Antimicrobial Activity of Isothiocyanates from Cruciferous Plants against Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
:1. Introduction
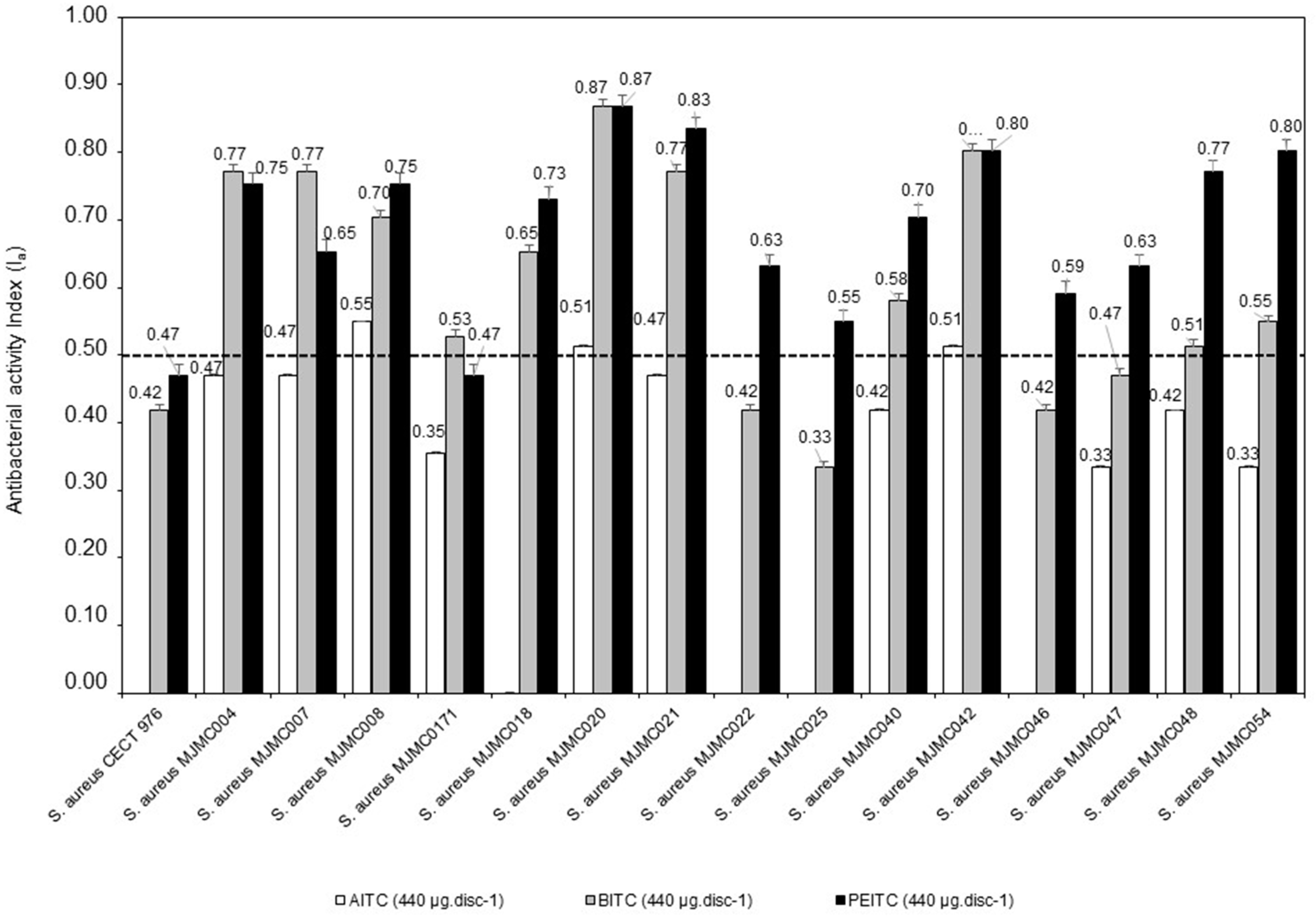
| Bacteria Isolates | AITC (440 µg·disc−1) | BITC (440 µg·disc−1) | PEITC (440 µg·disc−1) | Vancomycin (30 µg·disc−1) |
|---|---|---|---|---|
| S. aureus CECT 976 (reference strain) | - | 10.3 ± 0.6a | 11.3 ± 0.6a | 15.7 ± 0.6b |
| S. aureus MJMC004 | 11.3 ± 0.6a | 26.3 ± 0.6d | 24.3 ± 0.6c | 15.7 ± 0.0b |
| S. aureus MJMC007 | 11.3 ± 0.9a | 26.3 ± 0.6d | 17.3 ± 0.9c | 14.7 ± 0.0b |
| S. aureus MJMC008 | 13.3 ± 0.6a | 20.3 ± 0.6c | 24.3 ± 0.6d | 15.7 ± 0.0b |
| S. aureus MJMC0171 | 9.3 ± 0.6a | 12.7 ± 0.6c | 11.3 ± 0.6b | 14.7 ± 0.0d |
| S. aureus MJMC018 | - | 17.3 ± 0.6b | 22.3 ± 0.6c | 16.7 ± 0.0a |
| S. aureus MJMC020 | 12.3 ± 0.6a | 45.3 ± 1.6c | 45.3± 1.2c | 15.7 ± 0.0b |
| S. aureus MJMC021 | 11.3 ± 0.6a | 26.3 ± 0.6c | 36.3 ± 1.2d | 15.7 ± 0.0b |
| S. aureus MJMC022 | - | 10.3 ± 0.1a | 16.3 ± 0.6b | 15.7 ± 0.0b |
| S. aureus MJMC025 | - | 9.0 ± 0.1a | 13.3 ± 0.6b | 13.7 ± 0.0b |
| S. aureus MJMC040 | 10.3 ± 0.1a | 14.3 ± 0.6b | 20.3 ± 0.6c | 15.7 ± 0.0b |
| S. aureus MJMC042 | 12.3 ± 0.1a | 30.3 ± 1.0c | 30.3 ± 1.2c | 16.7 ± 0.0b |
| S. aureus MJMC046 | - | 10.3 ± 0.6a | 14.7 ± 0.6b | 16.7 ± 0.0c |
| S. aureus MJMC047 | 9.0 ± 0.6a | 11.3 ± 0.6b | 16.3 ± 0.6c | 15.7 ± 0.0c |
| S. aureus MJMC048 | 10.3 ± 0.1a | 12.3 ± 0.6b | 26.3 ± 0.6d | 15.7 ± 0.0c |
| S. aureus MJMC054 | 9.0 ± 0.1a | 13.3 ± 0.6b | 30.3 ± 1.2c | 15.7 ± 0.0b |
2. Results and Discussion
2.1. Chemical Structure of Isothiocyanates.
2.2. Antimicrobial Activity of ITCs against MRSA
| Bacteria Isolates | AITC | BITC | PEITC | |||
|---|---|---|---|---|---|---|
| MIC | Main Effect | MIC | Main Effect | MIC | Main Effect | |
| S. aureus CECT 976 (reference strain) | 110.0 ± 0.0b | bacteriostatic | 55.7 ± 0.0a | bactericidal | 110.0 ± 0.0b | bacteriostatic |
| S. aureus MJMC004 | 110.0 ± 0.0c | bacteriostatic | 2.9 ± 0.0a | bacteriostatic | 55.7 ± 0.0b | bacteriostatic |
| S. aureus MJMC007 | 36.7 ± 0.01b | bacteriostatic | 4.4 ± 0.0a | bacteriostatic | 7.3 ± 0.0a | bacteriostatic |
| S. aureus MJMC008 | 220.0 ± 0.0c | bactericidal | 23.5 ± 0.01a | bacteriostatic | 110.0 ± 0.0b | bacteriostatic |
| S. aureus MJMC0171 | 110.0 ± 0.0b | bactericidal | 55.7 ± 0.0a | bactericidal | 110.0 ± 0.0b | bactericidal |
| S. aureus MJMC018 | 220.0 ± 0.0b | bacteriostatic | 110.0 ± 0.0a | bactericidal | 183.3 ± 0.4b | bactericidal |
| S. aureus MJMC020 | 110.0 ± 0.0a | bactericidal | 110.0 ± 0.0a | bactericidal | 110.0 ± 0.0a | bacteriostatic |
| S. aureus MJMC021 | 110.0 ± 0.0b | bactericidal | 55.7 ± 0.0a | bactericidal | 110.0 ± 0.0b | bacteriostatic |
| S. aureus MJMC022 | 220.0 ± 0.0c | bacteriostatic | 110.0 ± 0.0a | bactericidal | 146.7 ± 0.04b | bacteriostatic |
| S. aureus MJMC025 | 110.0 ± 0.0a | bacteriostatic | 110.0 ± 0.0a | bactericidal | 110.0 ± 0.0a | bacteriostatic |
| S. aureus MJMC040 | 27.9 ± 0.0a | bacteriostatic | <7.3 ± 0.0 | bactericidal | 55.7 ± 0.0b | bacteriostatic |
| S. aureus MJMC042 | 110.0 ± 0.0b | bacteriostatic | 13.2 ± 0.0a | bactericidal | 146.7 ± 36.7b | bacteriostatic |
| S. aureus MJMC046 | 55.7 ± 0.0c | bacteriostatic | 7.3 ± 0.0a | bactericidal | 27.9 ± 0.0b | bacteriostatic |
| S. aureus MJMC047 | 220.0 ± 0.0b | bactericidal | 7.3 ± 0.0a | bactericidal | <7.3 ± 0.0 | bactericidal |
| S. aureus MJMC048 | 110.0 ± 0.0b | bacteriostatic | 55.7 ± 0.0a | bacteriostatic | 110.0 ± 0.0b | bacteriostatic |
| S. aureus MJMC054 | 110.0 ± 0.0b | bacteriostatic | 110.0 ± 0.0b | bacteriostatic | 110.0 ± 0.0b | bacteriostatic |
3. Experimental Section
3.1. Methicillin-Resistant Staphylococcus Aureus (MRSA) Isolates
3.2. Isothiocyanates (ITCs)
3.3. Antimicrobial Activity by Disc Diffusion Method
3.4. Antimicrobial Activity Minimum Inhibitory Concentration (MIC)
3.5. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schoenfeld, E.M.; McKay, M.P. Mastitis and methicillin-resistant Staphylococcus aureus (MRSA): The calm before the storm? J. Emerg. Med. 2010, 38, e31–e34. [Google Scholar]
- Kim, H.K.; Thammavongsa, V.; Schneewind, O.; Missiakas, D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 92–99. [Google Scholar]
- Montgomery, C.P.; Daniels, M.; Zhao, F.; Alegre, M.L.; Chong, A.S.; Daum, R.S. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect. Immun. 2014, 82, 2125–2134. [Google Scholar]
- Libert, M.; Elkholti, M.; Massaut, J.; Karmali, R.; Maskart, G.; Cherifi, S. Risk factors for methicillin-resistantce and outcome of Staphylococcus aureus blood stream infection in a Belgian university hospital. J. Hosp. Infect. 2008, 68, 17–24. [Google Scholar]
- Anderson, M.E.C.; Lefebvere, S.L.; Weese, S.J. Evaluation of prevalence and factors for methicillin-resistant Staphylococcus aureus colonization in veterinary personnel attending an international equine veterinary conference. Vet. Microbiol. 2008, 129, 410–417. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar]
- Vaughn, S.F.; Berhow, M.A. Glucosinolate hydrolysis products from various plant sources: pH effects, isolation, and purification. Ind. Crops Prod. 2005, 21, 193–202. [Google Scholar]
- Thejass, P.; Kuttan, G. Allyl isothiocyanate (AITC) and phenyl isothiocyanate (PITC) inhibit tumour-specific angiogenesis by downregulating nitric oxide (NO) and tumour necrosis factor-alpha (TNF-alpha) production. Nitric. Oxide-Biol. Chem. 2007, 16, 247–257. [Google Scholar]
- Qazi, A.; Pal, J.; Maitah, M.; Fulciniti, M.; Pelluru, D.; Nanjappa, P.; Lee, S.; Batchu, R.B.; Prasad, M.; Bryant, C.S.; et al. Anticancer activity of a broccoli derivative, sulforaphane, in Barrett adenocarcinoma: Potential use in hemoprevention and as adjuvant in chemotherapy. Transl. Oncol. 2010, 3, 389–399. [Google Scholar]
- O’Callaghan, K.J.; Stone, P.J.; Hu, X.; Griffiths, D.W.; Davey, M.R.; Cocking, E.C. Effects of glucosinolates and flavonoids on colonization of the roost of Brassica napus by Azorhizobium caulinodans ORS571. Appl. Environ. Microbiol. 2000, 66, 2185–2191. [Google Scholar]
- Hong, E.Y.; Kim, G.H. Anticancer and antibacterial activities of β-phenylethyl isothiocyanate in Brassica rapa L. Food Sci. Technol. Res. 2008, 14, 377–382. [Google Scholar]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar]
- Kim, M.G.; Lee, H.S. Growth-inhibiting activities of phenethylisothiocyanate and its derivatives against intestinal bacteria. J. Food Sci. 2009, 74, M467–M471. [Google Scholar]
- Wilson, A.E.; Bergaentzlé, M.; Bindler, F.; Marchioni, E. In vitro efficacies of various isothiocyanates from cruciferous vegetables as antimicrobial agents against foodborne pathogens and spoilage bacteria. Food Control 2013, 30, 318–324. [Google Scholar]
- Troncoso, R.; Espinoza, C.; Sánchez-Estrada, A.; Tiznado, M.E.; García, H.S. Analysis of the isothiocyanates present in cabbage leaves extract and their potential application to control Alternaria rot in bell peppers. Food Res. Int. 2005, 38, 701–708. [Google Scholar]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I. Glucosinolates in Brassica vegetables; the influence of the food supply chain on intake; bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219–S265. [Google Scholar]
- Herzallah, S.; Holley, R. Determination of sinigrin, sinalbin, allyl- and benzyl isothiocyanates by RP-HPLC in mustard powder extracts. LWT-Food Sci. Technol. 2012, 47, 293–299. [Google Scholar]
- Vermeulen, M.; van Den Berg, R.; Freidig, A.P.; van Bladeren, P.J.; Vaes, W.H.J. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. J. Agric. Food Chem. 2006, 54, 5350–5358. [Google Scholar]
- Matthäus, B.; Fiebig, H.J. Simultaneous determination of isothiocyanates, indoles, and oxazolidinethiones in myrosinase digests of rapeseeds and rapeseed meal by HPLC. J. Agric. Food Chem. 1996, 44, 3894e–3689e. [Google Scholar]
- Desbois, A.P.; Gemmell, C.G.; Coote, P.J. In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 2010, 35, 559–565. [Google Scholar]
- Jang, M.; Hong, E.Y.; Kim, G.H. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. J. Food Sci. 2010, 75, M412–M416. [Google Scholar]
- Bal, A.M.; Garau, J.; Gould, I.M.; Liao, C.H.; Mazzei, T. Vancomycin in the treatment of meticillin-resistant Staphylococcus aureus (MRSA) infection, End of an era? J. Glob. Antibiot. Res. 2013, 1, 23–30. [Google Scholar]
- Jacob, C.; Anwar, A. The chemistry behind redox regulation with a focus on sulphur redox systems. Physiol. Plant. 2008, 133, 469–480. [Google Scholar]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol. Biomark. Prev. 1998, 7, 1091–1100. [Google Scholar]
- Kassie, F.; Pool-Zobel, B.; Parzefall, W.; Knasmuller, S. Genotoxic effects of benzyl isothiocyanate, a natural chemopreventive agent. Mutagenesis 1999, 14, 595–604. [Google Scholar]
- Cockerill, F.R.; Wikle, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferrar, M.J.; Hardy, D.J.; Hecht, D.W.; Hindl, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard-9th ed.; Clinical and Laboratory Standards Institute: 950 West Valley Road, Suite 2500 Wayne, PA 19087, USA, 2012; p. 277. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamyn, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth; and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.; Aires, A.; Saavedra, M.J. Antimicrobial Activity of Isothiocyanates from Cruciferous Plants against Methicillin-Resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2014, 15, 19552-19561. https://doi.org/10.3390/ijms151119552
Dias C, Aires A, Saavedra MJ. Antimicrobial Activity of Isothiocyanates from Cruciferous Plants against Methicillin-Resistant Staphylococcus aureus (MRSA). International Journal of Molecular Sciences. 2014; 15(11):19552-19561. https://doi.org/10.3390/ijms151119552
Chicago/Turabian StyleDias, Carla, Alfredo Aires, and Maria José Saavedra. 2014. "Antimicrobial Activity of Isothiocyanates from Cruciferous Plants against Methicillin-Resistant Staphylococcus aureus (MRSA)" International Journal of Molecular Sciences 15, no. 11: 19552-19561. https://doi.org/10.3390/ijms151119552