Chrysin Protects against Focal Cerebral Ischemia/Reperfusion Injury in Mice through Attenuation of Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Results and Discussion
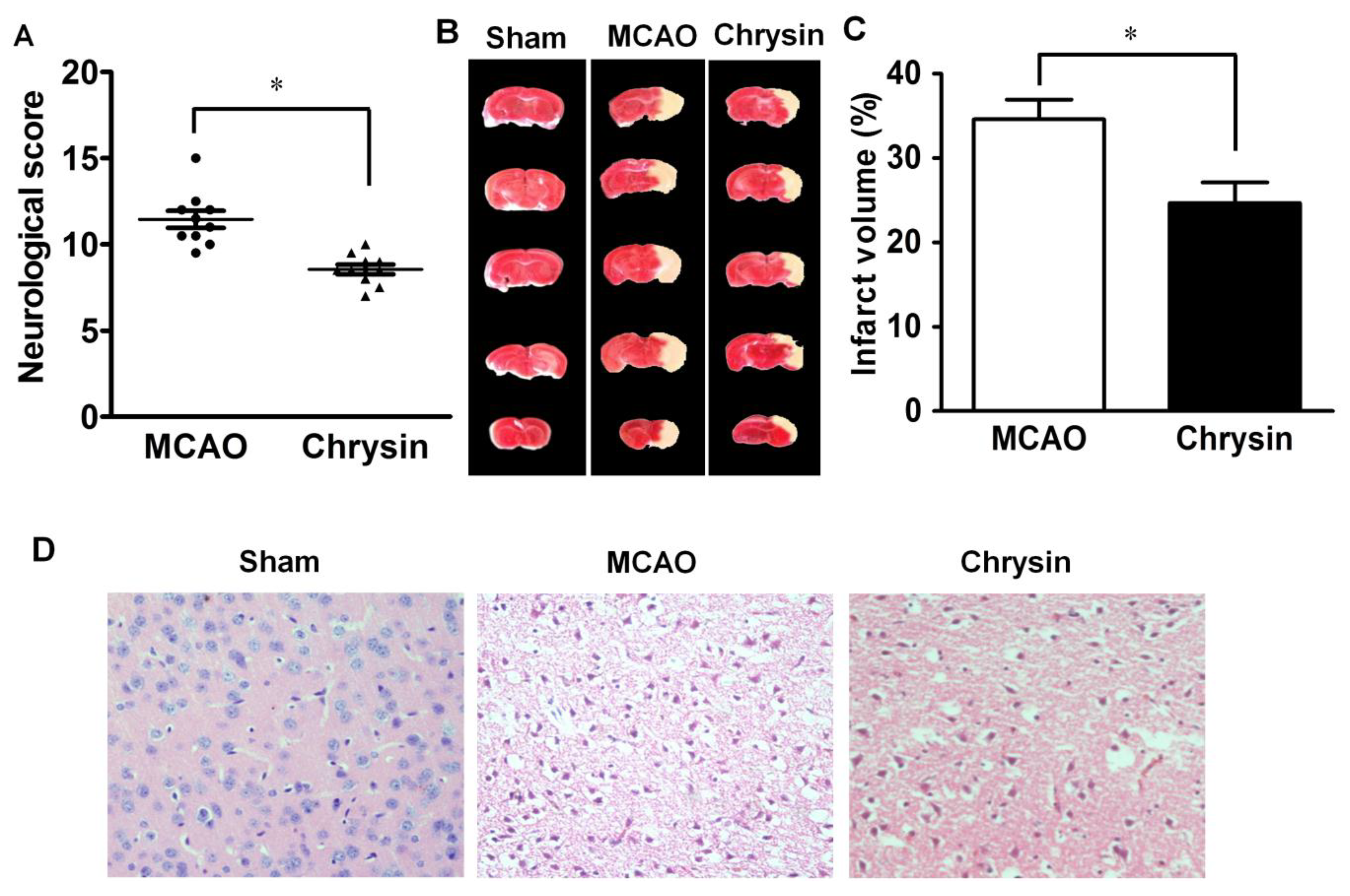
2.1. Effect of Chrysin on Neurological Deficits, Infarct Volume and Pathomorphological Changes
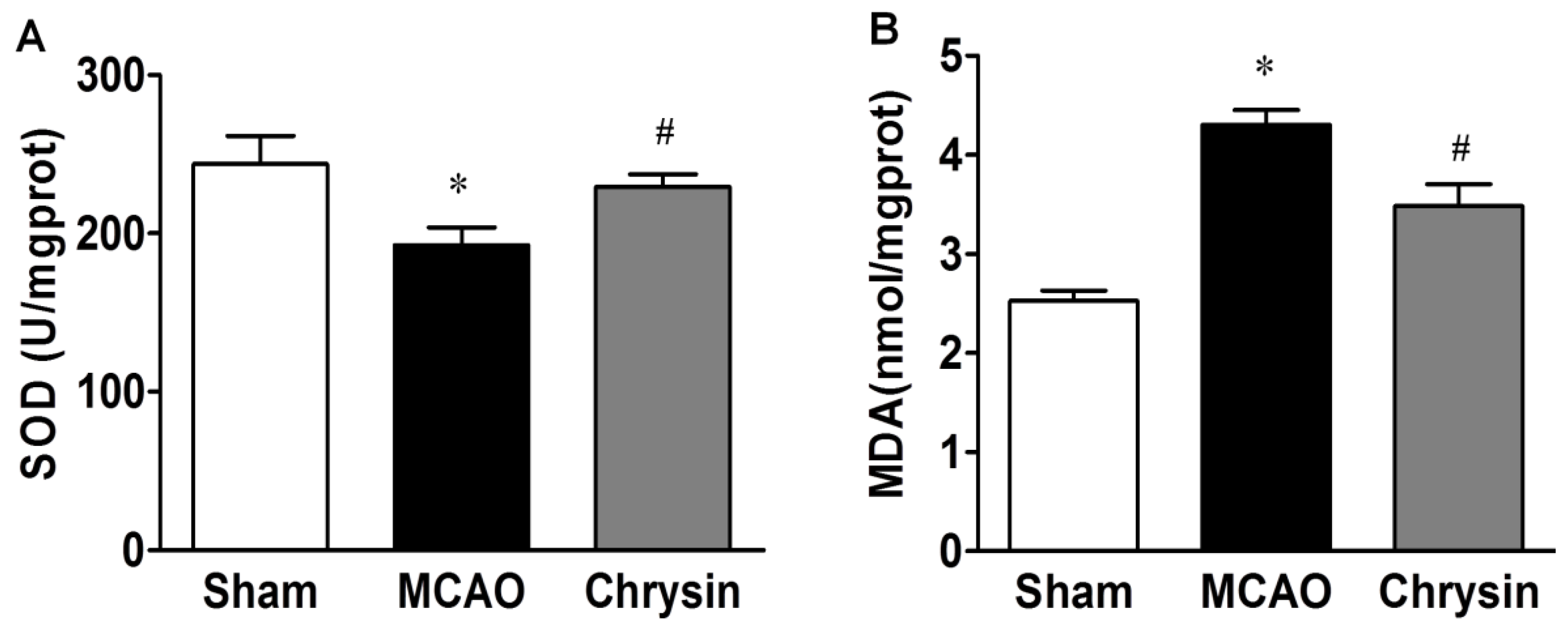
2.2. Effect of Chrysin on SOD Activity and MDA Content
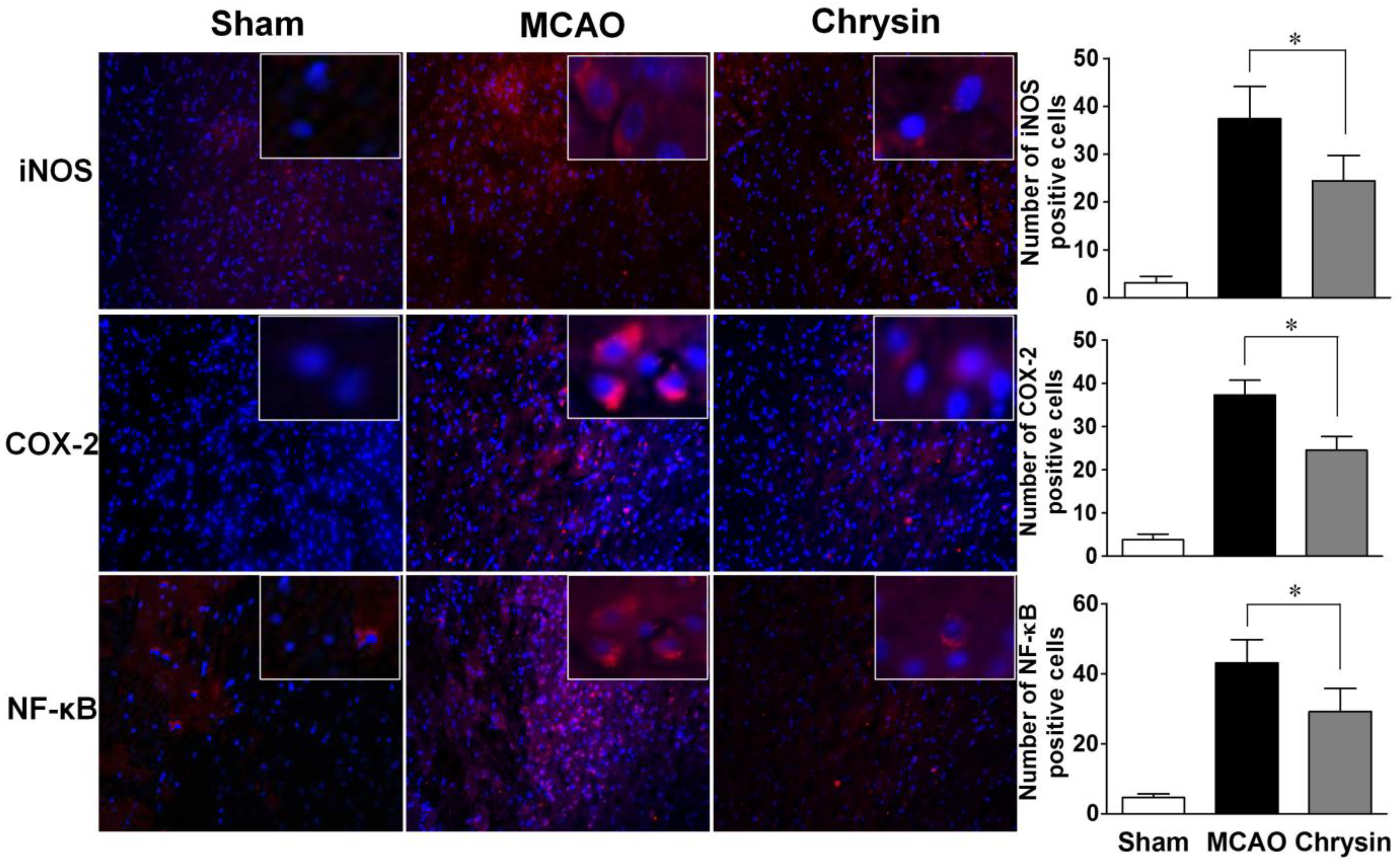
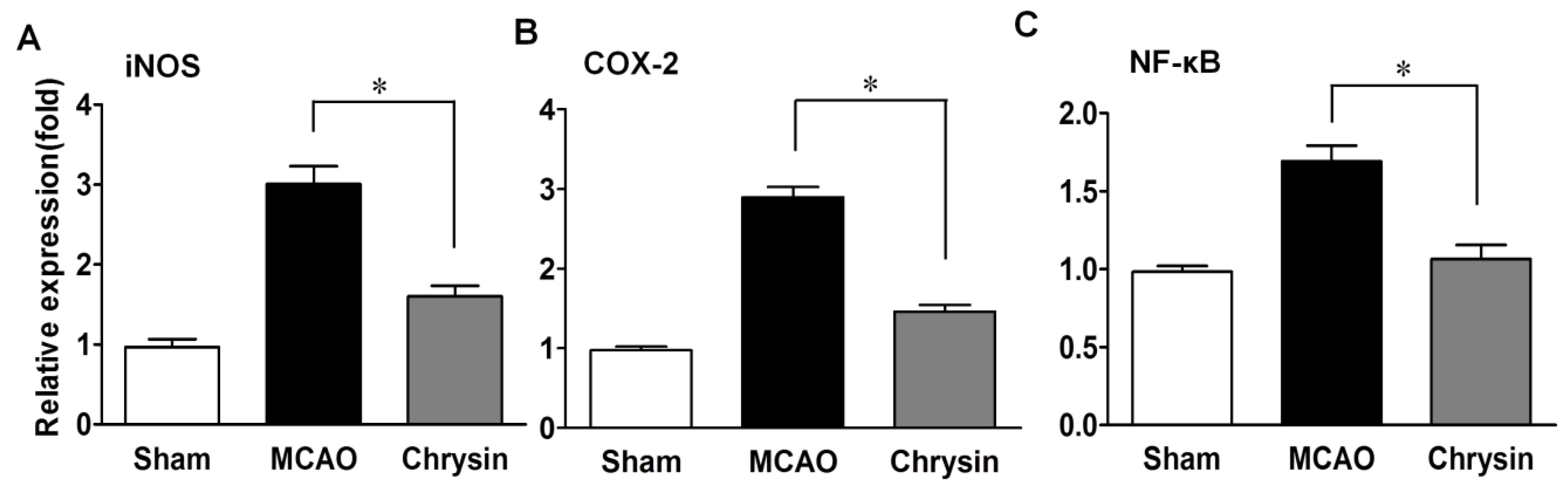
2.3. Effects of Chrysin on Levels of iNOS, COX-2 and NF-κB
2.4. Effect of Chrysin on the Expression of GFAP and Iba-1
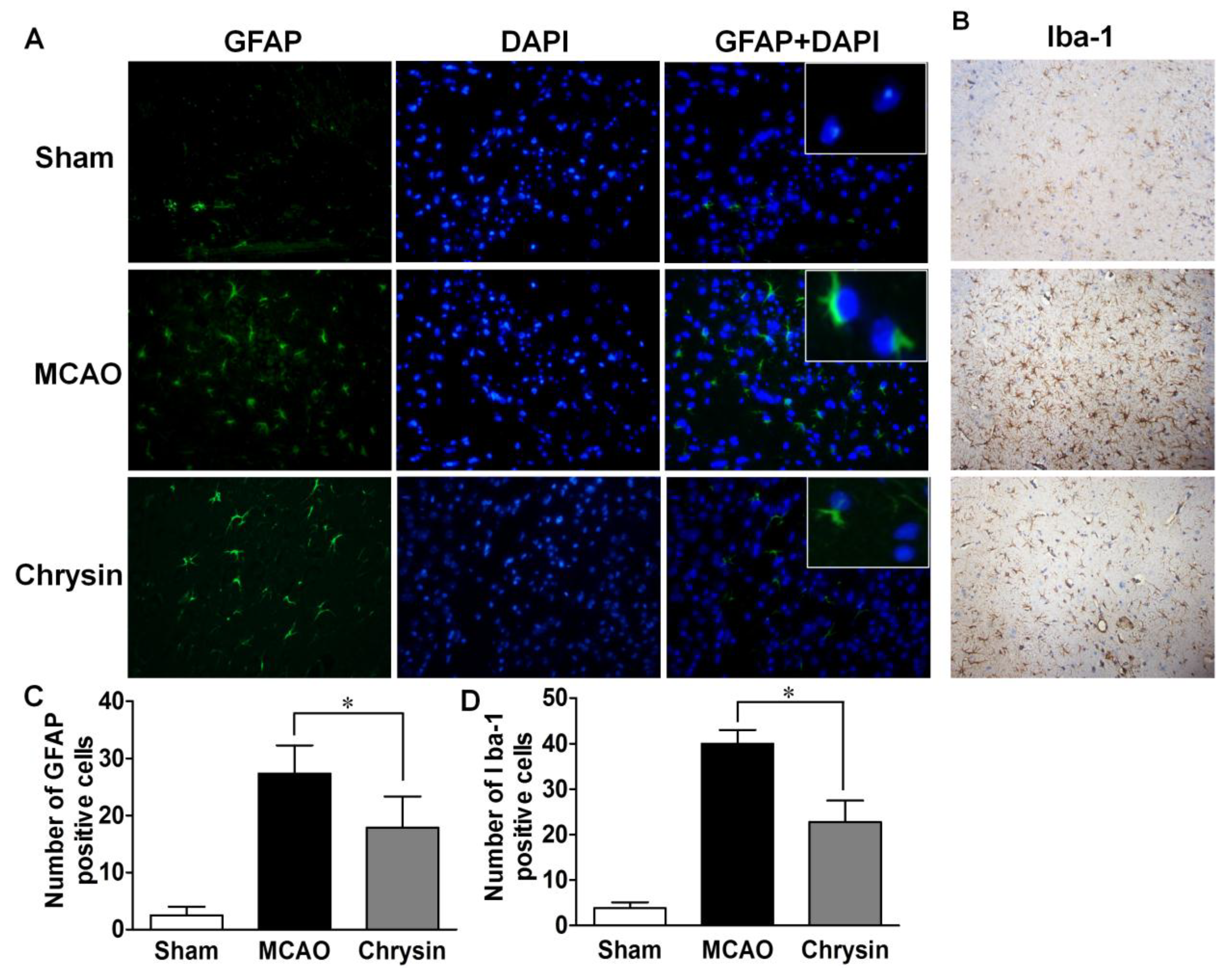
2.5. Effects of Chrysin Treatment on Cytokine Profiles
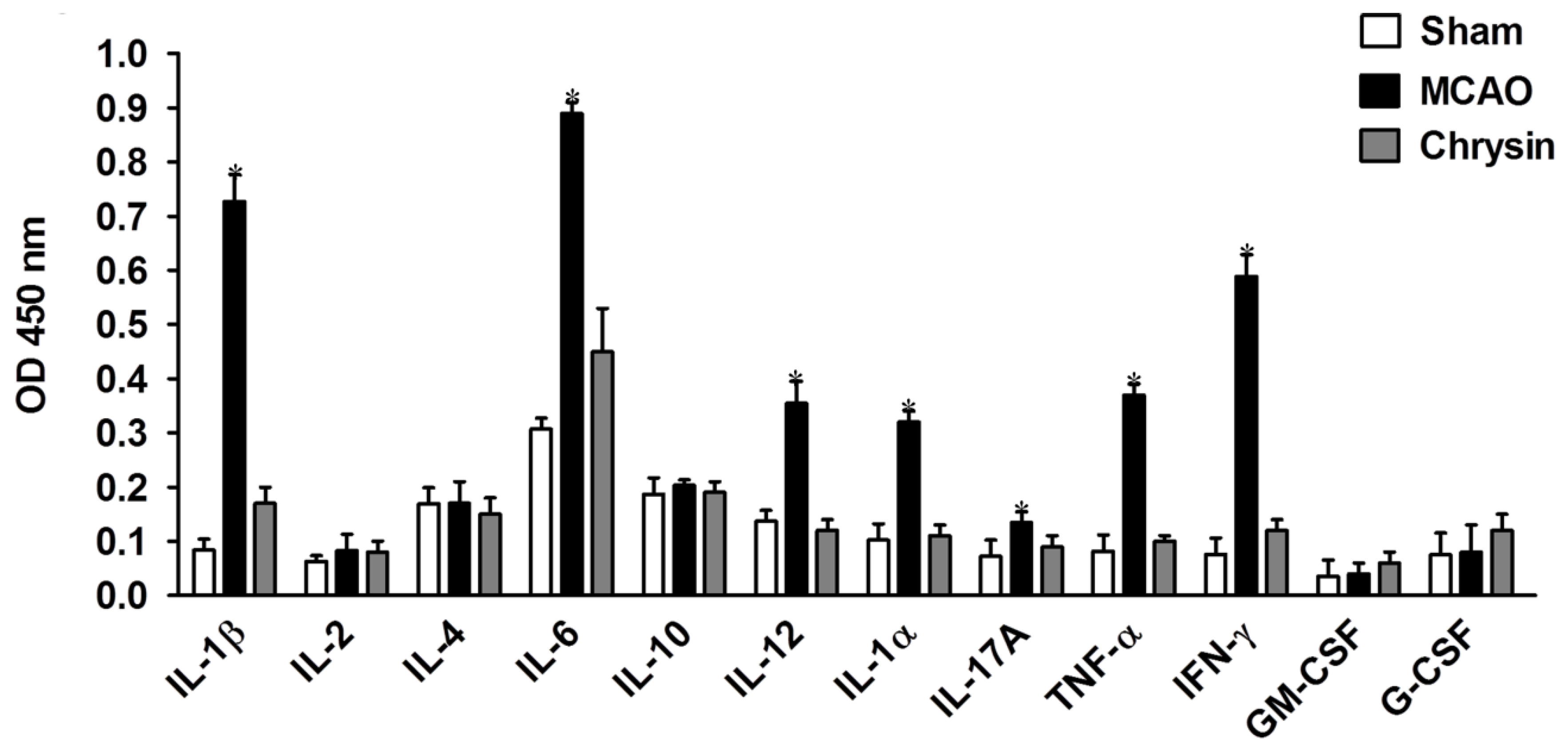
2.6. Discussion
3. Experimental Section
3.1. Animals and Reagents
3.2. MCAO Model and Clinical Evaluation
3.3. Infarct Volume Analysis
3.4. Pathological/Histological Analysis
3.5. Determination of SOD Activity and MDA Level
3.6. Immunofluorescence Analysis of iNOS, COX-2, NF-κB and GFAP
3.7. Immunohistochemical Detection of Iba-1
3.8. Quantitative Real-Time PCR
3.9. Measurement of Cytokines
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagakannan, P.; Shivasharan, B.D.; Thippeswamy, B.S.; Veerapur, V.P.; Bansal, P. Protective effect of hydroalcoholic extract of Mimusops elengi Linn. flowers against middle cerebral artery occlusion induced brain injury in rats. J. Ethnopharmacol. 2012, 140, 247–254. [Google Scholar]
- Yen, T.L.; Hsu, C.K.; Lu, W.J.; Hsieh, C.Y.; Hsiao, G.; Chou, D.S.; Wu, G.J.; Sheu, J.R. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J. Agric. Food Chem. 2012, 60, 1937–1944. [Google Scholar] [CrossRef]
- Ning, N.; Dang, X.; Bai, C.; Zhang, C.; Wang, K. Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J. Ethnopharmacol. 2012, 139, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Muzi, M.G.; Canini, A. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. Int. J. Oncol. 2010, 37, 973–981. [Google Scholar] [PubMed]
- Barbaric, M.; Miskovic, K.; Bojic, M.; Loncar, M.B.; Smolcic-Bubalo, A.; Debeljak, Z.; Medic-Saric, M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011, 135, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Qamar, W.; Lateef, A.; Tahir, M.; Rehman, M.U.; Ali, F.; Sultana, S. Chrysin protects against cisplatin-induced colon. toxicity via amelioration of oxidative stress and apoptosis: Probable role of p38MAPK and p53. Toxicol. Appl. Pharmacol. 2012, 258, 315–329. [Google Scholar]
- Cherkaoui-Tangi, K.; Lachkar, M.; Wibo, M.; Morel, N.; Gilani, A.H.; Lyoussi, B. Pharmacological studies on hypotensive, diuretic and vasodilator activities of chrysin glucoside from Calycotome villosa in rats. Phytother. Res. 2008, 22, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Jeong, G.S.; Kwon, K.B.; Ka, S.O.; Jang, H.Y.; Park, J.W.; Kim, Y.C.; Park, B.H. Sulfuretin protects against cytokine-induced beta-cell damage and prevents streptozotocin-induced diabetes. Exp. Mol. Med. 2010, 42, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Sultana, S. Chrysin modulates ethanol metabolism in Wistar rats: A promising role against organ toxicities. Alcohol. Alcohol. 2011, 46, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, H.; Pi, J.; Jiang, J.H.; Liu, L.; Bai, H.H.; Yang, P.H.; Cai, J.Y. Anti-tumor activity evaluation of novel chrysin–organogermanium (IV) complex in MCF-7 cells. Bioorg. Med. Chem. Lett. 2013, 23, 5544–5551. [Google Scholar] [CrossRef] [PubMed]
- Salmela, A.L.; Pouwels, J.; Kukkonen-Macchi, A.; Waris, S.; Toivonen, P.; Jaakkola, K.; Maki-Jouppila, J.; Kallio, L.; Kallio, M.J. The flavonoid eupatorin inactivates the mitotic checkpoint leading to polyploidy and apoptosis. Exp. Cell Res. 2012, 318, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.S.; Jiang, F.S.; Zhang, K.; Zhu, X.X.; Jin, B.; Lu, J.J.; Ding, Z.S. Flavonoids from the leaves of Carya cathayensis Sarg. inhibit vascular endothelial growth factor-induced angiogenesis. Fitoterapia 2014, 92, 34–40. [Google Scholar]
- Ha, S.K.; Moon, E.; Kim, S.Y. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-kappaB and JNK activations in microglia cells. Neurosci. Lett. 2010, 485, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Lateef, A.; Oday, O.H.; Qamar, W.; Ali, F.; Sultana, S. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-kappaB. Toxicol. Lett. 2013, 216, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Zhang, E.; Sun, A.; Ding, L.; Chou, G.; Wang, Z.; Mani, S. Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-kappaB signaling pathway. J. Pharmacol. Exp. Ther. 2013, 345, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Qamar, W.; Lateef, A.; Ali, F.; Rehman, M.U.; Tahir, M.; Sharma, S.; Sultana, S. Chrysin abrogates cisplatin-induced oxidative stress, p53 expression, goblet cell disintegration and apoptotic responses in the jejunum of Wistar rats. Br. J. Nutr. 2012, 108, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Wang, Y.H.; Bi, M.G.; Du, G.H. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur. J. Pharmacol. 2012, 680, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Li, R.C.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Dore, S. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Ashafaq, M.; Raza, S.S.; Khan, M.M.; Ahmad, A.; Javed, H.; Ahmad, M.E.; Tabassum, R.; Islam, F.; Siddiqui, M.S.; Safhi, M.M. Catechin hydrate ameliorates redox imbalance and limits inflammatory response in focal cerebral ischemia. Neurochem. Res. 2012, 37, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yue, R.; Zhang, J.; Shan, L.; Wang, R.; Zhang, W. Neuroprotective effects of bacopaside I in ischemic brain injury. Restor. Neurol. Neurosci. 2013, 31, 109–123. [Google Scholar] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sanberg, P.R.; Li, Y.; Wang, L.; Lu, M.; Willing, A.E.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001, 32, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, M.M.; Hoda, M.N.; Raza, S.S.; Khan, M.B.; Javed, H.; Ishrat, T.; Ashafaq, M.; Ahmad, M.E.; Safhi, M.M.; et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem. Res. 2011, 36, 1360–1371. [Google Scholar]
- Jia, J.; Liu, Y.; Zhang, X.; Liu, X.; Qi, J. Regulation of iNOS expression by NF-kappaB in human lens epithelial cells treated with high levels of glucose. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5070–5077. [Google Scholar] [CrossRef]
- Burke, S.J.; Updegraff, B.L.; Bellich, R.M.; Goff, M.R.; Lu, D.; Minkin, S.C., Jr.; Karlstad, M.D.; Collier, J.J. Regulation of iNOS gene transcription by IL-1beta and IFN-gamma requires a coactivator exchange mechanism. Mol. Endocrinol. 2013, 27, 1724–1742. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Back, K.O.; Kim, H.J.; Lee, S.Y.; Kook, K.H. Pirfenidone attenuates IL-1beta-induced COX-2 and PGE2 production in orbital fibroblasts through suppression of NF-kappaB activity. Exp. Eye Res. 2013, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Shi, B.; Chen, Y.; Zhang, Z.; Liu, T. Gardenia oil increases estradiol levels and bone material density by a mechanism associated with upregulation of COX-2 expression in an ovariectomized rat model. Exp. Ther Med. 2013, 6, 562–566. [Google Scholar] [PubMed]
- Yang, X.C.; Wang, X.; Luo, L.; Dong, D.H.; Yu, Q.C.; Wang, X.S.; Zhao, K. RNA interference suppression of A100A4 reduces the growth and metastatic phenotype of human renal cancer cells via NF-kB-dependent MMP-2 and bcl-2 pathway. Eur Rev. Med. Pharmacol. Sci. 2013, 17, 1669–1680. [Google Scholar] [PubMed]
- Wynants, M.; Vengethasamy, L.; Ronisz, A.; Meyns, B.; Delcroix, M.; Quarck, R. NF-kappaB pathway is involved in CRP-induced effects on pulmonary arterial endothelial cells in chronic thromboembolic pulmonary hypertension. Am. J. Physiol Lung Cell Mol. Physiol. 2013, 305, L934–L942. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Chen, L.; Xiao, J.; Wang, C.; Jiang, W.; Zhang, R.; Hao, J. Chrysin Protects against Focal Cerebral Ischemia/Reperfusion Injury in Mice through Attenuation of Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2014, 15, 20913-20926. https://doi.org/10.3390/ijms151120913
Yao Y, Chen L, Xiao J, Wang C, Jiang W, Zhang R, Hao J. Chrysin Protects against Focal Cerebral Ischemia/Reperfusion Injury in Mice through Attenuation of Oxidative Stress and Inflammation. International Journal of Molecular Sciences. 2014; 15(11):20913-20926. https://doi.org/10.3390/ijms151120913
Chicago/Turabian StyleYao, Yang, Li Chen, Jinting Xiao, Chunyang Wang, Wei Jiang, Rongxin Zhang, and Junwei Hao. 2014. "Chrysin Protects against Focal Cerebral Ischemia/Reperfusion Injury in Mice through Attenuation of Oxidative Stress and Inflammation" International Journal of Molecular Sciences 15, no. 11: 20913-20926. https://doi.org/10.3390/ijms151120913



