Th17/Treg Cells Imbalance and GITRL Profile in Patients with Hashimoto’s Thyroiditis
Abstract
:1. Introduction
2. Results
2.1. Enhancement of Th17 Cells in Peripheral Blood from HT Patients
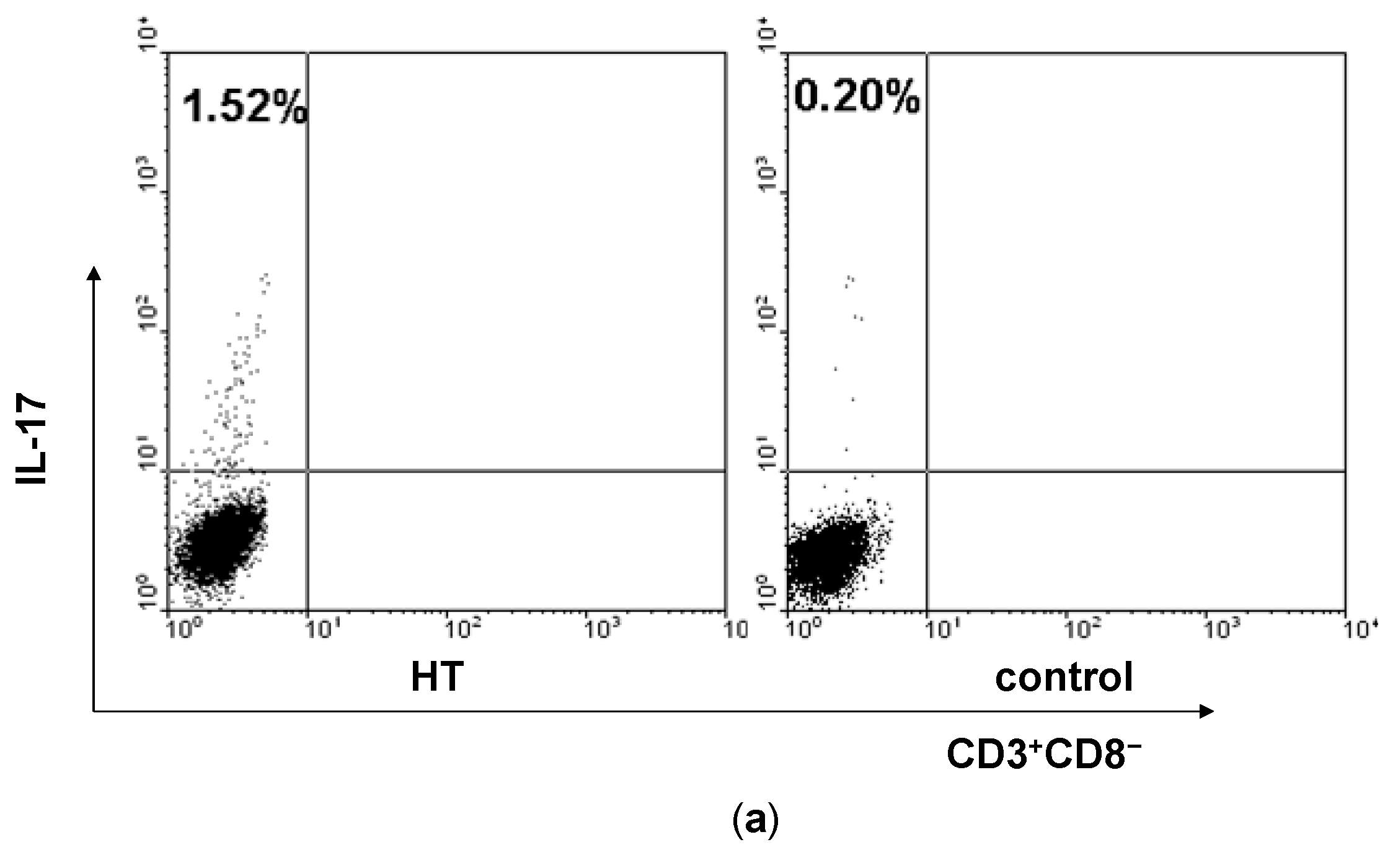
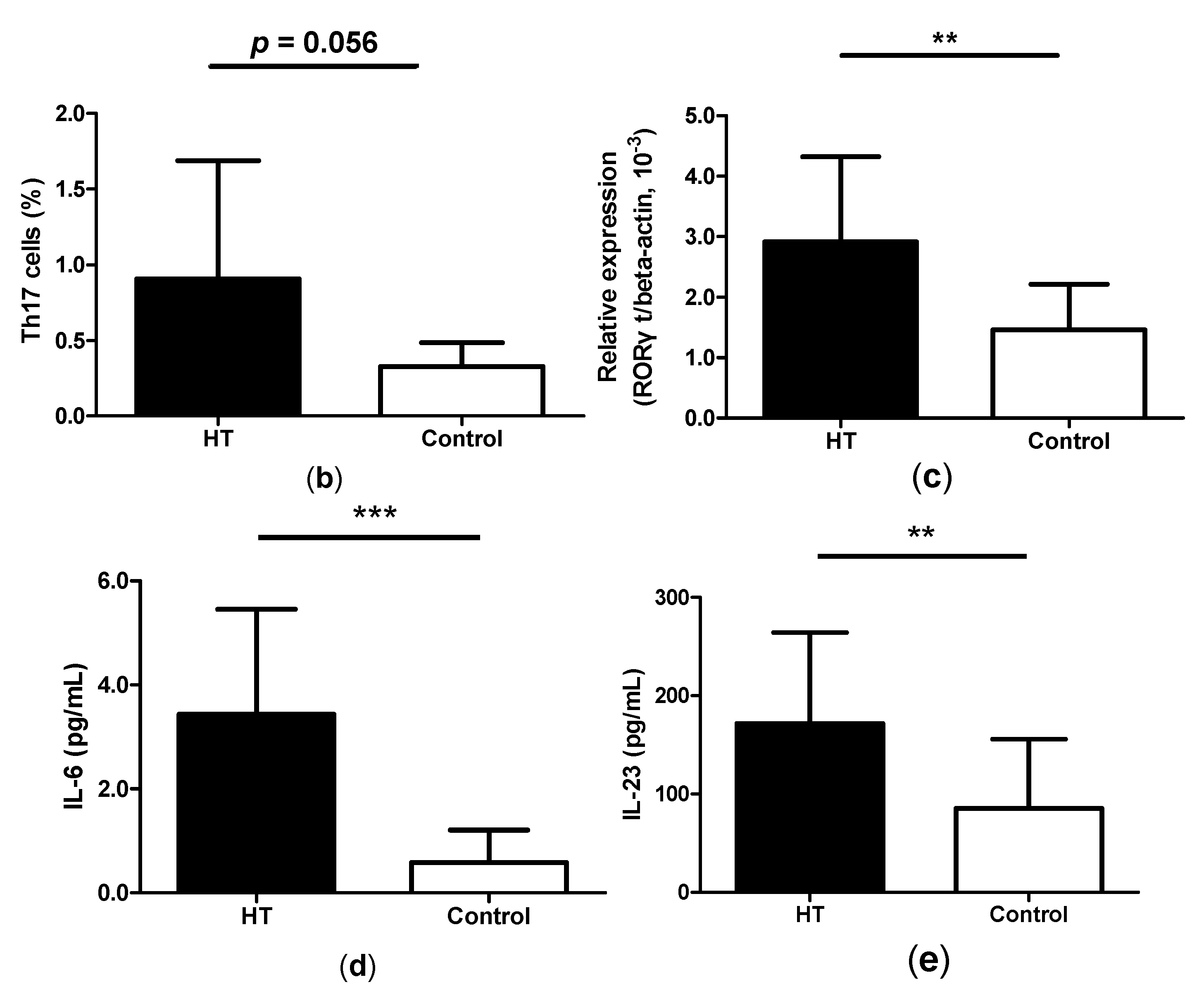
2.2. Reduction of Regulatory T Cells in Peripheral Blood from HT Patients
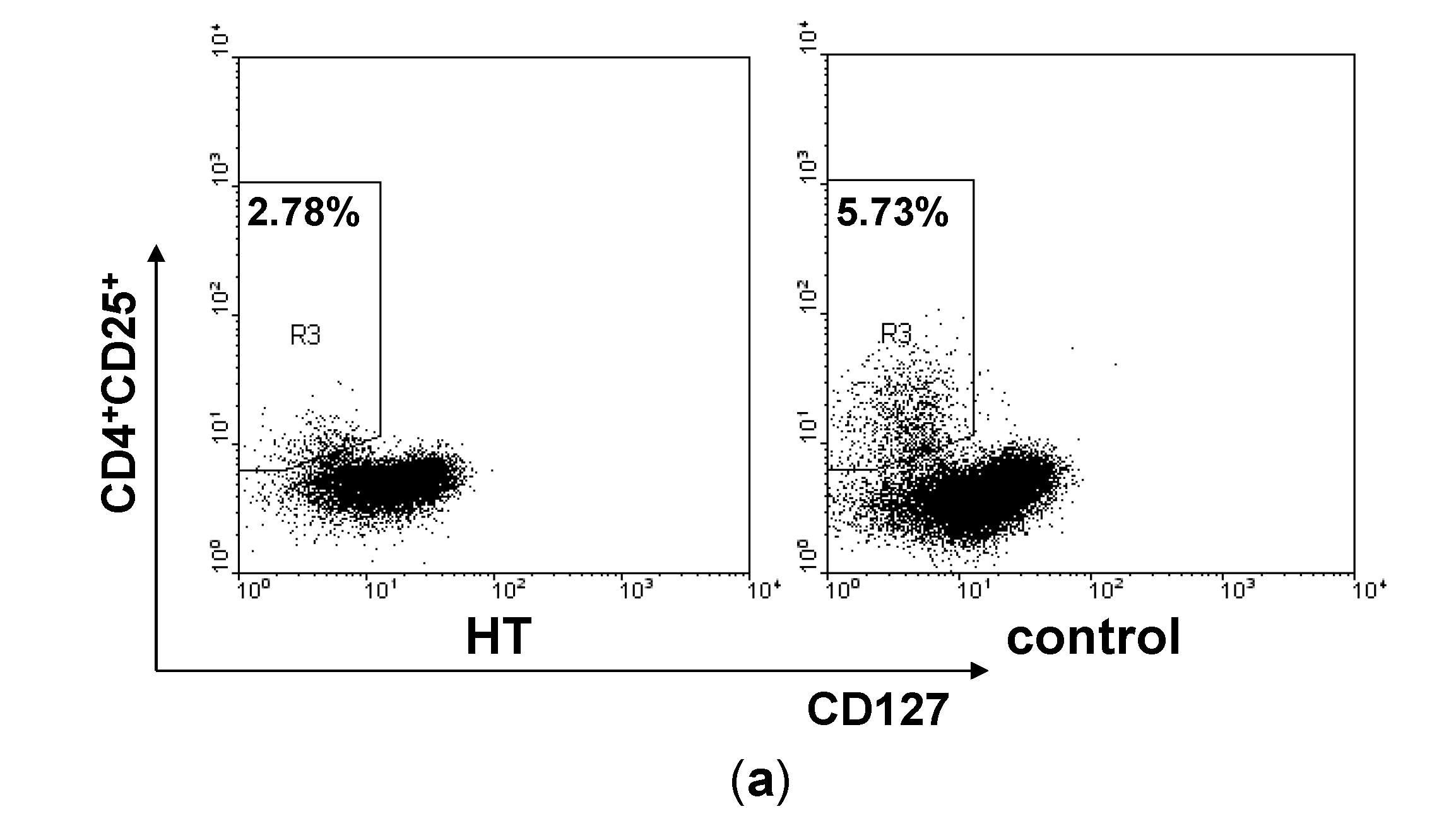

2.3. Correlation between Th17/Treg Balance and TgAb Levels in HT Patients

2.4. High Levels of GITRL in Patients with HT

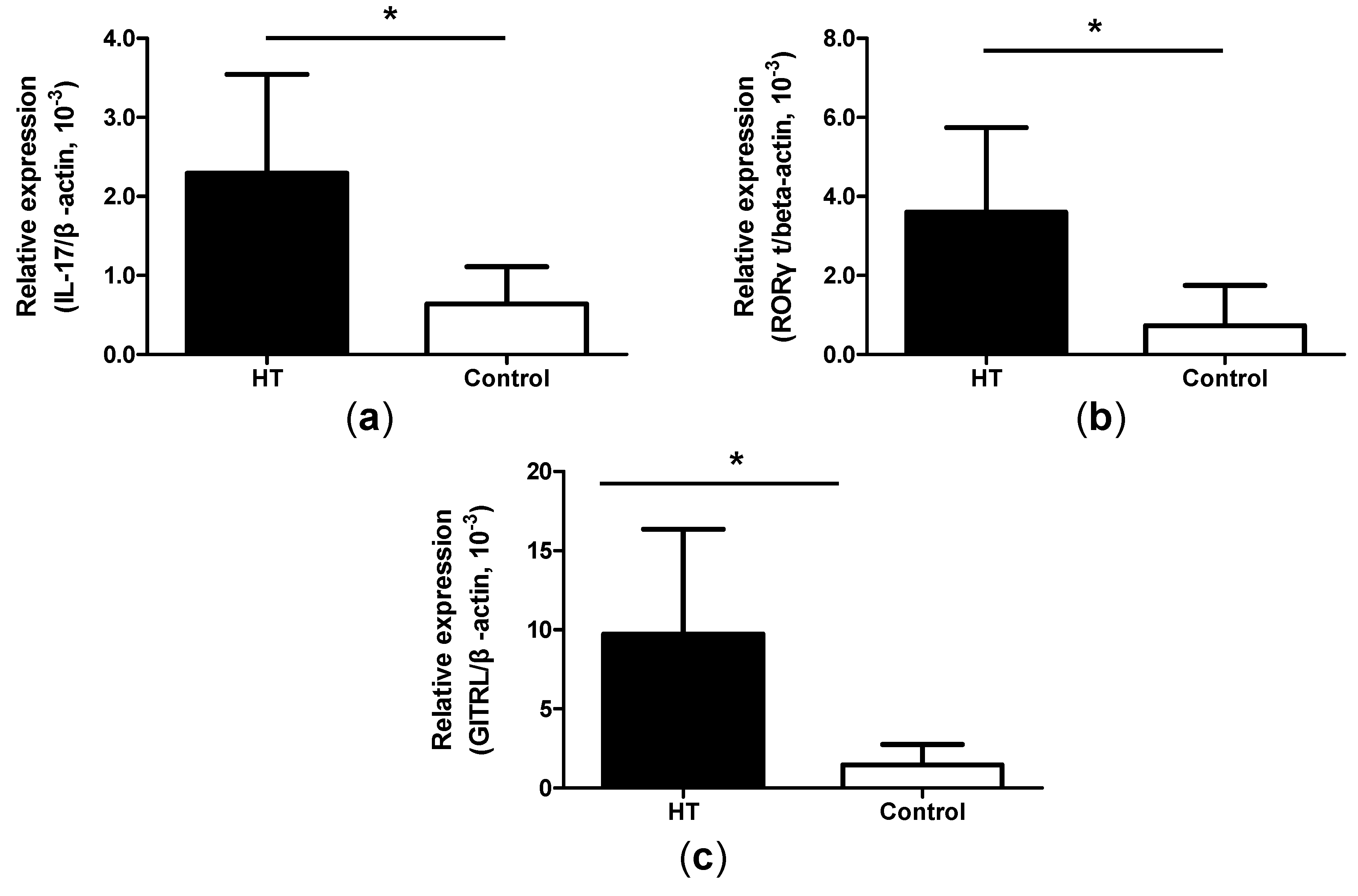
2.5. Increased Expression of IL-17, ROR-γt and GITRL mRNA in Thyroid Tissue from HT Patients
3. Discussion
4. Experimental Section
4.1. Individuals and Samples
| HT Patients | Healthy Controls | Range | |
|---|---|---|---|
| Number | 25 | 20 | |
| Gender (M/F) | 5/20 | 3/17 | |
| Age (year) | 47.2 ± 9.9 | 46.3 ± 7.2 | |
| Tg-Ab (IU/mL) | 677.1 ± 312.3 | 19.4 ± 9.3 | <30 |
| TPO-Ab (IU/mL) | 317.5 ± 206.7 | 16.6 ± 7.1 | <10 |
4.2. Cell Isolation and Stimulation in Vitro
4.3. Flow Cytometric Analysis
4.4. RNA Isolation and Real-Time PCR
4.5. Cytokine Quantification
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Abbreviations
| GITRL | glucocorticoid-induced TNF receptor family-related protein ligand |
| HT | Hashimoto’s thyroiditis. |
Conflicts of Interest
References
- Pearce, E.N.; Farwell, A.P.; Braverman, L.E. Thyroiditis. N. Engl. J. Med. 2003, 348, 2646–2655. [Google Scholar]
- Weetman, A.P. Autoimmune thyroid disease. Autoimmunity 2004, 37, 337–340. [Google Scholar]
- Zhu, C.; Ma, J.; Liu, Y.; Tong, J.; Tian, J.; Chen, J.; Tang, X.; Xu, H.; Lu, L.; Wang, S. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2012, 97, 943–950. [Google Scholar]
- Park, H. A distinct lineage of cd4 t cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar]
- Weaver, C.T.; Hatton, R.D.; Mangan, P.R.; Harrington, L.E. Il-17 family cytokines and the expanding diversity of effector t cell lineages. Annu. Rev. Immunol. 2007, 25, 821–852. [Google Scholar]
- Cascão, R.; Moura, R.A.; Perpétuo, I.; Canhão, H.; Sousa, E.V.; Mourão, A.F.; Rodrigues, A.M.; Pereira, J.P.; Queiroz, M.V.; Rosário, H.S. Identification of a cytokine network sustaining neutrophil and th17 activation in untreated early rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, 196–204. [Google Scholar]
- Seiderer, J.; Elben, I.; Diegelmann, J.; Glas, J.; Stallhofer, J.; Tillack, C.; Pfennig, S.; Jürgens, M.; Schmechel, S.; Konrad, A.; et al. Role of the novel Th17 cytokineIL-17F in inflammatory bowel disease (IBD): Upregulated colonic IL-17F expression in active crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm. Bowel Dis. 2008, 14, 437–445. [Google Scholar]
- Wang, S.; Shi, Y.; Yang, M.; Ma, J.; Tian, J.; Chen, J.; Mao, C.; Jiao, Z.; Ko, K.-H.; Baidoo, S.E.; et al. Gitrl exacerbates collagen-induced arthritis via enhancing the expansion of Th17 cells. Am. J. Pathol. 2012, 180, 1059–1067. [Google Scholar]
- Tzartos, J.S.; Manuel, A.F.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system infltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar]
- Tang, X.; Tian, X.; Zhang, Y.; Wu, W.; Tian, J.; Rui, K.; Tong, J.; Lu, L.; Xu, H.; Wang, S. Correlation between the frequency of Th17 cell and the expression of microrna-206 in patients with dermatomyositis. Clin. Dev. Immunol. 2013, 2013, 7. [Google Scholar]
- Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004, 22, 531–562. [Google Scholar]
- Wan, Y.Y.; Flavell, R.A. Tgf-beta and regulatory T cell in immunity and autoimmunity. J. Clin. Immunol. 2008, 28, 647–659. [Google Scholar]
- Wang, S.; Xu, H.; Wang, Y.; Ma, J.; Mao, C.; Shao, Q.; Ma, B.; Xu, W.; Yang, S. Regulatory T cells induced by rAAV carrying the forkhead box p3 gene prevent autoimmune thyroiditis in mice. Int. J. Mol. Med. 2006, 18, 1193–1199. [Google Scholar]
- Bettelli, E.; Carrire, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar]
- Dieckmann, D; Plottner, H.; Berchtold, S.; Berger, T.; Schuler, G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 2001, 193, 1303–1310. [Google Scholar]
- Kwon, B.; Yu, K.Y.; Ni, J.; Yu, G.L.; Jang, I.K.; Kim, Y.J.; Xing, L.; Liu, D.; Wang, S.X.; Kwon, B.S. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J. Biol. Chem. 1999, 274, 6056–6061. [Google Scholar]
- Kim, B.J.; Zhu, L.; Fariss, R.N.; Shen, D.F.; Mahesh, S.P.; Egwuagu, C.; Yu, C.-R.; Nagineni, C.N.; Chan, C.-C.; Nussenblatt, R.B. Constitutive and cytokine-induced GITR ligand expression on human retinal pigment epithelium and photoreceptors. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3170–3176. [Google Scholar]
- McHugh, R.S.; Whitters, M.; Piccirillo, C.A.; Young, D.A.; Shevach, E.M.; Collins, M.; Byrne, M.C. CD4+CD25+ immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced tnf receptor. Immunity 2002, 16, 311–323. [Google Scholar]
- Kamimura, Y.; Iwai, H.; Piao, J.; Hashiguchi, M.; Azuma, M. The glucocorticoid-induced TNF receptor-related protein (GITR)-GITR ligand pathway acts as a mediator of cutaneous dendritic cell migration and promotes T cell-mediated acquired immunity. J. Immunol. 2009, 182, 2708–2716. [Google Scholar]
- Nocentini, G.; Ronchetti, S.; Petrillo, M.G.; Riccardi, C. Pharmacological modulation of GITRL/GITR system: Therapeutic perspectives. Br. J. Pharmacol. 2012, 165, 2089–2099. [Google Scholar]
- Azuma, M. Role of the glucocorticoid-induced tnfr-related protein (GITR)-GITR ligand pathway in innate and adaptive immunity. Crit. Rev. Immunol. 2010, 30, 547–557. [Google Scholar]
- Santaguida, M.G.; Nardo, S.; del Duca, S.C.; Lococo, E.; Virili, C.; Gargano, L.; Lenti, L.; Centanni, M. Increased interleukin-4-positive lymphocytes in patients with hashimoto’s thyroiditis and concurrent non-endocrine autoimmune disorders. Clin. Exp. Immunol. 2011, 165, 148–154. [Google Scholar]
- Wang, S.; Baidoo, S.E.; Liu, Y.; Zhu, C; Tian, J.; Ma, J.; Tong, J.; Chen, J.; Tang, X.; Xu, H.; et al. T cell-derived leptin contributes to increased frequency of Th17 cells in female patients with hashimoto’s thyroiditis. Clin. Exp. Immunol. 2013, 171, 63–68. [Google Scholar]
- Shi, Y.; Wang, H.; Su, Z.; Chen, J.; Xue, Y.; Wang, S.; He, Z.; Yang, H.; Zhou, C.; Kong, F.; et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of hashimoto’s thyroiditis. Scand. J. Immunol. 2010, 72, 250–255. [Google Scholar]
- Baran, J.; Kowalczyk, D.; Ozog, M.; Zembala, M. Three-color flow cytometry detection of intracellular cytokines in peripheral blood mononuclear cells: Comparative analysis of phorbol myristate acetate-ionomycin and phytohemagglutinin stimulation. Clin. Diagn. Lab. Immunol. 2001, 8, 303–313. [Google Scholar]
- Faas, M.M.; Bouman, A.; Veenstra van Nieuwenhoven, A.L.; van der Schaaf, G.; Moes, H.; Heineman, M.J.; de Vos, P. Species differences in the effect of pregnancy on lymphocyte cytokine production between human and rat. J. Leukoc. Biol. 2005, 78, 946–953. [Google Scholar]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor rorgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupe, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(h)-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human t helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar]
- Annunziato, F.; Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S. Main features of human T helper 17 cells. Ann. N. Y. Acad. Sci. 1284, 66–70. [Google Scholar]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory t cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar]
- Ruggeri, R.M.; Saitta, S.; Cristani, M.; Giovinazzo, S.; Tigano, V.; Trimarchi, F.; Benvenga, S.; Gangemi, S. Serum interleukin-23 (IL-23) is increased in hashimoto’s thyroiditis. Endocr. J. 2014, 61, 359–363. [Google Scholar]
- Qin, Q.; Liu, P.; Liu, L.; Wang, R.; Yan, N.; Yang, J.; Wang, X.; Pandey, M.; Zhang, J.A. The increased but non-predominant expression of Th17- and Th1-specific cytokines in hashimoto’s thyroiditis but not in graves’ disease. Braz. J. Med. Biol. Res. 2012, 45, 1202–1208. [Google Scholar]
- Li, D.; Cai, W.; Gu, R.; Zhang, Y.; Zhang, H.; Tang, K.; Xu, P.; Katirai, F.; Shi, W.; Wang, L.; et al. Th17 cell plays a role in the pathogenesis of hashimoto’s thyroiditis in patients. Clin. Immunol. 2013, 149, 411–420. [Google Scholar]
- Figueroa-Vega, N.; Alfonso-Perez, M.; Benedicto, I.; Sanchez-Madrid, F.; Gonzalez-Amaro, R.; Marazuela, M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 2010, 95, 953–962. [Google Scholar]
- Glick, A.B.; Wodzinski, A.; Fu, P.; Levine, A.D.; Wald, D.N. Impairment of regulatory T-cell function in autoimmune thyroid disease. Thyroid 2013, 23, 871–878. [Google Scholar]
- Bossowski, A.; Moniuszko, M.; Dabrowska, M.; Sawicka, B.; Rusak, M.; Jeznach, M.; Wojtowicz, J.; Bodzenta-Lukaszyk, A.; Bossowska, A. Lower proportions of CD4+CD25high and CD4+Foxp3, but not CD4+CD25+CD127low Foxp3+ T cell levels in children with autoimmune thyroid diseases. Autoimmunity 2013, 46, 222–230. [Google Scholar]
- Nocentini, G.; Giunchi, L.; Ronchetti, S.; Krausz, L.T.; Bartoli, A.; Moraca, R.; Migliorati, G.; Riccardi, C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits t cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 6216–6221. [Google Scholar]
- Bianchini, R.; Bistoni, O.; Alunno, A.; Petrillo, M.G.; Ronchetti, S.; Sportoletti, P.; Bocci, E.B.; Nocentini, G.; Gerli, R.; Riccardi, C. CD4+CD25lowGITR+ cells: A novel human CD4+ T-cell population with regulatory activity. Eur. J. Immunol. 2011, 41, 2269–2278. [Google Scholar]
- Alunno, A.; Petrillo, M.G.; Nocentini, G.; Bistoni, O.; Bartoloni, E.; Caterbi, S.; Bianchini, R.; Baldini, C.; Nicoletti, I.; Riccardi, C.;et al. Characterization of a new regulatory CD4+ T cell subset in primary sjogren’s syndrome. Rheumatology (Oxford) 2013, 52, 1387–1396. [Google Scholar]
- Nocentini, G.; Alunno, A.; Petrillo, M.; Bistoni, O.; Bartoloni, E.; Caterbi, S.; Ronchetti, S.; Migliorati, G.; Riccardi, C.; Gerli, R. Expansion of regulatory GITR+CD25low/−CD4+ T cells in systemic lupus erythematosus patients. Arthritis Res. Ther. 2014, 16, 444. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tang, X.; Tian, J.; Zhu, C.; Peng, H.; Rui, K.; Wang, Y.; Mao, C.; Ma, J.; Lu, L.; et al. Th17/Treg Cells Imbalance and GITRL Profile in Patients with Hashimoto’s Thyroiditis. Int. J. Mol. Sci. 2014, 15, 21674-21686. https://doi.org/10.3390/ijms151221674
Liu Y, Tang X, Tian J, Zhu C, Peng H, Rui K, Wang Y, Mao C, Ma J, Lu L, et al. Th17/Treg Cells Imbalance and GITRL Profile in Patients with Hashimoto’s Thyroiditis. International Journal of Molecular Sciences. 2014; 15(12):21674-21686. https://doi.org/10.3390/ijms151221674
Chicago/Turabian StyleLiu, Yingzhao, Xinyi Tang, Jie Tian, Chenlu Zhu, Huiyong Peng, Ke Rui, Yungang Wang, Chaoming Mao, Jie Ma, Liwei Lu, and et al. 2014. "Th17/Treg Cells Imbalance and GITRL Profile in Patients with Hashimoto’s Thyroiditis" International Journal of Molecular Sciences 15, no. 12: 21674-21686. https://doi.org/10.3390/ijms151221674




