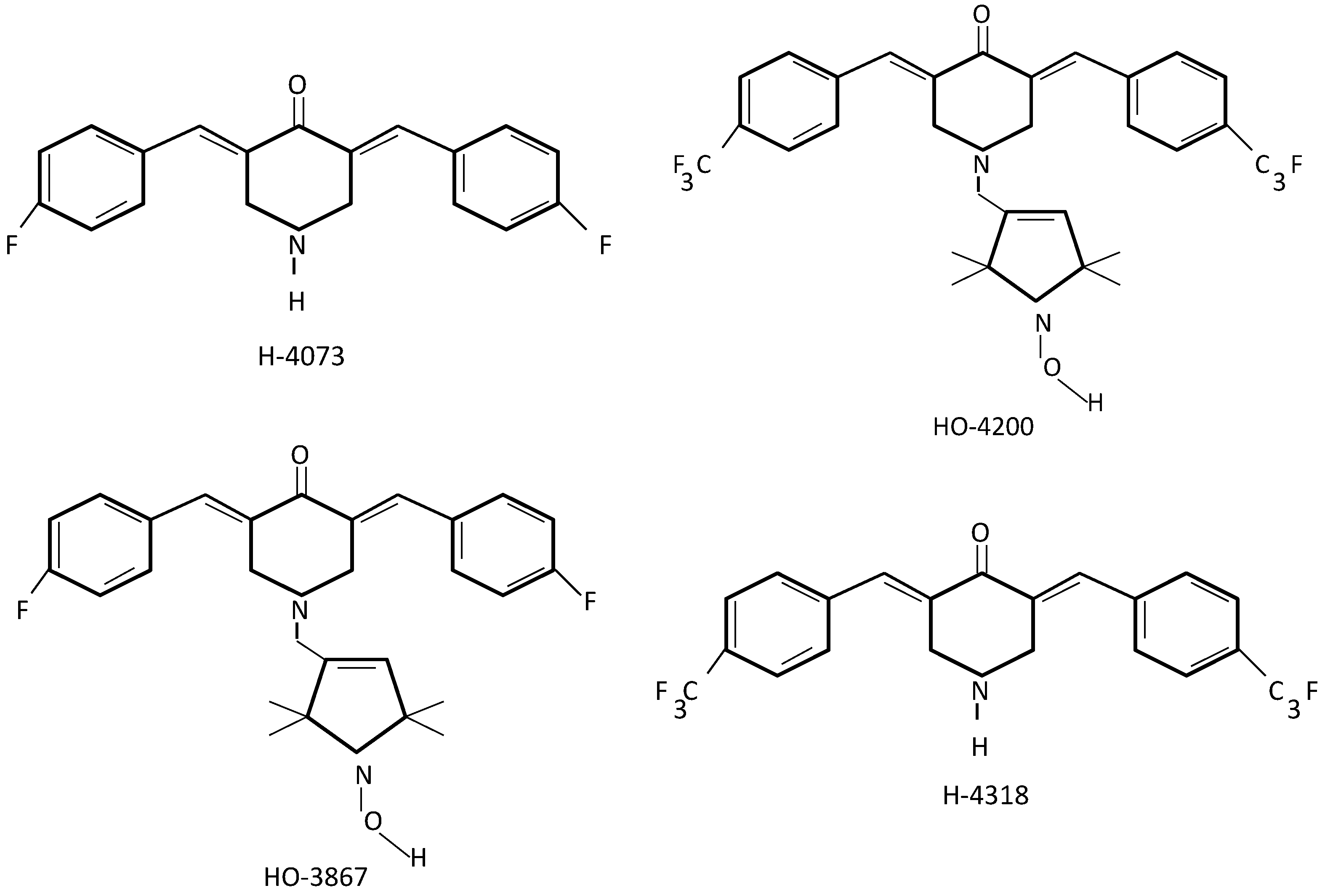
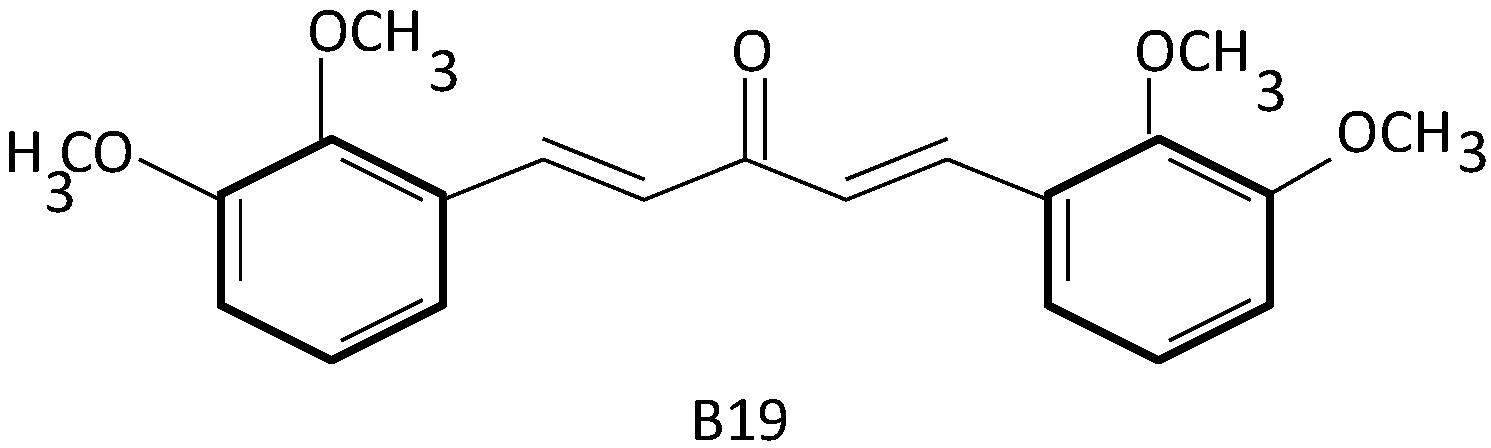
2.3. Curcumin and Its Analogues in Ovarian Cancer Drug Resistance
2.4. Curcumin and Clinical Studies
| Cancer | Inclusion Criteria | Intervention | Outcome Measures | Phase | Clinical Trial Number |
|---|---|---|---|---|---|
| Endometrial carcinoma | Recurrent with no life-threatening metastases | Curcuphyt (curcumin analogue), standard chemotherapy | Anti-inflammatory effect | Recruiting, 2 | NCT02017353 |
| Prostate cancer | Life expectancy > 5 years | Curcumin, curcumin analogue BCM95, radiotherapy | Radiosensitizing and radioprotective effect | Recruiting, data not shown | NCT01917890 |
| Breast cancer | Completed chemotherapy | Curcumin, radiotherapy | Level of NF-κβ DNA binding | Not yet recruiting, 2 | NCT01740323 |
| Colorectal cancer | Familial adenomatous polyposis, stage 0 | Curcumin | Number and size of polyps, side effect of curcumin, involved pathways | Recruiting | NCT00641147 |
| Lymphocytic lymphoma Lymphocytic leukemia | Stage 0,1,2 | Curcumin, vitamin D | Overall survival response, Overall survival rates, progression free survival | Not yet recruiting, 2 | NCT02100423 |
| Prostate cancer | Metastatic cancer, castration resistant | Curcumin, Taxotere | Time to progression, tumor response by RECIST criteria | Recruiting, 2 | NCT02095717 |
| Colorectal cancer | Metastatic cancer | Curcumin, chemotherapy | Neuropathic side-effect, disease response, disease survival, level of biomarkers | Recruiting, 1, 2 | NCT01490996 |
| Colon cancer | First diagnosed primary tumor without any treatment | Curcumin, curcumin conjugated with plant exosomes (Exo-cur) | Efficiency of plant exosomes in delivering curcumin to normal colon tissue and colon tumor | Recruiting, 1 | NCT01294072 |
| Intestinal adenomas | Familial adenomatous polyposis with an intact colon or with surgery | Curcumin (Calcumin) | Regression of intestinal adenomas | Recruiting, data not shown | NCT00927485 |
| Solid tumors | Advanced or metastatic cancer, life expectancy > 3 months | Liposomeal curcumin intravenous | Safety, tolerability and pharmacokinetic of liposomeal curcumin, tumor response by RECIST criteria | Recruiting, 1 | NCT02138955 |
| Breast cancer | Atypical ductal breast hyperplasia BRCA1 gene mutation BRCA2 gene mutation ductal breast carcinoma in situ lobular breast carcinoma in situ | Nanoemulsion formulation of curcumin | Adherence, tolerability and safety of curcumin, anti-inflammatory changes | Recruiting, pilot study | NCT01975363 |
| Colorectal cancer | Metastatic cancer | Curcumin, irinotecan | Safety, pharmacokinetics and effectiveness of irinotecan in combination with curcumin | Recruiting, 1 | NCT01859858 |
| Colorectal cancer | Familial adenomatous polyposis, stage 0 | Phospholipid curcumin, anthocyanin extract | Markers, apoptosis, cell proliferation | Recruiting, 2 | NCT01948661 |
| Prostate cancer | Stage T1–T3 | Curcumin, curcumin analogue BCM-95CG | Time of recurrence-free survival | Recruiting, 2 | NCT02064673 |
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: www.globocan.iarc.fr (accessed on 15 December 2013).
- Varga, D.; Deniz, M.; Schwentner, L.; Wiesmüller, L. Ovarian cancer: In search of better marker systems based on DNA repair defects. Int. J. Mol. Sci. 2013, 14, 640–673. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Salanti, G.; Pavlidis, N.; Paraskevaidis, E.; Ioannidis, J.P. Survival benefits with diverse chemotherapy regimens for ovarian cancer: meta-analysis of multiple treatments. J. Natl. Cancer Inst. 2006, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; van der Burg, M.E.; Griffin, C.L.; Guthrie, D.; Lamont, A.; Jayson, G.C.; Kristensen, G.; Mediola, C.; Coens, C.; Qian, W.; et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): A randomized trial. Lancet 2010, 376, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, G.C.; Jayson, G.C.; Clamp, A.R. Antiangiogenic drugs in ovarian cancer. Br. J. Cancer 2009, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Dhingra, R.; Singh, S.; Sharma, S.; Tomar, P.; Malhotra, M.; Bhardwaj, T.R. Afliberecept: A novel VEGF targeted agent to explore the future perspectives of anti-angiogenic theraphy for the treatment of multiple tumors. Mini-Rev. Med. Chem. 2013, 13, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Cannistra, S.A.; Matulonis, U.A.; Penson, R.T.; Hambleton, J.; Dupont, J.; Mackey, H.; Douglas, J.; Burger, R.A.; Armstrong, D.; Wenham, R.; et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol. 2007, 25, 5180–5186. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S. ICON-7 confirms first-line bevacizumab is beneficial. Available online: www.oncologypractice.com (accessed on 15 December 2013).
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Singh, H. Bevacizumab and ovarian cancer. Ther. Adv. Med. Oncol. 2013, 5, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Quinn, M.; Thigpen, T.; Vermorken, J.; Avall-Lundqvist, E.; Bookman, M.; Bowtell, D.; Brady, M.; Casado, A.; Cervantes, A.; et al. 2004 Consensus statements on the management of ovarian cancer: Final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann. Oncol. 2005, 16, viii7–viii12. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.; Harter, P.; Barinoff, J.; Beutel, B.; Kannisto, P.; Grabowski, J.P.; Heitz, J.; Kurzeder, C.; du Bois, A. Bevacizumab in the treatment of ovarian cancer. Adv. Ther. 2012, 29, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Mangerich, A.; Bürkle, A. How to kill tumor cells with inhibitors of poly(ADP-ribosyl)ation. Int. J. Cancer 2011, 128, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Underhill, C.; Toulmonde, M.; Bonnefoi, H. A review of PARP inhibitors: From bench to bedside. Ann. Oncol. 2011, 22, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Audeh, M.W.; Carmichael, J.; Penson, R.T.; Friedlander, M.; Powell, B.; Bell-McGuinn, K.M.; Scott, C.; Weitzel, J.N.; Oaknin, A.; Loman, N.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet 2010, 376, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Hao, Q. Olaparib: A promising PARP inhibitor in ovarian cancer theraphy. Arch. Gynecol. Obstet. 2013, 288, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.C.; Yap, T.A.; Boss, D.S.; Carden, C.P.; Mergui-Roelvink, M.; Gourley, C.; de Greve, J.; Lubinski, J.; Shanley, S.; Messiou, C.; et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 2010, 28, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Spannuth, W.A.; Sood, A.K.; Coleman, R.L. Farletuzumab in epithelial ovarian carcinoma. Expert Opin. Biol. Ther. 2010, 10, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.D.; Theti, D.S.; Wood, N.; Green, M.; Raynaud, F.; Valenti, M.; Forster, M.D.; Mitchell, F.; Bavetsias, V.; Henderson, E.; et al. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to α-folate receptor-overexpressing tumors. Cancer Res. 2005, 65, 11721–11728. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Calvert, H.; Kania, M.; Kaiser, C.; Zimmermann, A.H.; Sehouli, J.A. A randomised, double-blind, phase II study of two doses of pemetrexed in the treatment of platinum-resistant, epithelial ovarian primary peritoneal cancer. Eur. J. Cancer 2009, 45, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Naumann, R.W.; Coleman, R.L.; Burger, R.A.; Sausville, E.A.; Kutarska, E.; Ghamande, S.A.; Gabrail, N.Y.; Depasquale, S.E.; Nowara, E.; Gilbert, L.; et al. PRECEDENT: A randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, vs. PLD alone, in subjects with platinum-resistant ovarian cancer. J. Clin. Oncol. 2013, 31, 4400–4406. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, I.R.; Santhapuram, H.K.; Kleindl, P.J.; Howard, S.J.; Stanford, K.M.; Leamon, C.P. Design and regioselective synthesis of a new generation of target chemotherapeutics. Part 1: EC145, A folic acid conjugate of desacetylvinblastine monohydrazine. Bioorg. Med. Chem. Lett. 2006, 16, 5093–5096. [Google Scholar] [CrossRef] [PubMed]
- Boivin, M.; Lane, D.; Piché, A.; Rancourt, C. CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol. Oncol. 2009, 115, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Clark, S.; Wong, T.; Chen, Y.; Chen, Y.; Dennis, M.S.; Luis, E.; Zhong, F.; Bheddah, S.; Koeppen, H.; et al. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res. 2007, 67, 4924–4932. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.C.; Kumar, D.; Jaggi, M. Mucins in ovarian cancer diagnosis and therapy. J. Ovarian Res. 2009, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- McQuarrie, S.; Mercer, J.; Syme, A.; Suresh, M.; Miller, G. Preliminary results of nanopharmaceuticals used in the radioimmunotherapy of ovarian cancer. J. Pharm. Pharm. Sci. 2005, 7, 29–34. [Google Scholar] [PubMed]
- Hiss, D. Optimizing molecular-targeted therapies in ovarian cancer: The renewed surge of interest in ovarian cancer biomarkers and cell signaling pathways. J. Oncol. 2012, 2012, 737981. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sung, B.; Kim, J.H.; Prasad, S.; Li, S.; Aggarwal, B.B. Multitargetting by tumeric, the golden spice: From kitchen to clinic. Mol. Food Res. 2013, 57, 1510–1528. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, B.S.; Keum, K.S.; Yu, H.H.; Kim, Y.H.; Chang, B.S.; Ra, J.Y.; Moon, H.D.; Seo, B.R.; Choi, N.Y.; et al. Essential oil of Curcuma longa inhibits streptococcus mutants biofilm formation. J. Food Sci. 2011, 76, H226–H230. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, R.; Manikandan, R. Antioxidants and cataract. Free Radic. Res. 2013, 47, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Gopfert, E. The effect of cheliodonium-and turmeric root extract on upper abdominal pain due to functional disorders of the biliary system. Results from a placebo-controlled double-blind study. Med. Clin. 1999, 94, 425–430. [Google Scholar]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the reevaluation of curcumin (E 100) as a food additive. EFSA J. 2010, 8, 1679. [Google Scholar]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [PubMed]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Cheng, A.L; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.H.; Chen, X.; Sang, S.; Lee, M.J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related h-diketone derivatives: Effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.S.R.; Pavitra, E.; Nagaraju, G.P.; Ramesh, K.; El-Rayes, B.F.; Yu, J.S. Imaging and curcumin delivery in pancreatic cancer cell lines using PEGylated α-Gd2(MoO4)3 mesoporous particles. Dalton Trans. 2014, 43, 3330–3338. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Sahoo, S.K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef] [PubMed]
- Terlikowska, K.; Witkowska, A.; Terlikowski, S. Curcumin in chemoprevention of breast cancer. Postep. Hig. Med. Dosw. 2014, 68, 571–578. [Google Scholar] [CrossRef]
- Lv, J.; Shao, Q.; Wang, H.; Shi, H.; Wang, T.; Gao, W.; Song, B.; Zheng, G.; Kong, B.; Qu, X. Effects and mechanisms of curcumin and basil polysaccharide on the invasion of SKOV3 cells and dendritic cells. Mol. Med. Rep. 2013, 8, 1580–1586. [Google Scholar] [PubMed]
- Shi, M.; Cai, Q.; Yao, L.; Mao, Y.; Ming, Y.; Ouyang, G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell. Biol. Int. 2006, 30, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.D.; Tong, Q.S.; Wu, C.H. Growth inhibition and apoptosis inducing mechanisms of curcumin on human ovarian cancer cell line A2780. Chin. J. Integr. Med. 2006, 12, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Tong, Q.; Wu, C. Growth-inhibitory effects of curcumin on ovary cancer cells and its mechanisms. J. Huazhong Univ. Sci. Technol. 2004, 24, 55–58. [Google Scholar] [CrossRef]
- Pan, W.; Yang, H.; Cao, C.; Song, X.; Wallin, B.; Kivin, R.; Lu, S.; Hu, G.; Di, W.; Wan, Y. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol. Rep. 2008, 20, 1553–1559. [Google Scholar] [PubMed]
- Montopoli, M.; Ragazzi, E.; Froldi, G.; Caparrotta, L. Cell-cycle inhibition and apoptosis induced by curcumin and cisplatin or oxaliplatin in human ovarian carcinoma cells. Cell Prolif. 2009, 42, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Cao, C.; Lu, S.; Kivlin, R.; Amaral, A.; Kouttab, N.; Yang, H.; Chu, W.; Bi, Z.; Di, W.; et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008, 62, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Saydmohammed, M.; Joseph, D.; Syed, V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J. Cell. Biochem. 2010, 110, 447–456. [Google Scholar] [PubMed]
- Watson, J.L.; Greenshields, A.; Hill, R.; Hilchie, A.; Lee, P.W.; Giacomantonio, C.A.; Hoskin, D.W. Curcumin-induced apoptosis in ovarian carcinoma cells is p53-independent and involves p38 mitogen-activated protein kinase activation and down-regulation of Bcl-2 and survivin expression and Akt signaling. Mol. Carcinog. 2010, 49, 13–24. [Google Scholar] [PubMed]
- Zhao, S.-F.; Zhang, X.; Zhang, X.-J.; Shi, X.-Q.; Yu, Z.-J.; Kan, Q.-C. Induction of microRNA-9 mediates cytotoxicity of curcumin against SKOV3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 3363–3368. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Jeong, K.J.; Oha, W.J.; Sul, H.J.; Sohn, J.S.; Kim, Y.K.; Cho, D.Y.; Kang, J.K.; Park, C.G.; Lee, H.Y. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: Their inhibition by curcumin. Cancer Lett. 2010, 288, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rath, K.S.; McCann, G.A.; Cohn, D.E.; Rivera, B.K.; Kuppusamy, P.; Karuppaiyah, S. Safe and targeted anticancer therapy for ovarian cancer using a novel class of curcumin analogs. J. Ovarian Res. 2013, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Kálai, T.; Kuppusamy, M.L.; Balog, M.; Selvendiran, K.; Rivera, B.K.; Kuppusamy, P.; Hideg, K. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J. Med. Chem. 2011, 54, 5414–5421. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Ahmed, S.; Dayton, A.; Kuppusamy, M.L.; Tazi, M.; Bratasz, A.; Tong, L.; Rivera, B.K.; Kálai, T.; Hideg, K.; et al. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluoro-diarylidenylpiperidones: Differential cytotoxicity in healthy and cancer cells. Free Radic. Biol. Med. 2010, 48, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Dayton, A.; Selvendiran, K.; Kuppusamy, M.L.; Rivera, B.K.; Meduru, S.; Tamás, K.; Hideg, K.; Kuppusamy, P. Cellular uptake, retention and bioabsorption of HO-3867, a fluorinated curcumin analog with potential antitumor properties. Cancer Biol. Ther. 2010, 15, 1027–1032. [Google Scholar] [CrossRef]
- Samuni, Y.; Gamson, J.; Samuni, A.; Yamada, K.; Russo, A.; Krishna, M.C.; Mitchell, J.B. Factors influencing nitroxide reduction and cytotoxicity in vitro. Antioxid. Redox Signal. 2004, 6, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Krishna, M.C.; Kuppusamy, P.; Cook, J.A.; Russo, A. Protection against oxidative stress by nitroxides. Exp. Biol. Med. 2001, 226, 620–621. [Google Scholar]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002, 62, 307–312. [Google Scholar] [PubMed]
- Kuppusamy, P.; Wang, P.; Shankar, R.A.; Ma, L.; Trimble, C.E. In vivo topical EPR spectroscopy and imaging of nitroxide free radicals and polynitroxyl-albumin. Magn. Reson. Med. 1998, 40, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Tong, L.; Bratasz, A.; Kuppusamy, M.L.; Ahmed, S.; Ravi, Y.; Trigg, N.J.; Rivera, B.K.; Kálai, T.; Hideg, K.; et al. Anticancer efficacy of a difluorodiarylidenyl piperidone (HO-3867) in human ovarian cancer cells and tumor xenografts. Mol. Cancer Ther. 2010, 9, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Ahmed, S.; Dayton, A.; Ravi, Y.; Kuppusamy, M.L.; Bratasz, A.; Rivera, B.K.; Kálai, T.; Hideg, K.; Kuppusamy, P. HO-3867, a synthetic compound, inhibits migration and invasion of ovarian carcinoma cells through down-regulation of fatty acid synthase and focal adhesion kinase. Mol. Cancer Res. 2010, 8, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Ahmed, S.; Dayton, A.; Kuppusamy, M.L.; Rivera, B.K.; Kálai, T.; Hideg, K.; Kuppusamy, P. HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian carcinoma, leading to therapeutic synergy through STAT3 inhibition. Cancer Biol. Ther. 2011, 12, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Maher, D.M.; Sundram, V.; Bell, M.C.; Jaggi, M.; Chauhan, S.C. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J. Ovarian Res. 2010, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Xiao, J.; Zhang, H.; Chen, Q.; Wang, Z.; Shi, H.; Gong, L.; Chen, J.; Liu, Y.; Cao, R.; et al. B19, a novel monocarbonyl analogue of curcumin, induces human ovarian cancer cell apoptosis via activation of endoplasmic reticulum stress and the autophagy signaling pathway. Int. J. Biol. Sci. 2013, 9, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.Q.; Zhu, G.-H.; Wang, Y.-H.; Yu, X.-C.; Zhu, X.-B.; Liang, G.; Xiao, J.; Li, X.K. A novel mono-carbonyl analogue of curcumin induces apoptosis in ovarian carcinoma cells via endoplasmic reticulum stress and reactive oxygen species production. Mol. Med. Rep. 2012, 5, 739–744. [Google Scholar] [PubMed]
- Carew, J.S.; Nawrocki, S.T.; Cleveland, J.L. Modulating autophagy for therapeutic benefit. Autophagy 2007, 3, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Murphy, C.J.; Zhang, B.; Shen, Y.; van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Curcumin polymers as anticancer conjugates. Biomaterials 2010, 31, 7139–7149. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Annereau, J.P.; Lababidi, S.; Shankavaram, U.; Arciello, A.; Bussey, K.J.; Reinhold, W.; Guo, Y.; Kruh, G.D.; Reimers, M.; et al. Predicting drug sensitivity and resistance: Profiling ABC transporter genes in cancer cells. Cancer Cell 2004, 6, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Samimi, G.; Fink, D.; Varki, N.M.; Husain, A.; Hoskins, W.J.; Alberts, D.S.; Howell, S.B. Analysis of MLH1 and MSH2 expression in ovarian cancer before and after platinum drug-based chemotherapy. Clin. Cancer Res. 2000, 6, 1415–1421. [Google Scholar] [PubMed]
- Boudsocq, F.; Benaim, P.; Canitrot, Y.; Knibiehler, M.; Ausseil, F.; Capp, J.P.; Bieth, A.; Long, C.; David, B.; Massiot, G.; et al. Modulation of cellular response to cisplatin by a novel inhibitor of DNA polymerase β. Mol. Pharmacol. 2005, 67, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Canitrot, Y.; Cazaux, C.; Frechet, M.; Bouayadi, K.; Lesca, C.; Salles, B.; Hoffmann, J.S. Overexpression of DNA polymerase β in cell results in a mutator phenotype and a decreased sensitivity to anticancer drugs. Proc. Natl. Acad. Sci. USA 1998, 95, 12586–12590. [Google Scholar] [CrossRef] [PubMed]
- Dabholkar, M.; Vionnet, J.; Bostick-Bruton, F.; Yu, J.J.; Reed, E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum based chemotherapy. J. Clin. Investig. 1994, 94, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Helleman, J.; van Staveren, I.L.; Dinjens, W.N.; van Kuijk, P.F.; Ritstier, K.; Ewing, P.C.; van der Burg, M.E.; Stoter, G.; Berns, E.M. Mismatch repair and treatment resistance in ovarian cancer. BMC Cancer 2006, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Swisher, E.M.; Sakai, W.; Karlan, B.Y.; Wurz, K.; Urban, N.; Taniguchi, T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008, 68, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Tischkowitz, M.; Ameziane, N.; Hodgson, S.V.; Mathew, C.G.; Joenje, H.; Mok, S.C.; D’Andrea, A.D. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat. Med. 2003, 9, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Grompe, M.; D’Andrea, A.D. Fanconi anemia and DNA repair. Hum. Mol. Genet. 2001, 10, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.D.; Grompe, M. The Fanconi anemia/BRCA pathway. Nat. Rev. Cancer 2003, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Tang, J.; Stopfer, J.; Lilli, D.R.; Hamel, N.; Tischkowitz, M.; Monteiro, A.N.; Messick, T.E.; Powers, J.; Yonker, A. Biallelic deleterious BRCA1 mutations in woman with early-onset ovarian cancer. Cancer Discov. 2013, 3, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Chirnomas, D.; Taniguchi, T.; de la Vega, M.; Vaidya, A.P.; Vasserman, M.; Hartman, A.R.; Kennedy, R.; Foster, R.; Mahoney, J.; Seiden, M.V. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol. Cancer Ther. 2006, 5, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell. Biochem. 2007, 296, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Chearwae, W.; Wu, C.P.; Chu, H.Y.; Lee, T.R.; Ambudkar, S.V.; Limtrakul, P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother. Pharmacol. 2006, 57, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Amiji, M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Srivanias, G.; Harikrishna, D.; Mansoor, A. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J. Pharm. Sci. 2010, 99, 4630–4641. [Google Scholar] [CrossRef] [PubMed]
- Weir, N.M.; Selvendiran, K.; Kutala, V.K.; Tong, L.; Vishwanath, S.; Rajaram, M.; Tridandapani, S.; Anant, S.; Kuppusamy, P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol. Ther. 2007, 6, 1–7. [Google Scholar] [CrossRef]
- Chan, M.M.; Fong, D.; Soprano, K.J.; Holmes, W.F.; Heverling, H. Inhibition of growth and sensitization to cisplatin-mediated killing of ovarian cancer cells by polyphenolic chemopreventive agents. J. Cell. Physiol. 2002, 194, 63–70. [Google Scholar] [CrossRef]
- Yunos, N.M.; Beale, P.; Yu, J.Q.; Huq, F. Synergism from sequenced combinations of curcumin and epigallocatechin-3-gallate with cisplatin in the killing of human ovarian cancer cells. Anticancer Res. 2011, 31, 1131–1140. [Google Scholar] [PubMed]
- Ferrari, E.; Lazzari, S.; Marverti, G.; Pignedoli, F.; Spagnolo, F.; Saladini, M. Synthesis, cytotoxic and combined cDDP activity of new stable curcumin derivatives. Bioorg. Med. Chem. 2009, 17, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Yunos, N.M.; Beale, P.; Yu, J.Q.; Huq, F. Synergism from the combination of oxaliplatin with selected phytochemicals in human ovarian cancer cell lines. Anticancer Res. 2011, 31, 4283–4290. [Google Scholar] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer. Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar]
- Irving, G.R.B.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.; Scott, E.N.; Berry, D.P.; et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration—A clinical pilot study including assessment of patient acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Steward, W.P.; Gescher, A.J. Curcumin in cancer management: Recent results of analogue design and clinical studies and desirable future research. Mol. Nutr. Food Res. 2008, 52, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlikowska, K.M.; Witkowska, A.M.; Zujko, M.E.; Dobrzycka, B.; Terlikowski, S.J. Potential Application of Curcumin and Its Analogues in the Treatment Strategy of Patients with Primary Epithelial Ovarian Cancer. Int. J. Mol. Sci. 2014, 15, 21703-21722. https://doi.org/10.3390/ijms151221703
Terlikowska KM, Witkowska AM, Zujko ME, Dobrzycka B, Terlikowski SJ. Potential Application of Curcumin and Its Analogues in the Treatment Strategy of Patients with Primary Epithelial Ovarian Cancer. International Journal of Molecular Sciences. 2014; 15(12):21703-21722. https://doi.org/10.3390/ijms151221703
Chicago/Turabian StyleTerlikowska, Katarzyna M., Anna M. Witkowska, Malgorzata E. Zujko, Bozena Dobrzycka, and Slawomir J. Terlikowski. 2014. "Potential Application of Curcumin and Its Analogues in the Treatment Strategy of Patients with Primary Epithelial Ovarian Cancer" International Journal of Molecular Sciences 15, no. 12: 21703-21722. https://doi.org/10.3390/ijms151221703






