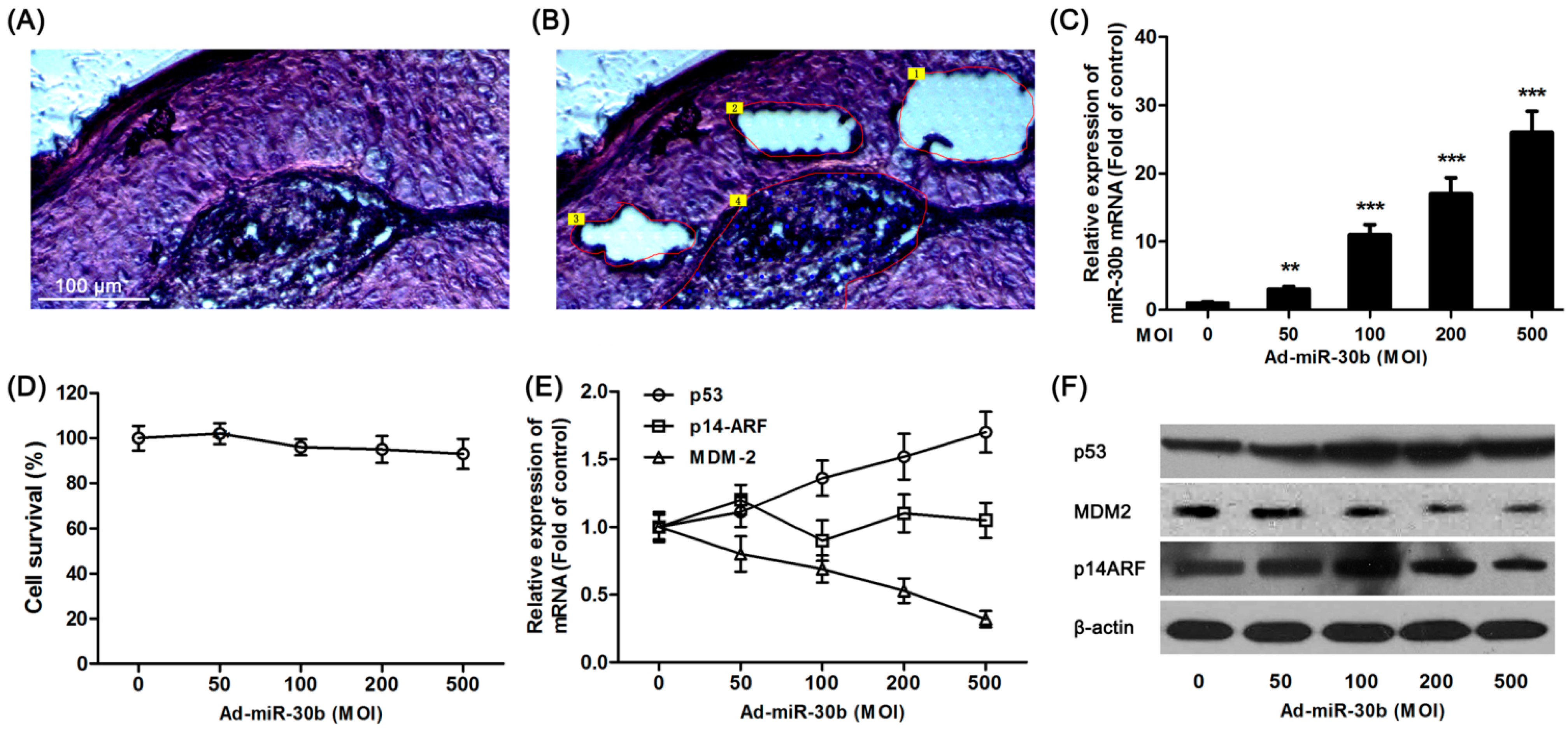
2.1. MiR-30b Expression Was Down-Regulated in Paracancerous Tissue of Laryngeal Carcinoma and Overexpression of MiR-30b Improved p53 Expression via Inhibition of MDM-2
Tumor tissue sections (
Figure 1A) from laryngeal carcinoma patients were divided into center area (
Figure 1B, Area 4), paracancerous (
Figure 1B, Area 1–3) and surgical margins (not shown) by LCM. Using extracted RNA from different regions of tissue, RT-PCR results showed that in total detected microRNAs, only the expression of miR-30b was significantly down-regulated in paracancerous tissue compared with surgical margins (
Table 1). In contrast, except for slightly increased expression of miR-370 and miR-16 in paracancerous tissue (not statistically significant), the expression of other microRNAs which were down-regulated in the center area of tumor changed less in paracancerous tissue (
Table 1).
To further investigate the effect of miR-30b on laryngeal carcinoma, HEp-2 cells were transfected by lentiviral vector-mediated expression of miR-30b and the infection efficiency was detected by qRT-PCR (
Figure 1C). As shown in
Figure 1D, there was no significant effect on cell survival in miR-30b overexpressed HEp-2 cells. Meanwhile, the
p53 expression in both mRNA and protein levels was found significantly increased in infected cells (
Figure 1E,F). To further investigate the expression of p14-ARF and MDM-2, which were related to the p53 pathway, western blot and RT-PCR results showed that although there was no difference in p14-ARF expression among miR-30b-overexpressed cells and the control group, MDM-2 expression was obviously down-regulated in both mRNA and protein levels (
Figure 1E,F).
Figure 1.
Overexpression of miR-30b regulated p53 expression. (A) Hematoxylin and eosin (H&E) staining of laryngeal carcinoma sections; (B) Using LCM (laser capture microdissection), sections were divided into center area (Area 4) paracancerous (paraneoplastic distance <5 mm, Area 1–3) and surgical margins (paraneoplastic distance >10 mm, not shown) by LCM. Scale bar = 100 μm and referred to (A) and (B) panels; (C) After HEp-2 cells were incubated with miR-30-lentivirus vector for 16 h in different multiplicity of infection (MOI), the expression of miR-30b was determined using qRT-PCR. ** p < 0.01, *** p < 0.001 compared to control group, n = 6; (D) After infected by miR-30-lentivirus vector for 16 h, HEp-2 cell viability was detected by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay; (E) After infected, the mRNA expression level of p53, MDM-2 and p14-ARF were investigated using qRT-PCR; and (F) The expression of p53, MDM-2 and p14-ARF in protein level were detected by western blot after infected. All experiments were repeated at least three times.
Figure 1.
Overexpression of miR-30b regulated p53 expression. (A) Hematoxylin and eosin (H&E) staining of laryngeal carcinoma sections; (B) Using LCM (laser capture microdissection), sections were divided into center area (Area 4) paracancerous (paraneoplastic distance <5 mm, Area 1–3) and surgical margins (paraneoplastic distance >10 mm, not shown) by LCM. Scale bar = 100 μm and referred to (A) and (B) panels; (C) After HEp-2 cells were incubated with miR-30-lentivirus vector for 16 h in different multiplicity of infection (MOI), the expression of miR-30b was determined using qRT-PCR. ** p < 0.01, *** p < 0.001 compared to control group, n = 6; (D) After infected by miR-30-lentivirus vector for 16 h, HEp-2 cell viability was detected by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay; (E) After infected, the mRNA expression level of p53, MDM-2 and p14-ARF were investigated using qRT-PCR; and (F) The expression of p53, MDM-2 and p14-ARF in protein level were detected by western blot after infected. All experiments were repeated at least three times.
![Ijms 15 19729 g001]()
Table 1.
Relative expression of microRNAs in center area and paracancerous compared with surgical margins.
Table 1.
Relative expression of microRNAs in center area and paracancerous compared with surgical margins.
| MicroRNAs | Fold Change (Center Area) | Fold Change (Paracancerous) |
|---|
| miR-30b | 0.262 | 0.385 |
| miR-149 | 0.355 | 0.921 |
| miR-26a | 0.391 | 1.032 |
| miR-145 | 0.108 | 0.873 |
| miR-34a | 0.514 | 0.932 |
| miR-125a | 0.319 | 0.830 |
| miR-370 | 0.577 | 1.131 |
| miR-203 | 0.110 | 0.779 |
| miR-195 | 0.273 | 0.903 |
| miR-16 | 0.863 | 1.255 |
| miR-144 | 0.375 | 0.943 |
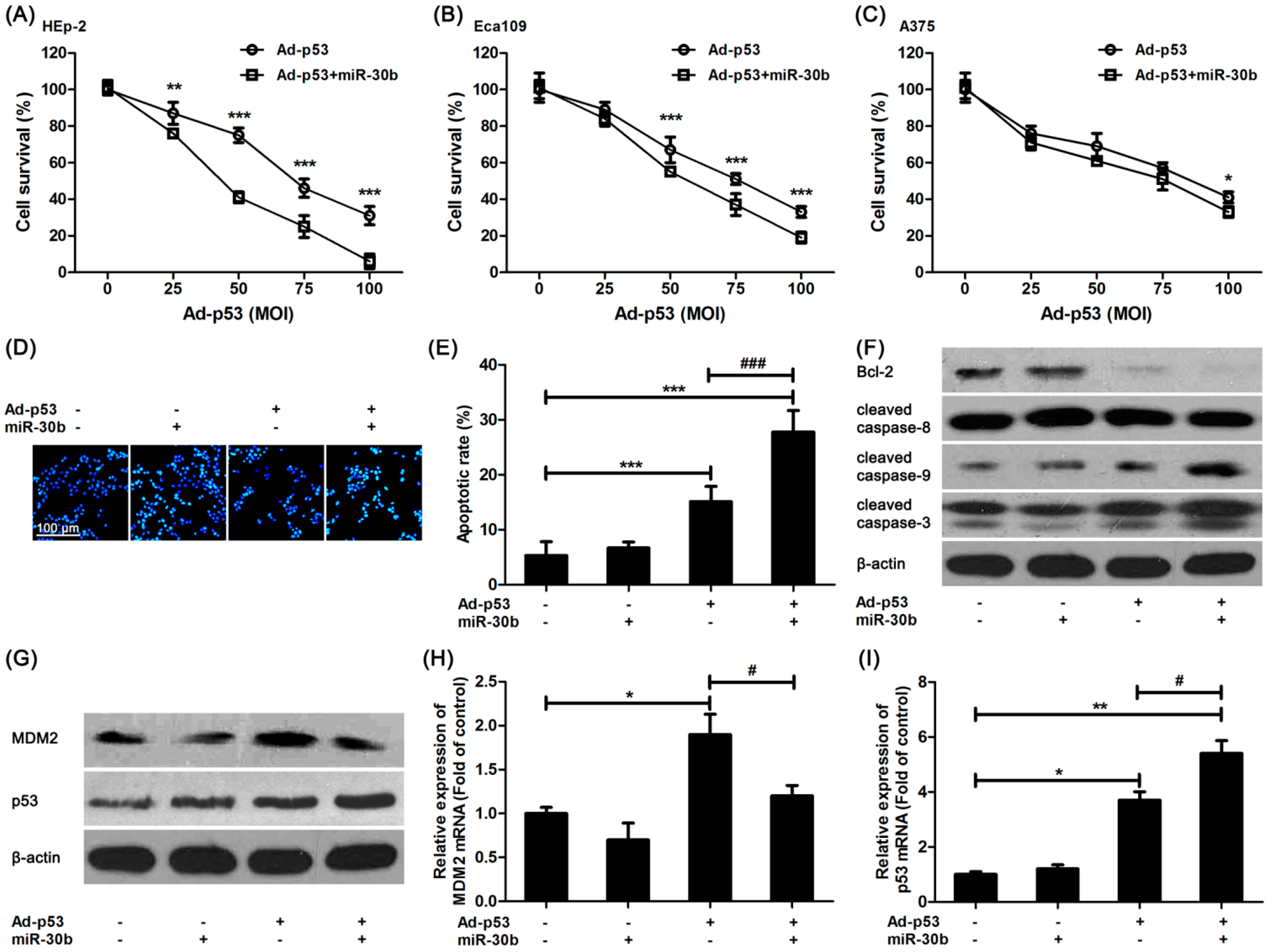
2.2. MiR-30b Overexpression Enhanced Ad-p53-Induced Apoptosis in Laryngeal Carcinoma and Other Tumor Cell Lines
It was shown that in miR-30b overexpressed HEp-2 cells, the damage of Ad-
p53 on HEp-2 cells was increased compared to that in control HEp-2 cells (
Figure 2A), and a similar result was found in another esophageal squamous cell line, Eca109 (
Figure 2B). However, this intensive effect of miR-30b in A375 cells was not as efficacious as the others (
Figure 2C). We further detected the Ad-
p53-induced apoptosis in miR-30b overexpressed and normal HEp-2 cells.
Figure 2D,E exhibited that the number of Ad-
p53-induced apoptotic cells was increased obviously in miR-30b overexpressed cells in comparison with normal HEp-2 cells. Moreover, the expression of one important anti-apoptotic protein, Bcl-2, was down-regulated in Ad-
p53-treated cells, especially in the miR-30b overexpressed group, while the caspase-3 activation level was significantly improved (
Figure 2F). It was also confirmed that caspase-9 activation but not caspase-8 might be involved in this process (
Figure 2F).
The expression level of MDM-2 and p53 was also detected by using western blot and RT-PCR. As shown in
Figure 2G,H, MDM-2 expression was improved in Ad-
p53-treated cells. However, the increased expression was restored in the miR-30b overexpressed group (
Figure 2G,H), which was in consistence with the result in
Figure 1F. As a result, there was a higher expression level of p53 in the Ad-
p53-treated miR-30b overexpressed group (
Figure 2G,I).
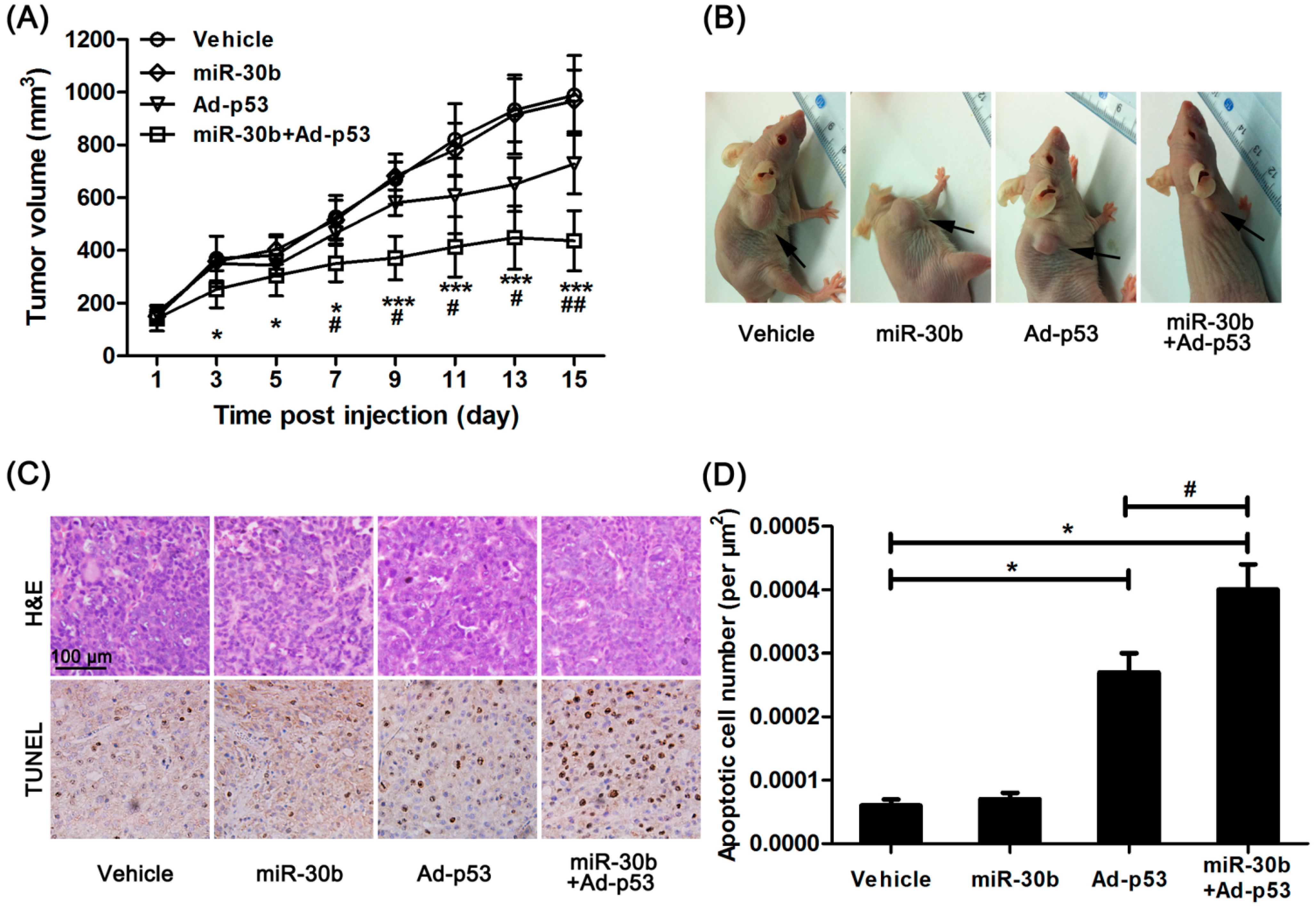
2.3. Overexpression of MiR-30b Could Improve the Sensitivity of Ad-p53 in HEp-2-Transplated Nude Mice
We further investigated the effect of miR-30b on Ad-
p53-treated HEp-2 cells
in vivo. Normal HEp-2 and miR-30b stably infected HEp-2 cells were implanted in nude mice, and Ad-
p53 was given to mice by multiple-center intratumoral injection. After 15 days treatment, the tumor growth originating from HEp-2 cells in nude mice was inhibited by Ad-
p53 treatment, while this effect was obviously enhanced in the miR-30b overexpressed group (
Figure 3A,B). Additionally, HE staining showed the presence of well-differentiated tumors with extensive necrosis in the miR-30b overexpressed group (
Figure 3C). Moreover, under Ad-
p53-treatment, the apoptotic cell number was also significantly higher in the miR-30b overexpressed group compared to normal HEp-2-implanted nude mice (
Figure 3C,D).
Figure 2.
Overexpression of miR-30b improved Ad-p53-induced cell apoptosis via enhancing p53 pathway. (A) After HEp-2 cells were infected by miR-30-lentivirus vector for 16 h, Ad-p53 vector at different MOIs was added into culture medium for another 24 h, then MTT assay was performed to detect cell viability; (B) Similar MTT assay was performed in Eca109 cells; (C) Similar MTT assay was performed in A375 cells. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to Ad-p53-treated alone cells, n = 6; (D) After HEp-2 cells were infected by miR-30-lentivirus vector (200 MOI) for 16 h and stimulated with Ad-p53 (50 MOI), the cells were stained with Hoechst33342 and bright nuclei indicated apoptotic cells. Scale bar = 100 μm and referred to all panels; (E) Statistical analysis of Hoechst staining, *** p < 0.001 compared to untreated HEp-2 cells, ### p < 0.001 compared to Ad-p53-treated alone group, n = 4; (F,G) HEp-2 cells were treated as in Hoechst staining, western blot was performed. β-actin was used for loading control. All blots were repeated for at least three times; (H,I) HEp-2 cells were treated as in Hoechst staining, qRT-PCR was performed to detect the mRNA expression of p53 and MDM-2. * p < 0.05, ** p < 0.01 compared to untreated HEp-2 cells, # p < 0.05 compared to Ad-p53-treated alone group, n = 3.
Figure 2.
Overexpression of miR-30b improved Ad-p53-induced cell apoptosis via enhancing p53 pathway. (A) After HEp-2 cells were infected by miR-30-lentivirus vector for 16 h, Ad-p53 vector at different MOIs was added into culture medium for another 24 h, then MTT assay was performed to detect cell viability; (B) Similar MTT assay was performed in Eca109 cells; (C) Similar MTT assay was performed in A375 cells. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to Ad-p53-treated alone cells, n = 6; (D) After HEp-2 cells were infected by miR-30-lentivirus vector (200 MOI) for 16 h and stimulated with Ad-p53 (50 MOI), the cells were stained with Hoechst33342 and bright nuclei indicated apoptotic cells. Scale bar = 100 μm and referred to all panels; (E) Statistical analysis of Hoechst staining, *** p < 0.001 compared to untreated HEp-2 cells, ### p < 0.001 compared to Ad-p53-treated alone group, n = 4; (F,G) HEp-2 cells were treated as in Hoechst staining, western blot was performed. β-actin was used for loading control. All blots were repeated for at least three times; (H,I) HEp-2 cells were treated as in Hoechst staining, qRT-PCR was performed to detect the mRNA expression of p53 and MDM-2. * p < 0.05, ** p < 0.01 compared to untreated HEp-2 cells, # p < 0.05 compared to Ad-p53-treated alone group, n = 3.
![Ijms 15 19729 g002]()
Figure 3.
Overexpression of miR-30b improved the anti-tumor effect of Ad-p53 in vivo. (A) After Ad-p53 injection, tumor volume was calculated every two days, * p < 0.05, *** p < 0.005 compared to normal HEp-2 cell-implanted nude mice, # p < 0.05, ## p < 0.01 compared to Ad-p53-treated implanted mice, n = 8; (B) After 15 days injection, the tumor in nude mice was pictured, the black arrow in the representative figure indicating tumor position; (C) After the experiment, the tumor tissue was removed and made into sections. H&E and TdT-mediated dUTP nick end labeling (TUNEL) staining were performed. Brown showed TUNEL-positive nuclei, and all nuclei were stained with hematoxylin. Scale bar = 100 μm and referred to all panels; and (D) Apoptotic cell number was calculated as TUNEL-positive number divided by total cells. * p < 0.05 compared to normal HEp-2 cell-implanted nude mice, # p < 0.05 compared to Ad-p53-treated implanted mice, n = 8.
Figure 3.
Overexpression of miR-30b improved the anti-tumor effect of Ad-p53 in vivo. (A) After Ad-p53 injection, tumor volume was calculated every two days, * p < 0.05, *** p < 0.005 compared to normal HEp-2 cell-implanted nude mice, # p < 0.05, ## p < 0.01 compared to Ad-p53-treated implanted mice, n = 8; (B) After 15 days injection, the tumor in nude mice was pictured, the black arrow in the representative figure indicating tumor position; (C) After the experiment, the tumor tissue was removed and made into sections. H&E and TdT-mediated dUTP nick end labeling (TUNEL) staining were performed. Brown showed TUNEL-positive nuclei, and all nuclei were stained with hematoxylin. Scale bar = 100 μm and referred to all panels; and (D) Apoptotic cell number was calculated as TUNEL-positive number divided by total cells. * p < 0.05 compared to normal HEp-2 cell-implanted nude mice, # p < 0.05 compared to Ad-p53-treated implanted mice, n = 8.
2.4. Discussion
It is widely accepted that laryngeal carcinoma is a significant cause of morbidity and mortality nowadays. Because of frequently occurring local or regional recurrence and distant metastases, traditional treatments including adjuvant radiotherapy after surgery and chemotherapy could not improve survival obviously [
3,
9,
10]. Thus, to further understand the molecular concepts of laryngeal carcinoma and search for potential therapies has been encouraging and urgent. Laryngeal carcinoma has been proven to arise from a common premalignant progenitor which has genetic alterations followed by activation of proto-oncogenes and inactivation of tumor-suppressor genes [
3]. After observing abnormal epithelium in the mucosa adjacent to head and neck cell carcinoma, Slaughter
et al. [
4] had coined the term “field cancerization” since 1953. Some investigations, including microsatellite analysis and
p53 mutational, revealed the genetic alterations in the adjacent position to primary carcinoma, thus providing evidence that field cancerization could be an important factor in the recurrence of laryngeal carcinoma after therapy [
11,
12].
Therefore, to detect the possible genetic alterations in the cells of the surgical margins, we compared some microRNAs expression in paracancerous tissue to tumor center or normal margins of resection. Recently studies have revealed that microRNAs could be potential biomarkers in cancer research because of its unique expression profiles in tumor tissue [
13]. More importantly, aberrant microRNAs expression was related to early cancerization and has been used for cancer detection and prognosis [
14]. Some of them could also be possible therapeutic targets in cancers. For example, miR-24 [
8], miR-34a [
15] and some other microRNAs had been found significantly down-regulated in laryngeal carcinoma and could be regarded as tumor suppressors. However, the expression of microRNAs in paracancerous tissue and the relationship with field cancerization has been studied less. Using LCM technology, we isolated the paracancerous tissue, tumor center and surgical margins and detected the expression of some reported tumor suppressor microRNAs. We found that only miR-30b was still significantly down-regulated not only in tumor tissue but also in paracancerous tissue among all detected microRNAs.
Thus, further studies were performed to investigate the possible effect of miR-30b in laryngeal carcinoma HEp-2 cell line. miR-30b has been reported to affect lactation and involution in the developing mouse mammary gland [
16]. Zhu
et al. [
17] has also reported that miR-30b could increase apoptosis in gastric cancer cells. How miR-30b is integrated into tumor cell apoptosis remains unknown. In this study, we found overexpression of miR-30b in HEp-2 cells could increase the expression of
p53, one important tumor suppressor gene [
18]. In this process, MDM2, a p53-specific E3 ubiquitin ligase which could promote p53 degradation [
19], was down-regulated in miR-30b overexpressed HEp-2 cells. However, using an online microRNA target prediction tool (Target Scan Human 6.2), it was found that there were fewer matched nucleotides between miR-30b and MDM2. The luciferase reporter assay of 3'-UTR was also constructed to verify the direct binding of miR-30b followed these comments. Disappointingly, the 3'-UTR of MDM2 does not respond to miR-30b (Data was not shown). Meanwhile, there was no different expression of a MDM2 inhibitor p14-ARF between each group. We speculated that miR-30b could affect MDM2-p53 interaction to indirectly regulate MDM2 and
p53 expression [
19], or another binding site of miR-30b might exist in the open reading frame of MDM2 [
20]. Together, results inferred that miR-30b might affect MDM2-p53 interaction, thereby participating in apoptotic process.
However, overexpression of miR-30b has less effect on control HEp-2 cells. Insufficient
p53 expression might explain this appearance. As miR-30b could inhibit MDM2 expression, therefore, we investigated whether overexpression of miR-30b could enhance the effect of adenoviral
p53 gene therapy, which has been used in clinical cancer therapy [
21]. As expected, the pro-apoptotic effect of Ad-
p53 was obviously enhanced in miR-30b-overexpression laryngeal carcinoma cell line HEp-2 and another esophageal cancer cell, Eca109. However, the intensive pro-apoptotic effect in A375 human amelanotic melanoma cells was not as efficient as in HEp-2 and Eca109 cells. The different p53 pathway might contribute to this result [
22]. To further investigate the Ad-
p53-induced cell apoptosis process, mitochondrial apoptosis-associated proteins were detected in this study. Several evidences suggested that p53 signals leads to regulation of Bcl-2 expression and caspase-9-associated mitochondrial apoptosis [
23,
24]. We found that overexpression of miR-30b obviously enhanced Ad-
p53-induced apoptosis via caspase-9 activation but not caspase-8. The expression of anti-apoptotic protein Bcl-2 was also inhibited in Ad-
p53-treated miR-30b overexpression cells. To further investigate the possible effect of miR-30b on anti-tumor therapy
in vivo, we compared the effect of Ad-
p53 on normal HEp-2 cells and miR-30b stably overexpressed cells implanted in nude mice. It was also proven that overexpression of miR-30b could significantly enhance the anti-tumor and pro-apoptotic effect of Ad-
p53 in HEp-2 implanted nude mice.