Cobalt Alleviates GA-Induced Programmed Cell Death in Wheat Aleurone Layers via the Regulation of H2O2 Production and Heme Oxygenase-1 Expression
Abstract
:1. Introduction
2. Results
2.1. Cobalt Delays Gibberellic Acid (GA)-Induced Programmed Cell Death (PCD)
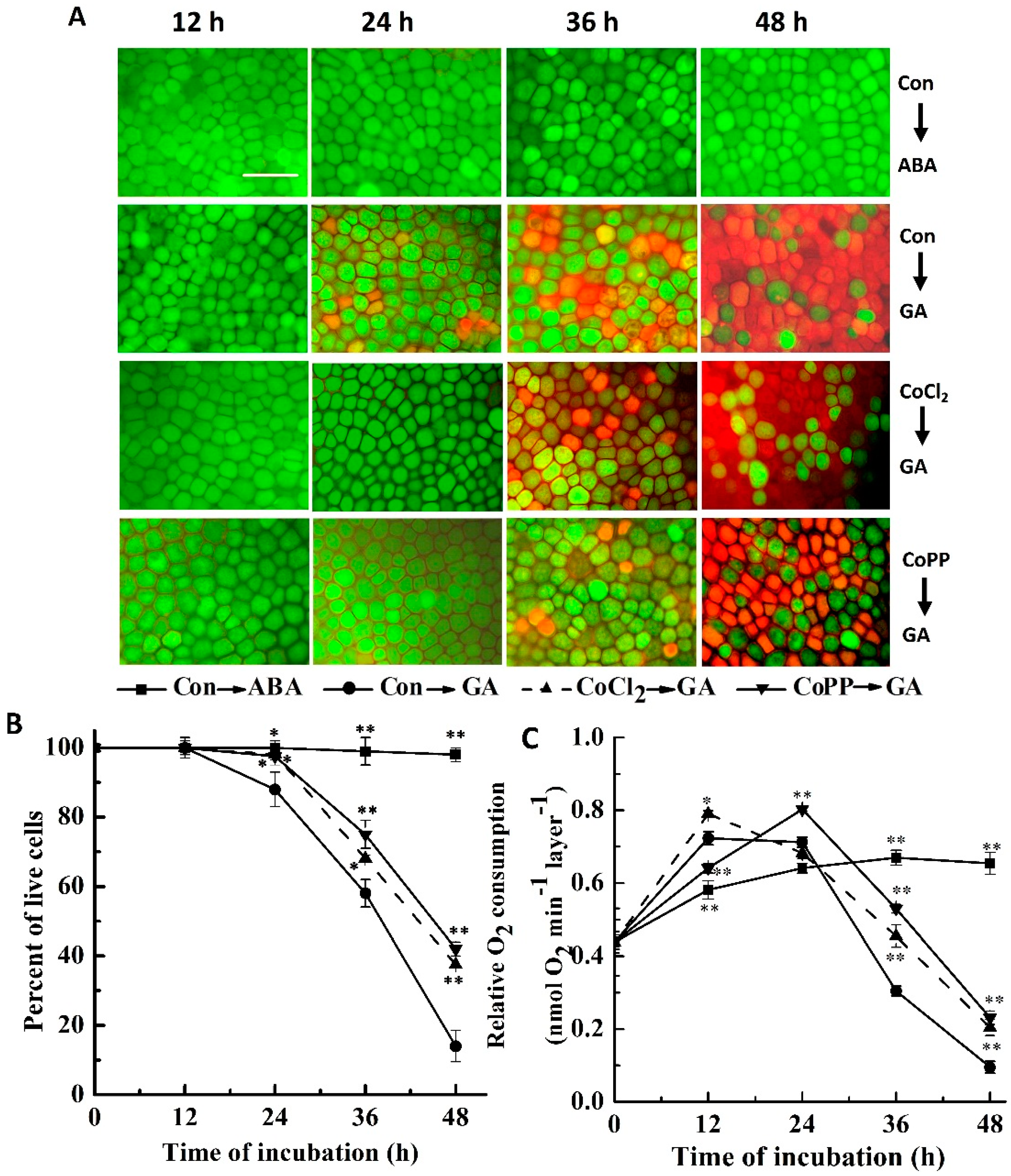
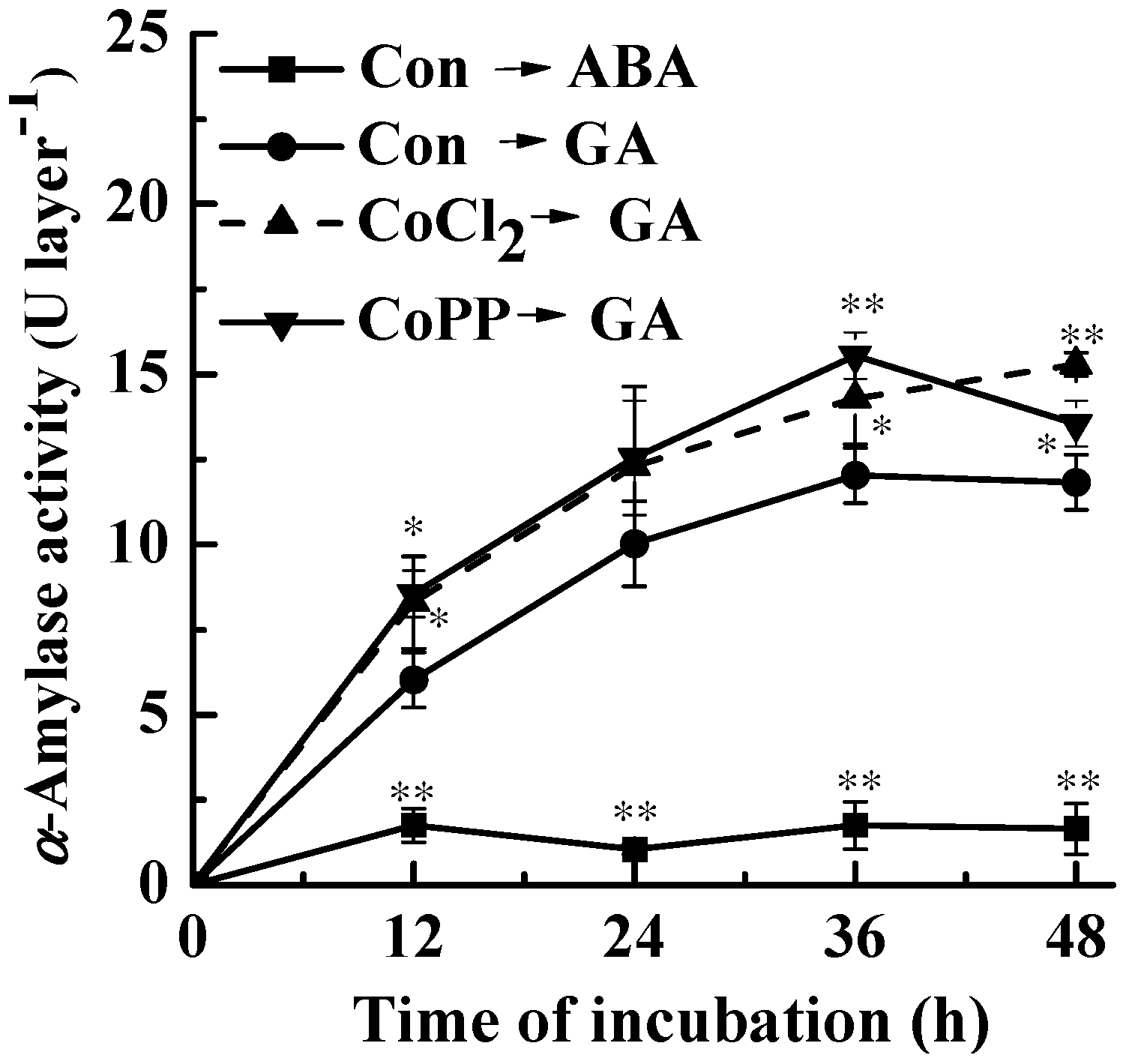
2.2. Up-Regulation of α-Amylase Caused by Cobalt
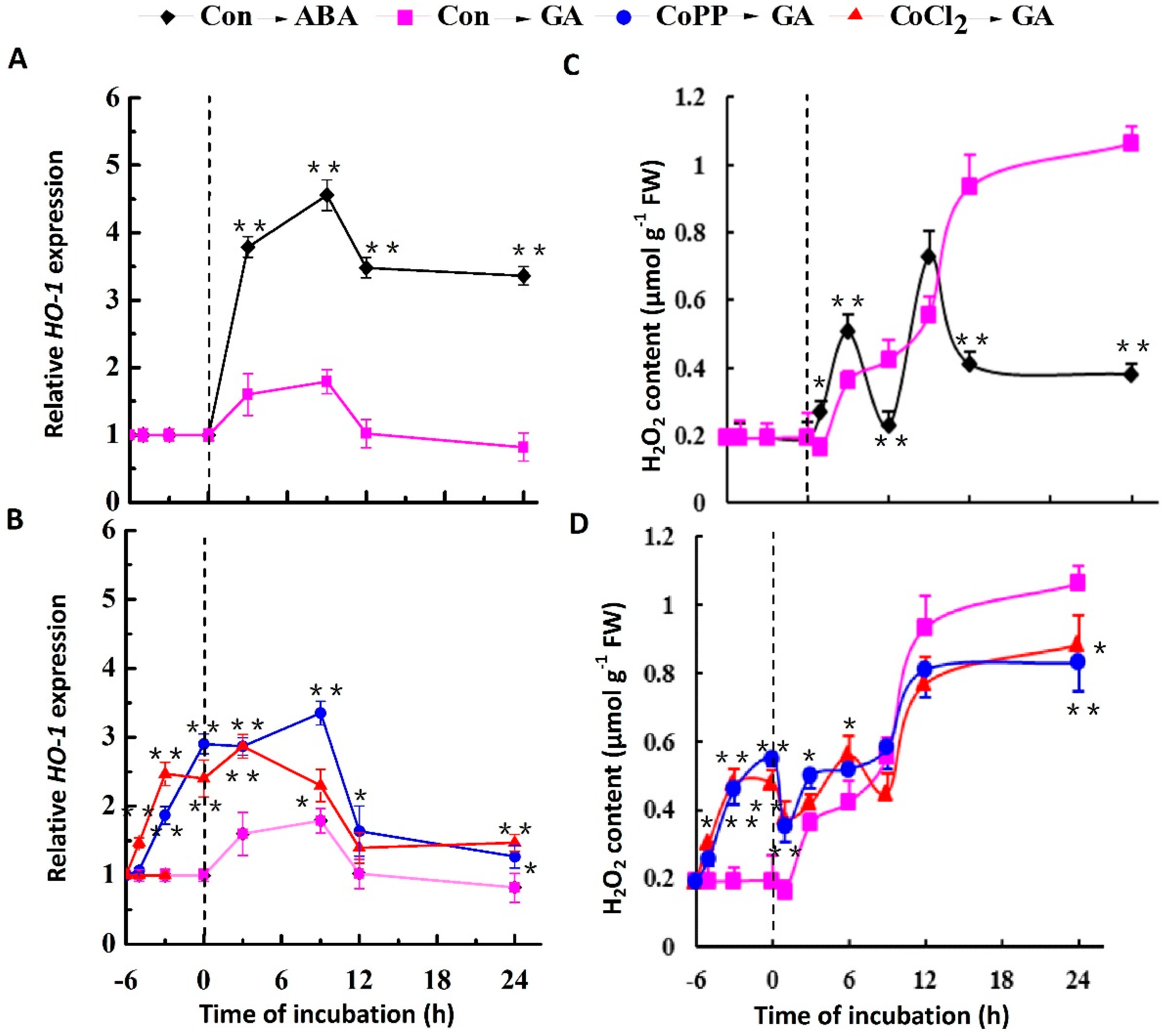
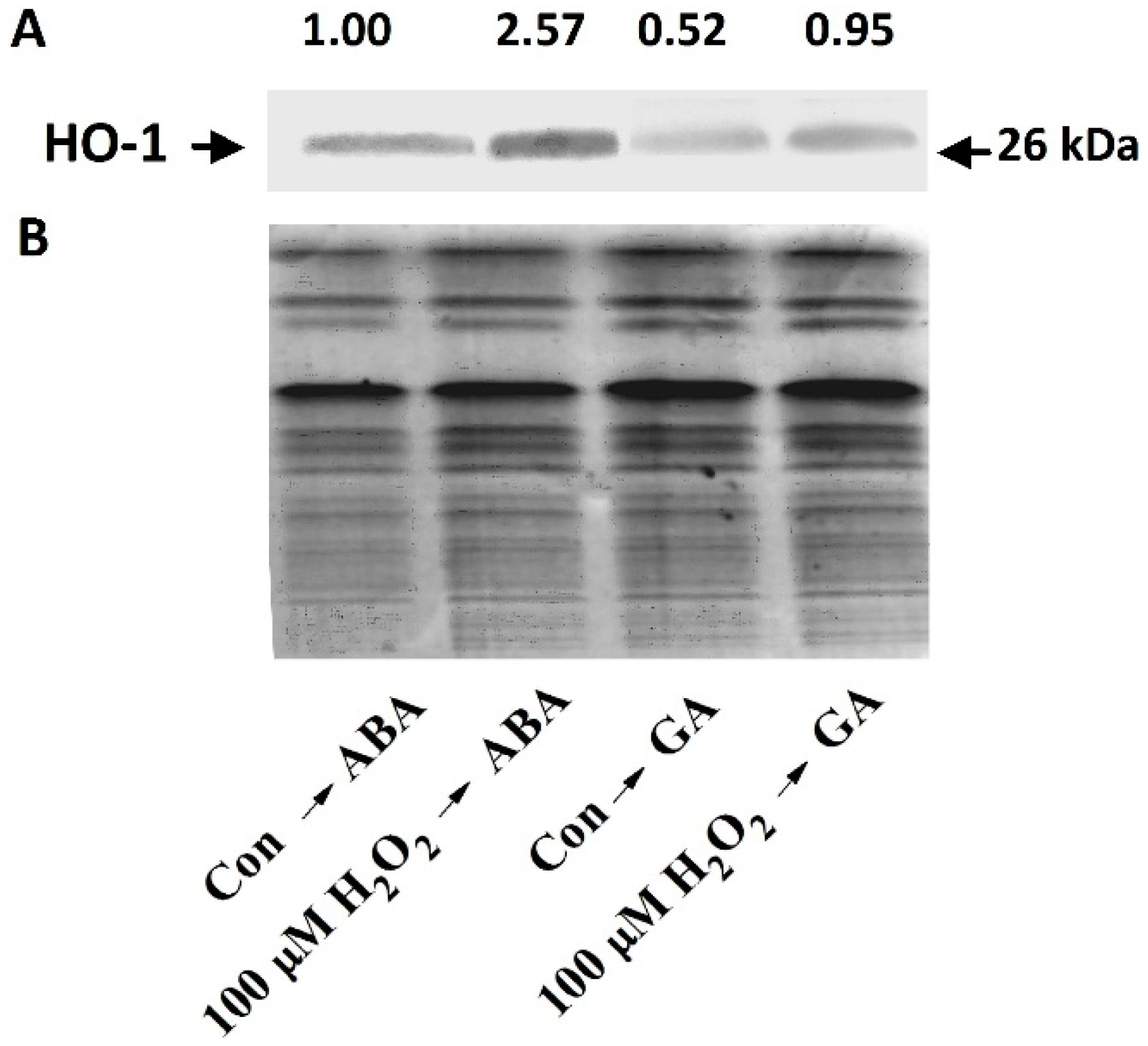
2.3. Stimulation of HO-1 Gene Expression and H2O2 Production by Cobalt
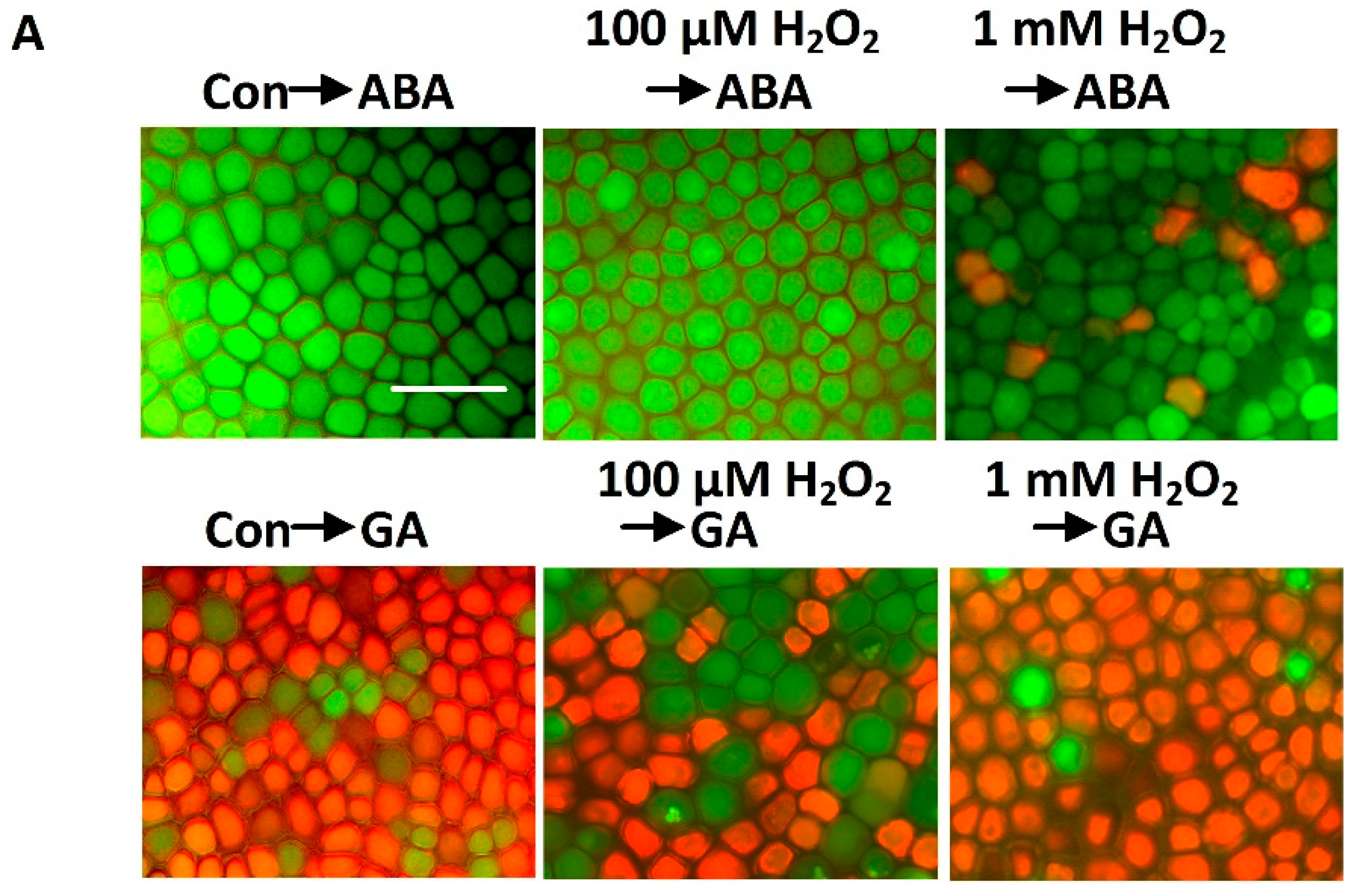
2.4. Exogenous H2O2 Mimics the Effect of Cobalt on Delaying GA-Induced PCD

2.5. Decreased H2O2 Production Intensifies PCD in Wheat Aleurone Layers
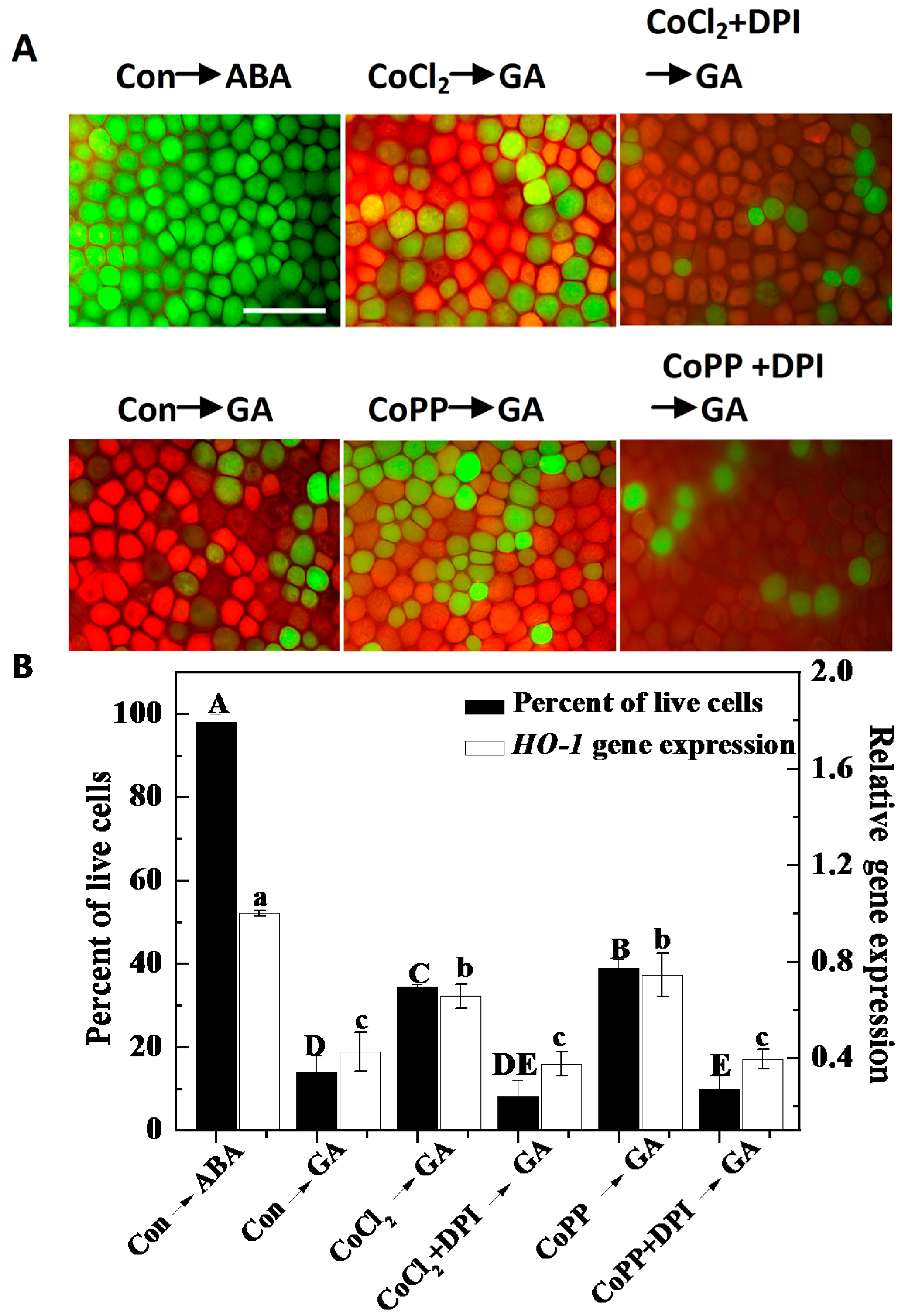
2.6. Cobalt Action Is Sensitive to ZnPPIX
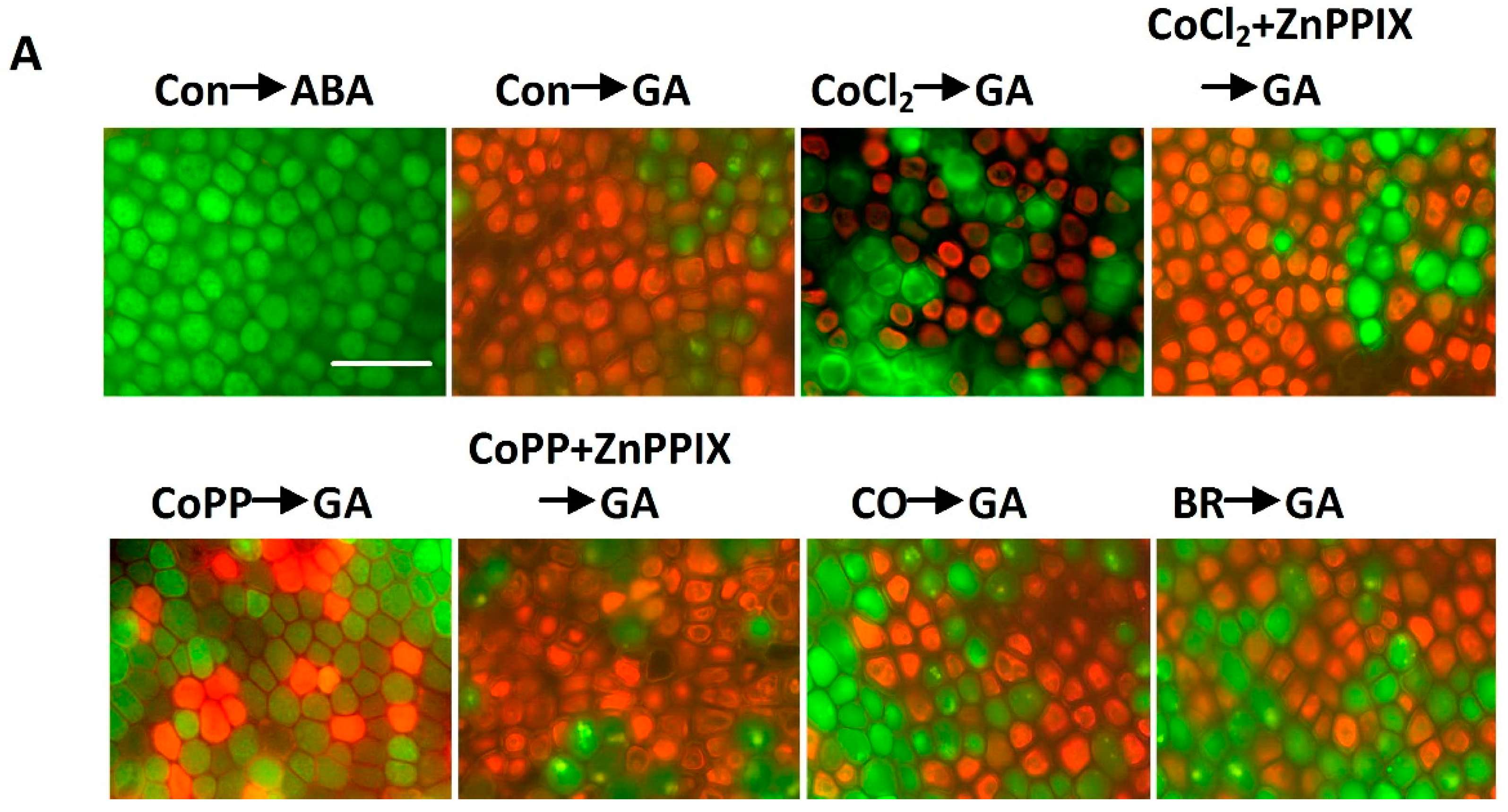
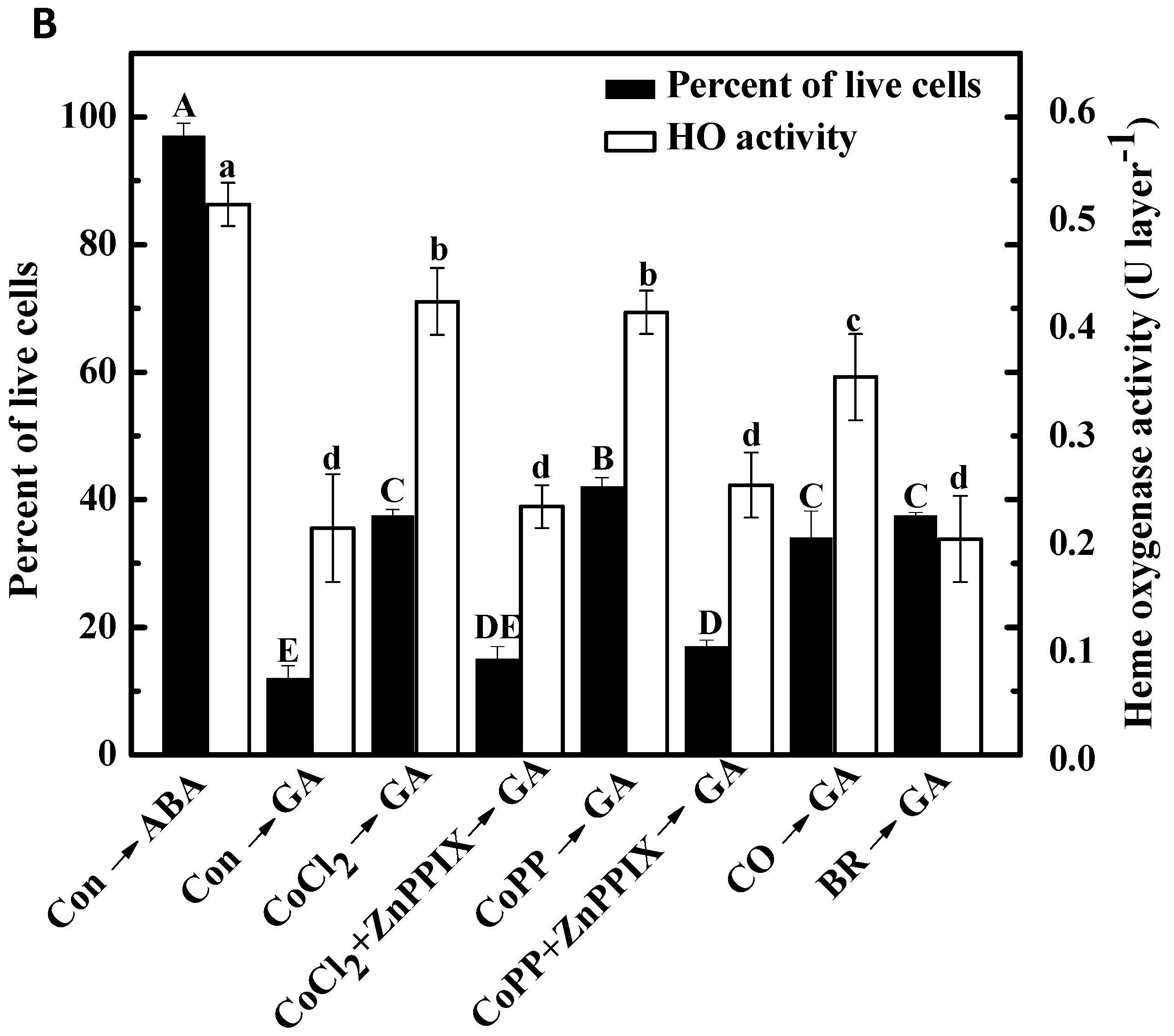
2.7. Decrease of H2O2 Accumulation Induced by Cobalt

2.8. Changes of Antioxidant Enzyme Activities and Gene Expression
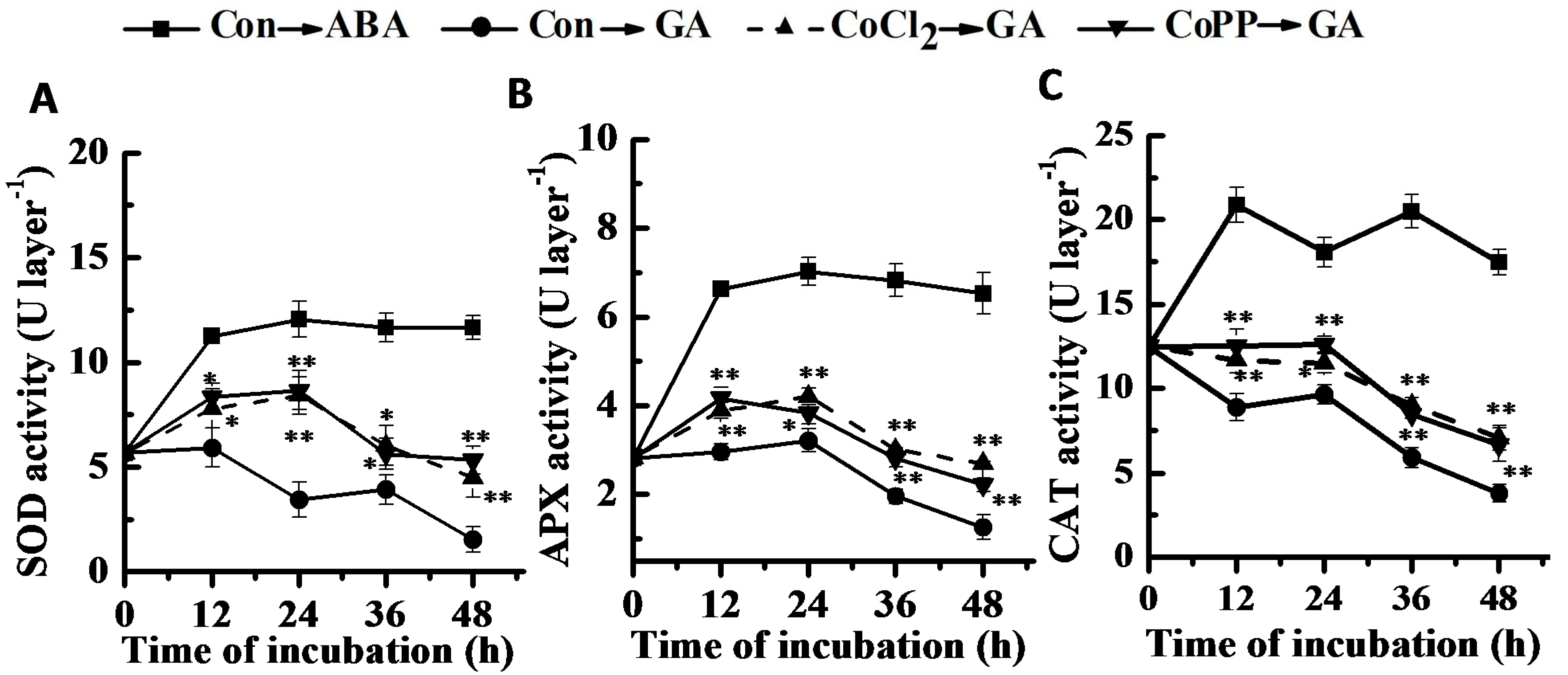
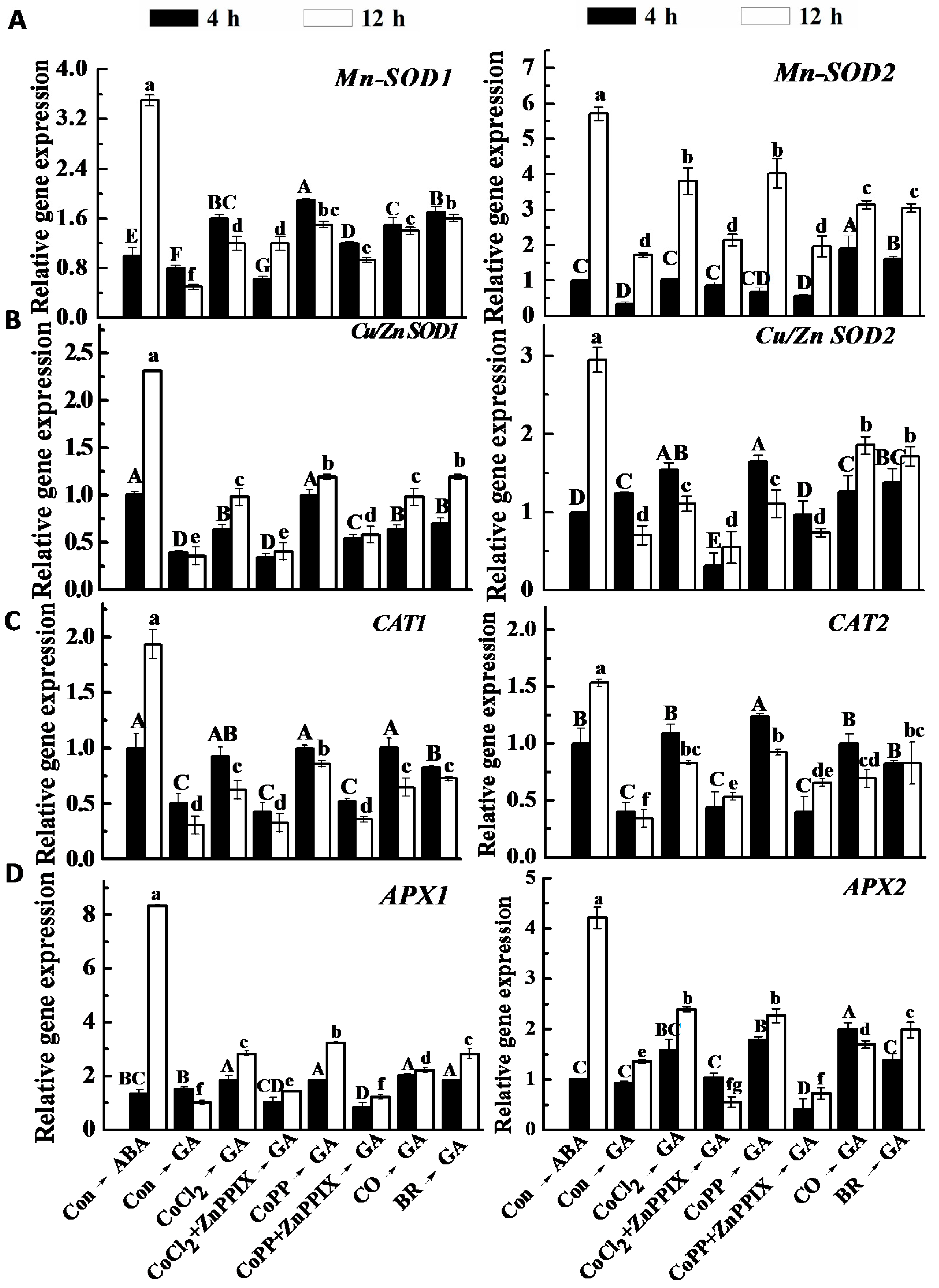
3. Discussion
4. Experimental Section
4.1. Plant Materials and Chemicals
4.2. Preparation of 1% CO Aqueous Solution
4.3. Determination of Cell Viability
4.4. O2 Consumption
4.5. Determination of HO Activity and Western Blot Analysis for the HO-1 Protein
4.6. Determination of the Activities of Other Enzymes
4.7. RNA Isolation, cDNA Synthesis and qRT-PCR Analysis
4.8. Visualization and Measurement of H2O2 Production
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Chandler, P.M.; Zwar, J.A.; Jacobsen, J.V.; Higgins, T.J.V.; Inglis, A.S. The effect of gibberellic acid and abscisic acid on α-amylase mRNA levels in barley aleurone layers: Studies using an α-amylase cDNA clone. Plant Mol. Biol. 1984, 3, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Jones, R.L.; Jacobsen, J.V. Regulation of synthesis and transport of secreted proteins in cereal aleurone. Int. Rev. Cytol. 1991, 126, 49–88. [Google Scholar] [PubMed]
- Bethke, P.C.; Lonsdale, J.E.; Fath, A.; Jones, R.L. Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell 1999, 11, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Fath, A.; Bethke, P.C.; Jones, R.L. Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol. 2001, 126, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Fath, A.; Bethke, P.; Beligni, V.; Jones, R. Active oxygen and cell death in cereal aleurone cells. J. Exp. Bot. 2002, 53, 1273–1282. [Google Scholar] [CrossRef]
- Kliewer, M.; Evans, H.J. Effect of cobalt deficiency on the B12 coenzyme content of Rhizobium meliloti. Arch. Biochem. Biophys. 1962, 97, 428–429. [Google Scholar] [CrossRef]
- Kliewer, M.; Evans, H.J. Cobamide coenzyme contents of soybean nodules & nitrogen fixing bacteria in relation to physiological conditions. Plant Physiol. 1963, 38, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Sharma, A.; Talukder, G. Effects of cobalt on plants. Bot. Rev. 1994, 60, 149–181. [Google Scholar] [CrossRef]
- Kenesarina, N.A. The effect of mineral fertilizers on cobalt content in potato plants. Izv. Akad. Nauk. Kaz. SSR. Ser. Biol. 1972, 6, 31–35. [Google Scholar]
- Joshi, P.K.; Bhatt, D.N.; Kulkarni, J.H. Groundnut root nodulation as affected by micronutrient application and Rhizobium inoculation. Int. J. Trop. Agric. 1987, 5, 199–202. [Google Scholar]
- Gad, N.; Atta-Aly, M.A. Effect of cobalt on the formation, growth and development of adventitious roots in tomato and cucumber cuttings. J. Appl. Sci. Res. 2006, 2, 423–429. [Google Scholar]
- Xu, S.; Zhang, B.; Cao, Z.Y.; Ling, T.F.; Shen, W.B. Heme oxygenase is involved in cobalt chloride-induced lateral root development in tomato. Biometals 2011, 24, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.Y.; Chao, Y.; Kao, C.H. Cobalt chloride-induced lateral root formation in rice: The role of heme oxygenase. J. Plant Physiol. 2013, 170, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Tosh, S.; Choudhuri, M.A.; Chatterjee, S.K. Retardation of lettuce (Lactuca sativa) leaf senescence by cobalt ions. Indian. J. Exp. Biol. 1979, 17, 1134–1136. [Google Scholar]
- Tarabrin, V.P.; Teteneva, T.R. Presowing treatment of seeds and its effect on drought resistance of woody plant seedlings. Sov. J. Ecol. 1979, 10, 204–211. [Google Scholar]
- Koval’Skii, V.V.; Grinkevich, N.I.; Gribovskaya, I.F.; Dinevich, L.S.; Shandova, A.N. Cobalt in medicinal plants and its effect on the accumulation of biologically active compounds. Restit. Resur. 1971, 7, 503–510. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, G.S.; Verma, K. Haem oxygenase (HO): An overlooked enzyme of plant metabolism and defence. J. Exp. Bot. 2010, 61, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signaling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Noriega, G.O.; Balestrasse, K.B.; Batlle, A.; Tomaro, M.L. Heme oxygenase exerts a protective role against oxidative stress in soybean leaves. Biochem. Biophys. Res. Commun. 2004, 323, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, J.; Chen, X.; Gao, Z.; Xuan, W.; Xu, S.; Ding, X.; Shen, W. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2008, 177, 155–166. [Google Scholar] [PubMed]
- Jin, Q.; Zhu, K.; Xie, Y.; Shen, W. Heme oxygenase-1 is involved in ascorbic acid-induced alleviation of cadmium toxicity in root tissues of Medicago sativa. Plant Soil 2013, 366, 605–616. [Google Scholar] [CrossRef]
- Xie, Y.J.; Zhang, C.; Lai, D.W.; Sun, Y.; Samma, M.K.; Zhang, J.; Shen, W. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J. Plant Physiol. 2014, 171, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.; Lin, J.H.; Schwartzman, M.L.; Levere, R.D.; Shibahara, S. The physiological significance of heme oxygenase. Int. J. Biochem. 1988, 20, 543–558. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ishikawa, K.; Itabe, H.; Maruyama, Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol. Cell Biochem. 2006, 291, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ling, T.; Han, Y.; Liu, K.; Zheng, Q.; Huang, L.; Yuan, X.; He, Z.; Hu, B.; Fang, L.; et al. Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant Cell Environ. 2008, 31, 1864–1881. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Yuan, F.; Hu, S.; He, P. Effects of hematin and carbon monoxide on the salinity stress responses of Cassia obtusifolia L. seeds and seedlings. Plant Soil 2012, 359, 85–105. [Google Scholar] [CrossRef]
- Suhita, D.; Raghavendra, A.S.; Kwak, J.M.; Vavasseur, A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol. 2004, 134, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Y.; Huang, B.K.; Wang, Q.Y.; Xuan, W.; Ling, T.F.; Zhang, B.; Chen, X.; Nie, L.; Shen, W.B. Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin. Sci. Bull. 2007, 52, 2365–2373. [Google Scholar] [CrossRef]
- Noriega, G.O.; Yannarelli, G.G.; Balestrasse, K.B.; Batlle, A.; Tomaro, M.L. The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta 2007, 226, 1155–1163. [Google Scholar] [CrossRef]
- Razem, F.A.; Hill, R.D. Hydrogen peroxide affects abscisic acid binding to ABAP1 in barley aleurones. Biochem. Cell Biol. 2007, 85, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhang, J.; Xuan, W.; Xie, Y. Up-regulation of heme oxygenase-1 contributes to the amelioration of aluminum-induced oxidative stress in Medicago sativa. J. Plant Physiol. 2013, 170, 1328–1336. [Google Scholar] [CrossRef]
- Yannarelli, G.G.; Noriega, G.O.; Batlle, A.; Tomaro, M.L. Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 2006, 224, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Ding, X.; Xu, S.; Wang, R.; Xuan, W.; Cao, Z.Y.; Chen, J.; Wu, H.H.; Ye, M.B.; Shen, W.B. Endogenous hydrogen peroxide plays a positive role in the up-regulation of heme oxygenase and acclimation to oxidative stress in wheat seedling leaves. J. Integr. Plant Biol. 2009, 51, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Beligni, M.V.; Fath, A.; Bethke, P.C.; Lamattina, L.; Jones, R.L. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 2002, 129, 1642–1650. [Google Scholar] [CrossRef]
- Wu, M.; Huang, J.; Xu, S.; Ling, T.; Xie, Y.; Shen, W. Haem oxygenase delays programmed cell death in wheat aleurone layers by modulation of hydrogen peroxide metabolism. J. Exp. Bot. 2011, 62, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Kutsuna, N.; Hasezawa, S. Dynamic organization of vacuolar and microtubule structures during cell cycle progression in synchronized tobacco BY-2 cells. Plant Cell Physiol. 2002, 43, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Hong-Hermesdorf, A.; Stierhof, Y.D.; Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006, 18, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kutsuna, N.; Kanazawa, Y.; Kondo, N.; Hasezawa, S.; Sano, T. Intra-vacuolar reserves of membranes during stomatal closure: The possible role of guard cell vacuoles estimated by 3-D reconstruction. Plant Cell Physiol. 2007, 48, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Weaver, M.S.; Cao, B.; Dennis, J.E.; van Biber, B.; Laflamme, M.A.; Allen, M.D. Cobalt protoporphyrin pretreatment protects human embryonic stem cell-derived cardiomyocytes from hypoxia/reoxygenation injury in vitro and increases graft size and vascularization in vivo. Stem Cells Transl. Med. 2014, 3, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Józkowicz, A. Carbon monoxide—A “new” gaseous modulator of gene expression. Acta Biochim. Pol. 2003, 50, 31–47. [Google Scholar] [PubMed]
- Han, B.; Xu, S.; Xie, Y.J.; Huang, J.J.; Wang, L.J.; Yang, Z.; Zhang, C.H.; Sun, Y.; Shen, W.B.; Xie, G.S. ZmHO-1, a maize haem oxygenase-1 gene, plays a role in determining lateral root development. Plant Sci. 2012, 184, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tawaratsumida, T.; Kondo, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Zheng, S.H.; Yuasa, T.; Iwaya-Inoue, M. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 2012, 158, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Padmanaban, G.; Sharma, P.S. Regulation of heme biosynthesis in Neurospora crassa. J. Biol. Chem. 1969, 244, 4241–4246. [Google Scholar] [PubMed]
- Wilks, A. Heme oxygenase: Evolution, structure, and mechanism. Antioxid. Redox Signal. 2002, 4, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, L.; Zhang, B.; Han, B.; Xie, Y.; Yang, J.; Zhong, W.; Chen, H.; Wang, R.; Wang, N.; et al. RNAi knockdown of rice SE5 gene is sensitive to the herbicide methyl viologen by the down-regulation of antioxidant defense. Plant Mol. Biol. 2012, 80, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D.; Kappas, A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc. Nat. Acad. Sci. USA 1974, 71, 4293–4297. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Villalon, P.; Martasek, P.; Abraham, N.G. Regulation of heme oxygenase gene expression by cobalt in rat liver and kidney. Eur. J. Biochem. 1990, 192, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Christova, T.Y.; Gorneva, G.A.; Taxirov, S.I.; Duridanova, D.B.; Setchenska, M.S. Effect of cisplatin and cobalt chloride on antioxidant enzymes in the livers of Lewis lung carcinoma-bearing mice: protective role of heme oxygenase. Toxicol. Lett. 2003, 138, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Ö.; Büyükbingöl, Z. Effect of cobalt on the oxidative status in heart and aorta of streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2003, 21, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Jones, R.L. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 2001, 25, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jabs, T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999, 57, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chao, Y.Y.; Hsu, Y.Y.; Kao, C.H. Heme oxygenase is involved in H2O2-induced lateral root formation in apocynin-treated rice. Plant Cell Rep. 2013, 32, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.; Cappelluti, S.; Cervantes-Cervantes, M.; Rodriguez, M.; Bush, D.S. Okadaic acid, a protein phosphatase inhibitor, blocks calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant Cell 1996, 8, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Mrva, K.; Wallwork, M.; Mares, D.J. α-Amylase and programmed cell death in aleurone of ripening wheat grains. J. Exp. Bot. 2006, 57, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Balla, J.; Otterbein, L.; Smith, R.N.; Brouard, S.; Lin, Y.; Csizmadia, E.; Sevigny, J.; Robson, S.C.; Vercellotti, G.; et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J. Immunol. 2001, 166, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Haga, M.; Tyagi, S.; Yamashita, K.; Graça-Souza, A.V.; Ollinger, R.; Czismadia, E.; May, G.A.; Ifedigbo, E.; Otterbein, L.E.; et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J. 2004, 18, 771–772. [Google Scholar] [PubMed]
- Jin, Q.; Zhu, K.; Cui, W.; Xie, Y.; Han, B.; Shen, W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2013, 36, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.K.; Jin, Q.J.; Xie, Y.J.; Liu, Y.H.; Lin, Y.T.; Shen, W.B.; Zhou, Y.J. Characterization of a wheat heme oxygenase-1 gene and its response to different abiotic stresses. Int. J. Mol. Sci. 2011, 12, 7692–7707. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, F.; Zhang, C.; Xie, Y.; Han, B.; Huang, J.; Shen, W. Heme oxygenase-1 is involved in nitric oxide- and cGMP-induced α-Amy2/54 gene expression in GA-treated wheat aleurone layers. Plant Mol. Biol. 2013, 81, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Varner, J.E. The bioassay of gibberellins. Planta 1966, 72, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Palma, K.; Kermode, A.R. Metabolism of hydrogen peroxide during reserve mobilization and programmed cell death of barley (Hordeum vulgare L.) aleurone layer cells. Free Radic. Biol. Med. 2003, 35, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, S.; Xuan, W.; Ling, T.; Cao, Z.; Huang, B.; Sun, Y.; Fang, L.; Liu, Z.Y.; Zhao, N. Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa. Plant Sci. 2007, 172, 544–555. [Google Scholar] [CrossRef]
- Shen, W.B.; Wang, R.; Wang, Y.H.; Zheng, T.Q.; Wan, J.M. A novel method of extracting total RNA from rice embryo samples. Hereditas 2003, 25, 208–210. [Google Scholar] [PubMed]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; de Lorenzo, G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1385. [Google Scholar] [CrossRef]
- De Michele, R.; Vurro, E.; Rigo, C.; Costa, A.; Elviri, L.; di Valentin, M.; Careri, M.; Zottini, M.; Sanità di Toppi, L.; lo Schiavo, F. Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol. 2009, 150, 217–228. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Li, J.; Wang, F.; Li, F.; Yang, J.; Shen, W. Cobalt Alleviates GA-Induced Programmed Cell Death in Wheat Aleurone Layers via the Regulation of H2O2 Production and Heme Oxygenase-1 Expression. Int. J. Mol. Sci. 2014, 15, 21155-21178. https://doi.org/10.3390/ijms151121155
Wu M, Li J, Wang F, Li F, Yang J, Shen W. Cobalt Alleviates GA-Induced Programmed Cell Death in Wheat Aleurone Layers via the Regulation of H2O2 Production and Heme Oxygenase-1 Expression. International Journal of Molecular Sciences. 2014; 15(11):21155-21178. https://doi.org/10.3390/ijms151121155
Chicago/Turabian StyleWu, Mingzhu, Jiale Li, Fangquan Wang, Feng Li, Jun Yang, and Wenbiao Shen. 2014. "Cobalt Alleviates GA-Induced Programmed Cell Death in Wheat Aleurone Layers via the Regulation of H2O2 Production and Heme Oxygenase-1 Expression" International Journal of Molecular Sciences 15, no. 11: 21155-21178. https://doi.org/10.3390/ijms151121155




