Some Characteristics of Free Cell Population in the Airways of Rats after Intratracheal Instillation of Copper-Containing Nano-Scale Particles
Abstract
:1. Introduction
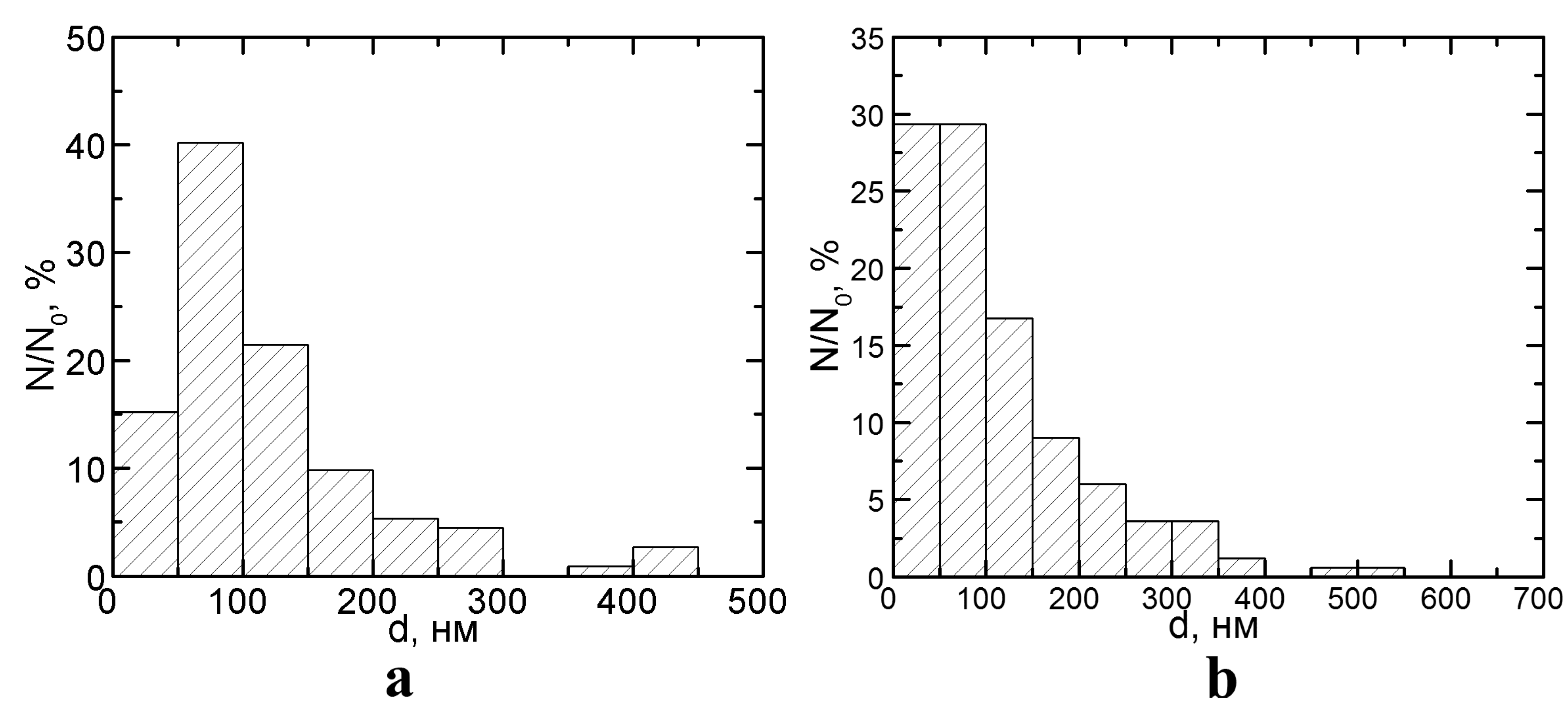
2. Results and Discussion
2.1. Optical Microscopy Data
| Particles Administered | Number of Cells * (×106) | NL/AM | ||
|---|---|---|---|---|
| Total | Neutrophil Leukocytes (NL) | Alveolar Macrophages (AM) | ||
| Nanoparticles | 12.42 ± 1.89 *,● | 9.8 ± 2.16 *,● | 2.44 ± 0.38 * | 4.76 ± 1.39 *,● |
| Submicron particles | 6.79 ± 1.28 * | 3.64 ± 0.90 * | 3.06 ± 0.86 * | 1.39 ± 0.16 * |
| None (controls) | 1.06 ± 0.14 | 0.052 ± 0.01 | 0.95 ± 0.18 | 0.06 ± 0.01 |
2.2. Some Indices of BALF Biochemistry
| Indices | Groups of Rats Instilled Intratracheally with | ||
|---|---|---|---|
| Water (Control) | Nanoparticles | Submicron Particles | |
| Glucose, mmol/L | 0.56 ± 0.00 | 1.36 ± 0.25 *,● | 0.56 ± 0.00 |
| Urea nitrogen, mmol/L | 0.36 ± 0.00 | 0.68 ± 0.10 *,● | 0.36 ± 0.00 |
| Uric acid, µmol/L | 14.67 ± 0.33 | 16.75 ± 0.50 *,● | 14.25 ± 0.25 |
| Amylase, mcmol/L·min | 30.00 ± 0.00 | 140.25 ± 3.50 *,● | 30.00 ± 0.00 |
| Alkaline phosphatase, mcmol/L·min | 20.33 ± 2.60 | 29.75 ± 3.77 | 27.00 ± 1.68 |
| ALT, mcmol/L·min | 21.33 ± 0.67 | 25.25 ± 0.29 *,● | 22.00 ± 0.41 |
| AST, mcmol/L·min | 7.33 ± 1.20 | 39.75 ± 2.29 *,● | 19.50 ± 1.66 * |
| De Ritis ratio | 0.34 ± 0.05 | 1.57 ± 0.08 *,● | 0.88 ± 0.07 * |
| γ-Glutamyl transferase, mcmol/L·min | 5.33 ± 0.33 | 14.50 ± 0.87 * | 10.25 ± 0.63 * |
| Lactate dehydrogenase, mcmol/L·min | 166.67 ± 30.33 | 821.50 ± 9.50 *,● | 601.75 ± 54.65 * |
| Calcium, mmol/L | 0.12 ± 0.00 | 0.25 ± 0.02 *,● | 0.12 ± 0.00 |
| Magnesium, mmol/L | 0.12 ± 0.00 | 0.16 ± 0.01 *,● | 0.12 ± 0.00 |
| Phosphorus, mmol/L | 0.14 ± 0.01 | 0.25 ± 0.02 *,● | 0.14 ± 0.00 |
| Iron, µmol/L | 0.40 ± 0.00 | 0.83 ± 0.21 *,● | 0.40 ± 0.00 |
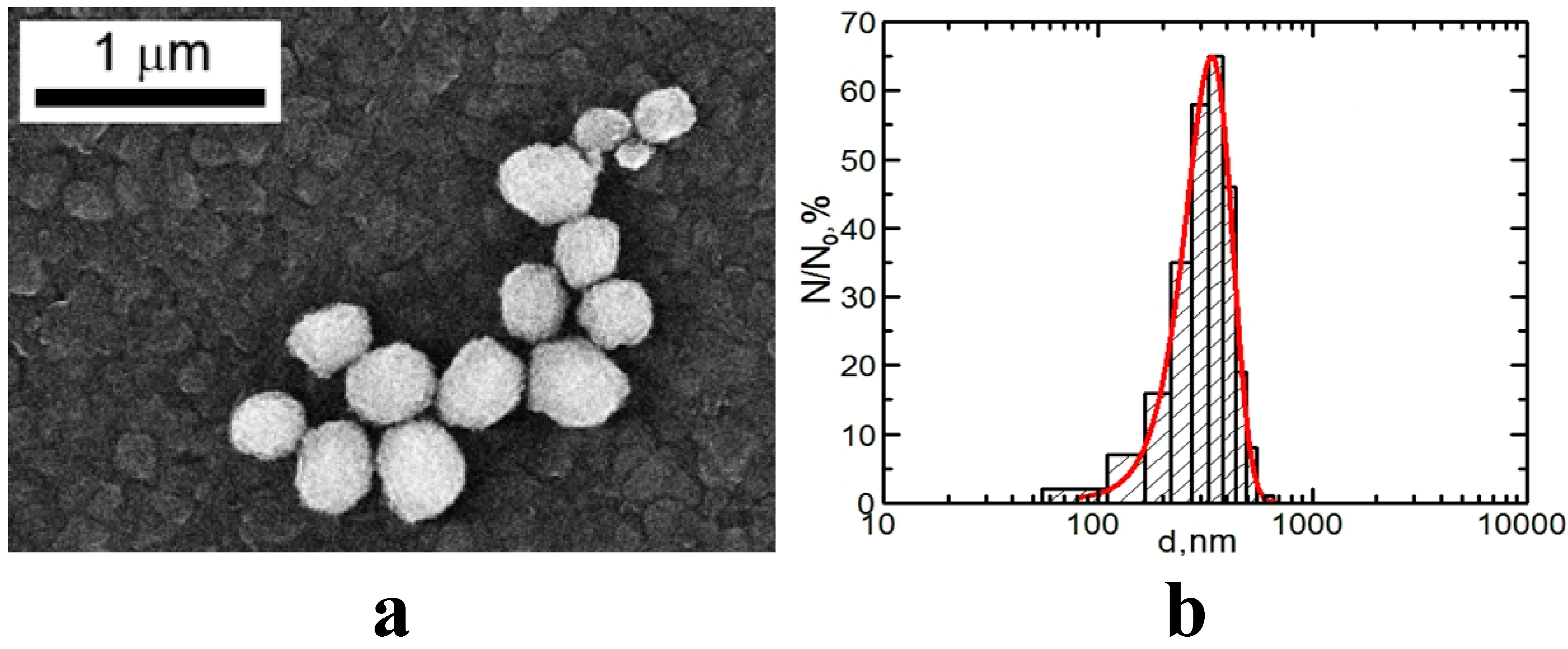
2.3. Semi-Contact Atomic Force Microscopy (sc-AFM) and Transmission Electron Microscopy (TEM) Data
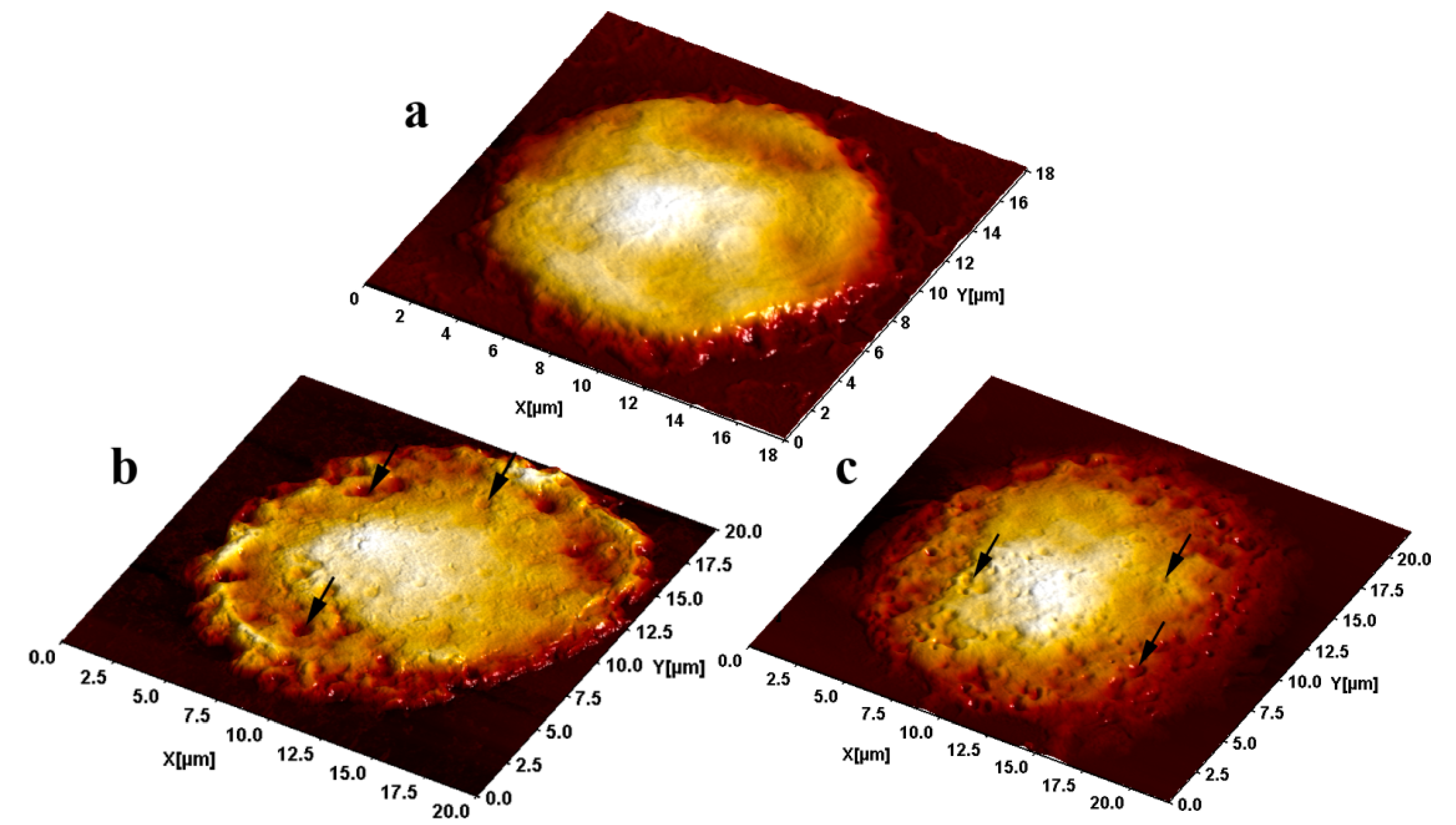
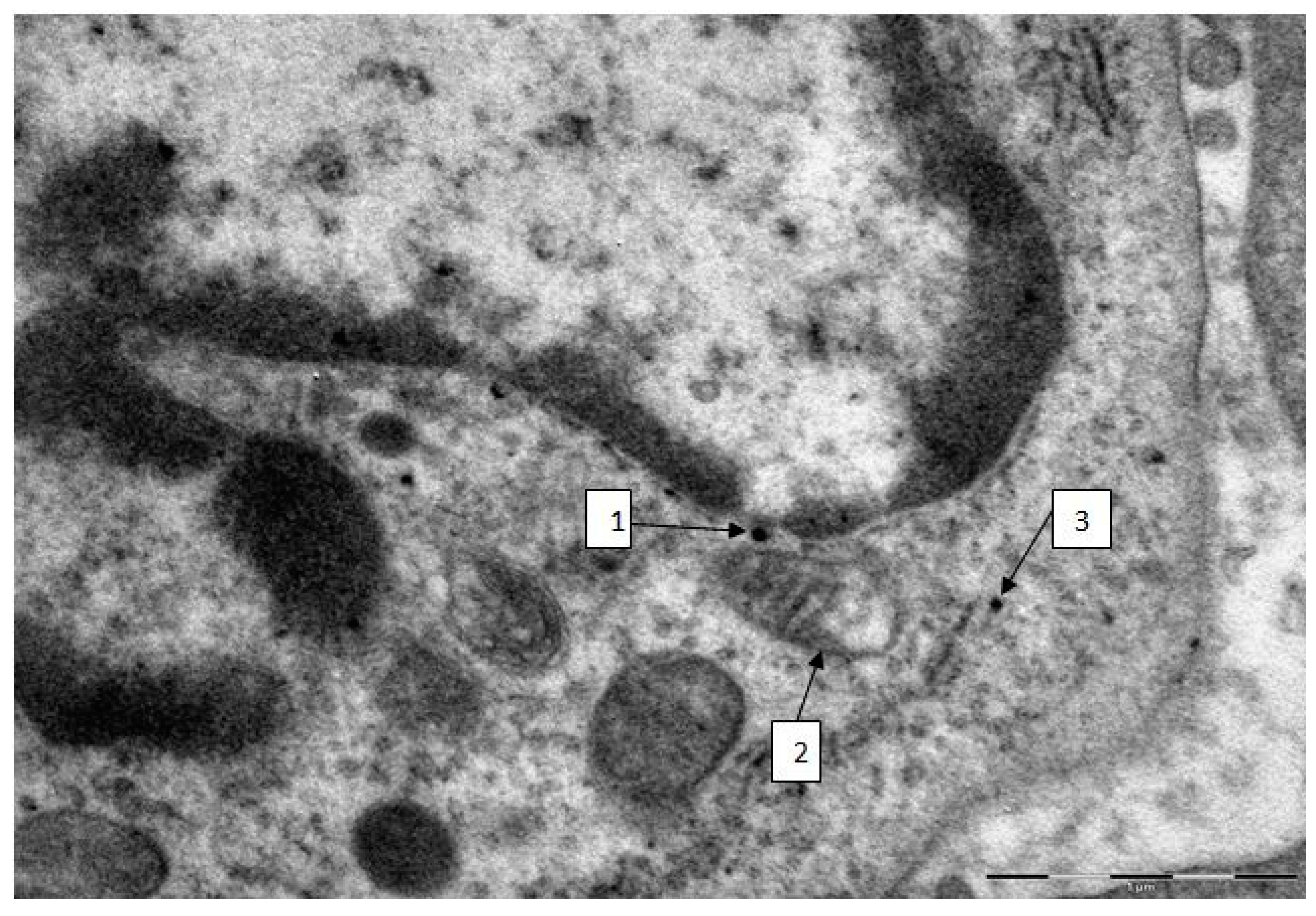
3. Experimental Section
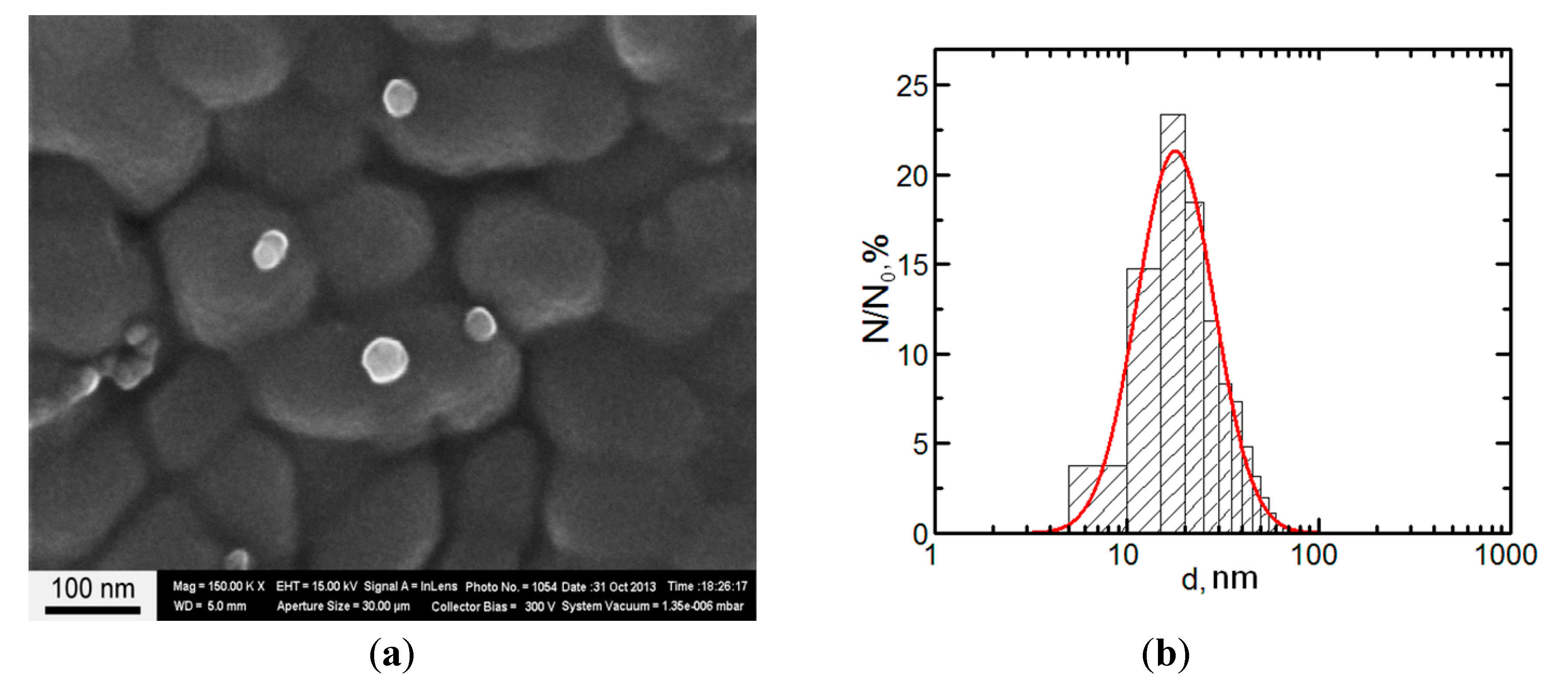
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kahru, A. Sub-toxic effects of CuO nanoparticles on bacteria:Kinetics, role of Cu ions and possible mechanisms of action. Environ. Pollut. 2012, 169, 81–89. [Google Scholar]
- Pang, C.; Selck, H.; Misra, S.K.; Berhanu, D.; Dybowska, A.; Valsami-Jones, E.; Forbes, V.E. Effects of sediment-associated copper to the deposit-feeding snail, Potamopyrgus antipodarum: A comparison of Cu added in aqueous form or as nano- and micro-CuO particles. Aquat. Toxicol. 2012, 15, 114–122. [Google Scholar]
- Studer, A.M.; Limbach, L.K.; van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 169, 169–174. [Google Scholar]
- Cronholm, P.; Karlsson, H.L.; Hedberg, J.; Lowe, T.A.; Winnberg, L.; Elihn, K.; Wallinder, I.O.; Möller, L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small 2013, 8, 970–982. [Google Scholar]
- Cuillel, M.; Chevallet, M.; Charbonnier, P.; Fauquant, C.; Pignot-Paintrand, I.; Arnaud, J.; Cassio, D.; Michaud-Soret, I.; Mintz, E. Interference of CuO nanoparticles with metal homeostasis in hepatocytes under sub-toxic conditions. Nanoscale 2014, 16, 1707–1715. [Google Scholar]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 25, 109–120. [Google Scholar]
- Liao, M.; Liu, H. Gene expression profiling of nephrotoxicity from copper nanoparticles in rats after repeated oral administration. Environ. Toxicol. Pharmacol. 2012, 34, 67–80. [Google Scholar]
- Pan, X.; Redding, J.E.; Wiley, P.A.; Wen, L.; McConnell, J.S.; Zhang, B. Mutagenicity evaluation of metal oxide nanoparticles by the bacterial reverse mutation assay. Chemosphere 2010, 79, 113–116. [Google Scholar]
- Song, M.F.; Li, Y.S.; Kasai, H.; Kawai, K. Metal nanoparticle-induced micronuclei and oxidative DNA damage in mice. J. Clin. Biochem. Nutr. 2012, 50, 211–216. [Google Scholar]
- Gomes, T.; Araújo, O.; Pereira, R.; Almeida, A.C.; Cravo, A.; Bebianno, M.J. Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2013, 84, 51–59. [Google Scholar]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar]
- Akhtar, M.J.; Kumar, S.; Alhadlaq, H.A.; Alrokayan, S.A.; Abu-Salah, K.M.; Ahamed, M. Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol. Ind. Health 2013. doi:10.1177/0748233713511512. [Google Scholar]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar]
- Xu, J.; Li, Z.; Xu, P.; Xiao, L.; Yang, Z. Nanosized copper oxide induces apoptosis through oxidative stress in podocytes. Arch. Toxicol. 2013, 87, 1067–1073. [Google Scholar]
- Privalova, L.I.; Katsnelson, B.A.; Loginova, N.V.; Gurvich, V.B.; Shur, V.Y.; Valamina, I.E.; Makeyev, O.H.; Sutunkova, M.P.; Minigalieva, I.A.; Kireyeva, E.P. Subchronic toxicity of copper oxide nanoparticles and its attenuation with the help of a combination of bioprotectors. Int. J. Mol. Sci. 2014, 15, 12379–12406. [Google Scholar]
- Katsnelson, B.A.; Privalova, L.I.; Kuzmin, S.V.; Degtyareva, T.D.; Sutunkova, M.P.; Yeremenko, O.S.; Minigalieva, I.A.; Kireyeva, E.P.; Khodos, M.Y.; Kozitsina, A.N.; et al. Some peculiarities of pulmonary clearance mechanisms in rats after intratracheal instillation of magnetite (Fe3O4) suspensions with different particle sizes in the nanometer and micrometer ranges: Are we defenseless against nanoparticles? Int. J. Occup. Environ. Health 2010, 16, 508–524. [Google Scholar]
- Katsnelson, B.A.; Privalova, L.I.; Degtyareva, T.D.; Sutunkova, M.P.; Yeremenko, O.S.; Minigalieva, I.A.; Kireyeva, E.P.; Kozitsina, A.N.; Malakhova, N.A.; Glazyrina, J.A.; et al. Experimental estimates of the toxicity of iron oxide Fe3O4 (magnetite) nanoparticles. Cent. Eur. J. Occup. Environ. Med. 2010, 16, 47–63. [Google Scholar]
- Katsnelson, B.A.; Privalova, L.I.; Sutunkova, M.P.; Tulakina, L.G.; Pichugova, S.V.; Beikin, J.B.; Khodos, M.Y. The “in vivo” interaction between iron oxide Fe3O4 nanoparticles and alveolar macrophages. Bull. Exp. Biol. Med. 2012, 152, 627–631. [Google Scholar]
- Katsnelson, B.A.; Privalova, L.I.; Sutunkova, M.P.; Khodos, M.Y.; Shur, V.Y.; Shishkin, E.I.; Tulakina, L.G.; Pichugova, S.V.; Beikin, J.B. Uptake of some metallic nanoparticles by, and their impact on pulmonary macrophages in vivo as viewed by optical, atomic force, and transmission electron microscopy. J. Nanomed. Nanotechnol. 2012, 3, 1–8. [Google Scholar]
- Katsnelson, B.A.; Privalova, L.I.; Gurvich, V.B.; Makeyev O.H., Shur, V.Y.; Beikin, J.B.; Sutunkova, M.P.; Kireyeva, E.P.; Minigalieva, I.A.; Loginova, N.V.; et al. Comparative in vivo assessment of some adverse bio-effects of equidimensional gold and silver nanoparticles and the attenuation of nanosilver’s effects with a complex of innocuous bioprotectors. Int. J. Mol. Sci. 2013, 14, 2449–2483. [Google Scholar]
- Privalova, L.I.; Katsnelson, B.A.; Osipenko, A.B.; Yushkov, B.H.; Babushkina, L.G. Response of a phagocyte cell system to products of macrophage breakdown as a probable mechanism of alveolar phagocytosis adaptation to deposition of particles of different cytotoxicity. Environ. Health Perspect. 1980, 35, 205–218. [Google Scholar]
- Katsnelson, B.A.; Privalova, L.I. Recruitment of phagocytizing cells into the respiratory tract as a response to the cytotoxic action of deposited particles. Environ. Health Perspect. 1984, 55, 313–325. [Google Scholar]
- Katsnelson, B.A.; Konysheva, L.K.; Privalova, L.Y.; Sharapova, N.Y. Quartz dust retention in rat lungs under chronic exposure simulated by a multicompartmental model: Further evidence of the key role of the cytotoxicity of quartz particles. Inhalat. Toxicol. 1997, 9, 703–715. [Google Scholar]
- Privalova, L.I.; Katsnelson, B.A.; Sharapova, N.Y.; Kislitsina, N.S. On the relationship between activation and the breakdown of macrophages in pathogenesis of silicosis. Med. Lav. 1995, 86, 511–521. [Google Scholar]
- Zhang, Q.; Yukinori, K.; Sato, K.; Nakakuki, K.; Koyahama, N.; Donaldson, K. Differences in the extent of inflammation caused by intratracheal exposure to three ultrafine metals: Role of free radicals. J. Toxicol. Environ. Health 1998, 53, 423–438. [Google Scholar]
- Zhang, Q.; Yukinori, K.; Zhu, X.; Sato, K.; Mo, Y.; Kluz, T.; Domaldson, K. Comparative toxicity ofstandard nickel and ultrafine nickel after intratracheal instillation. J. Occip. Health 2003, 45, 23–30. [Google Scholar]
- Fröhlich, E. Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles. J. Curr. Drug. Metab. 2013, 14, 976–988. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Privalova, L.I.; Katsnelson, B.A.; Loginova, N.V.; Gurvich, V.B.; Shur, V.Y.; Beikin, Y.B.; Sutunkova, M.P.; Minigalieva, I.A.; Shishkina, E.V.; Pichugova, S.V.; et al. Some Characteristics of Free Cell Population in the Airways of Rats after Intratracheal Instillation of Copper-Containing Nano-Scale Particles. Int. J. Mol. Sci. 2014, 15, 21538-21553. https://doi.org/10.3390/ijms151121538
Privalova LI, Katsnelson BA, Loginova NV, Gurvich VB, Shur VY, Beikin YB, Sutunkova MP, Minigalieva IA, Shishkina EV, Pichugova SV, et al. Some Characteristics of Free Cell Population in the Airways of Rats after Intratracheal Instillation of Copper-Containing Nano-Scale Particles. International Journal of Molecular Sciences. 2014; 15(11):21538-21553. https://doi.org/10.3390/ijms151121538
Chicago/Turabian StylePrivalova, Larisa I., Boris A. Katsnelson, Nadezhda V. Loginova, Vladimir B. Gurvich, Vladimir Y. Shur, Yakov B. Beikin, Marina P. Sutunkova, Ilzira A. Minigalieva, Ekaterina V. Shishkina, Svetlana V. Pichugova, and et al. 2014. "Some Characteristics of Free Cell Population in the Airways of Rats after Intratracheal Instillation of Copper-Containing Nano-Scale Particles" International Journal of Molecular Sciences 15, no. 11: 21538-21553. https://doi.org/10.3390/ijms151121538




