Exercise Preconditioning Protects against Spinal Cord Injury in Rats by Upregulating Neuronal and Astroglial Heat Shock Protein 72
Abstract
:1. Introduction
2. Results
2.1. Spinal Cord HSP 72 Was Higher in Exercise-Preconditioned (EP+) Rats
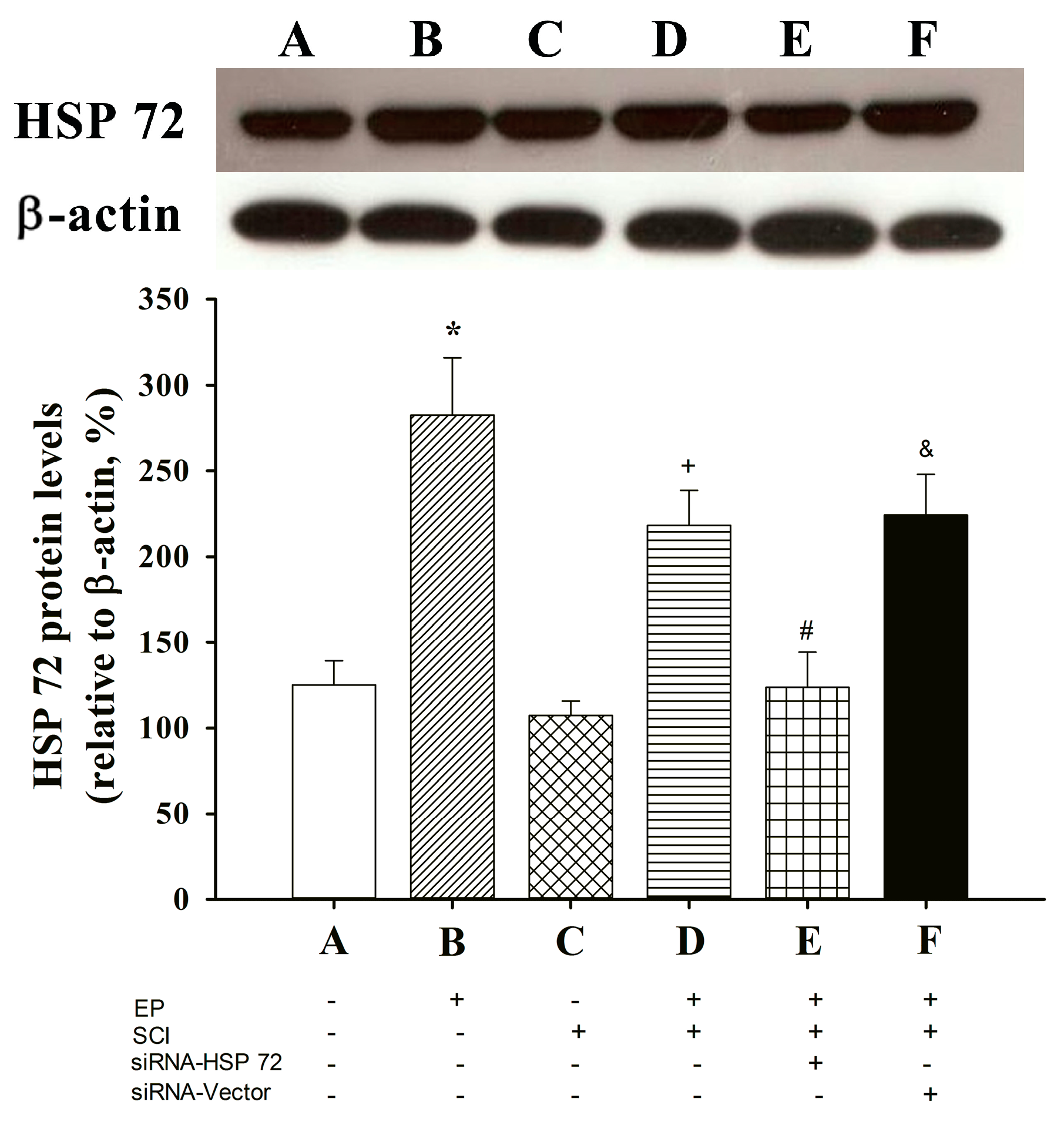
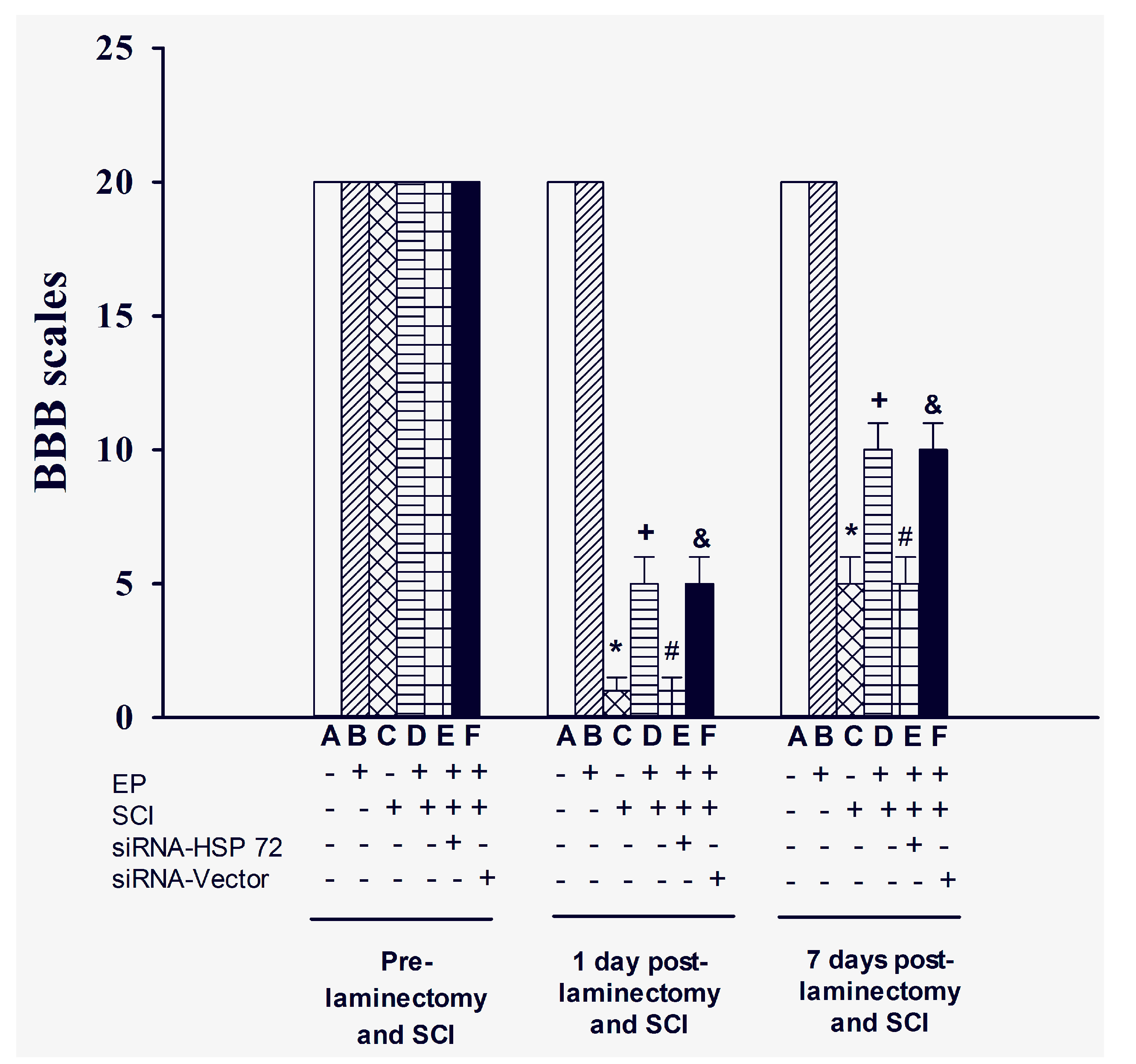
2.2. Functional Recovery after SCI
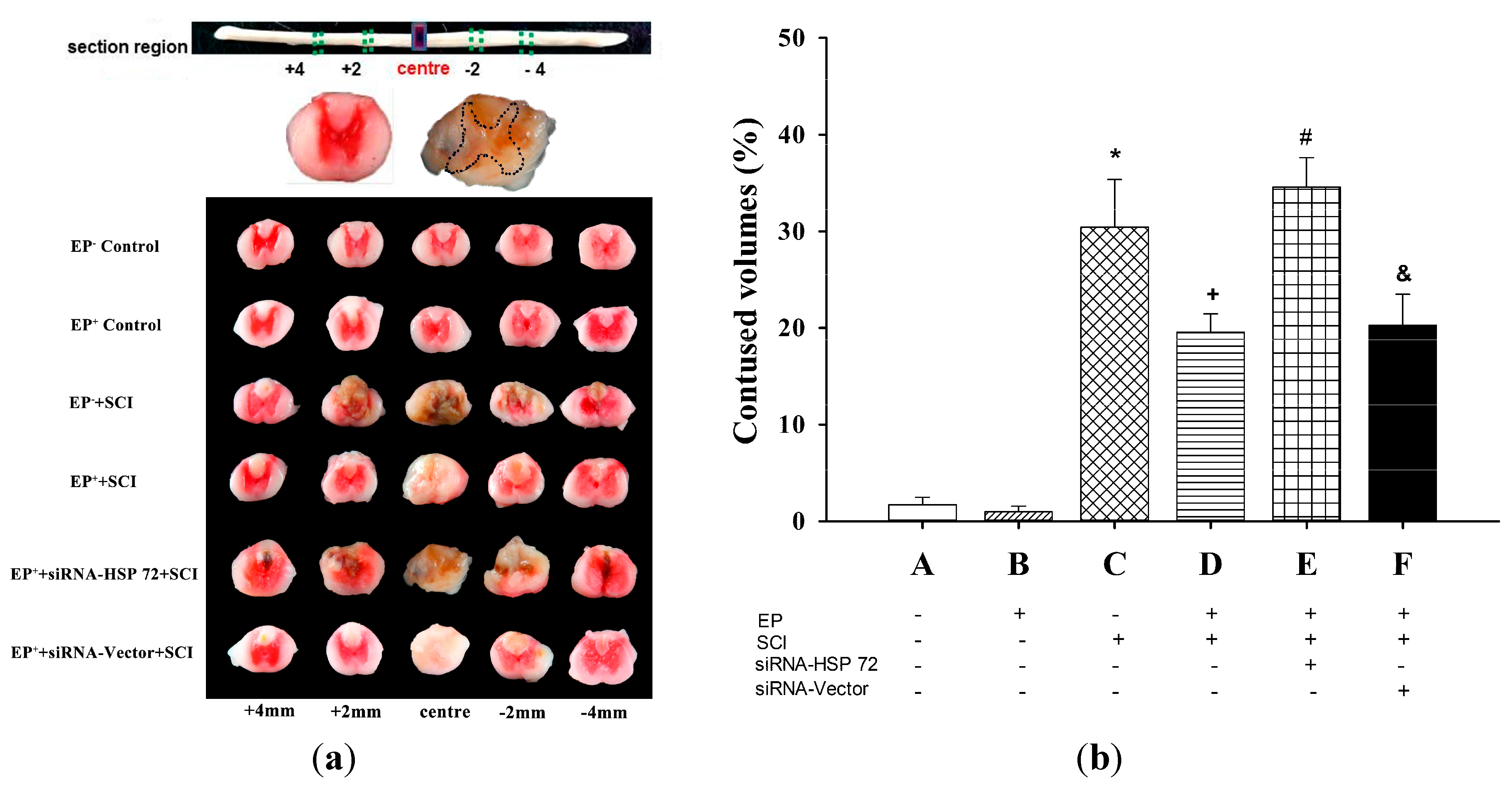
2.3. The Volume of Gray Matter Contusions in the Injured Spinal Cords Was Significantly Smaller in the EP+ Groups
2.4. Post-Laminectomy Levels of Gray Matter Neuronal and Astroglial Apoptosis Were Significantly Lower in the EP+ Groups
2.5. Post-Laminectomy Gray Matter Neuronal Loss Was Significantly Lower in the EP+ Group
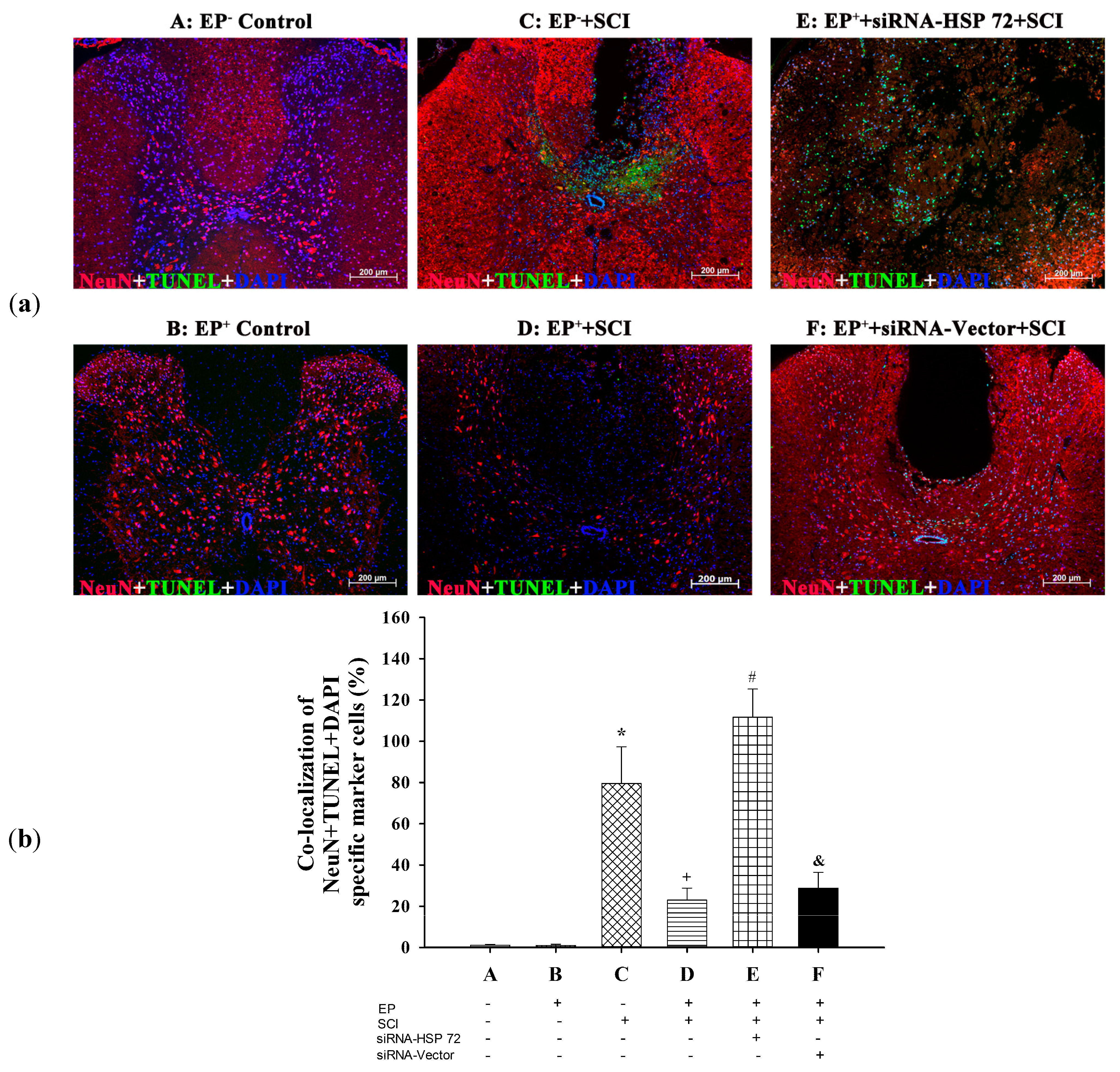
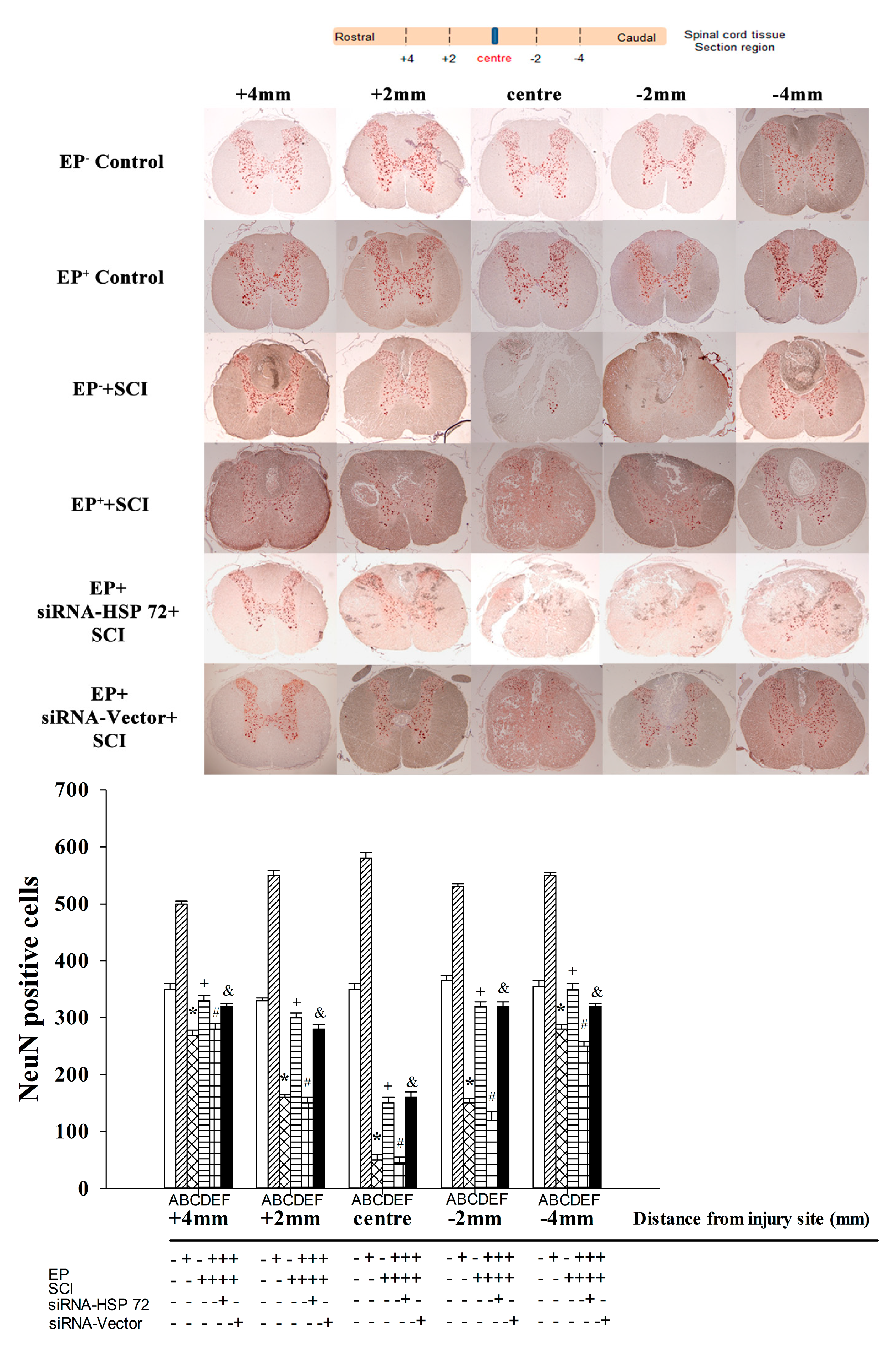
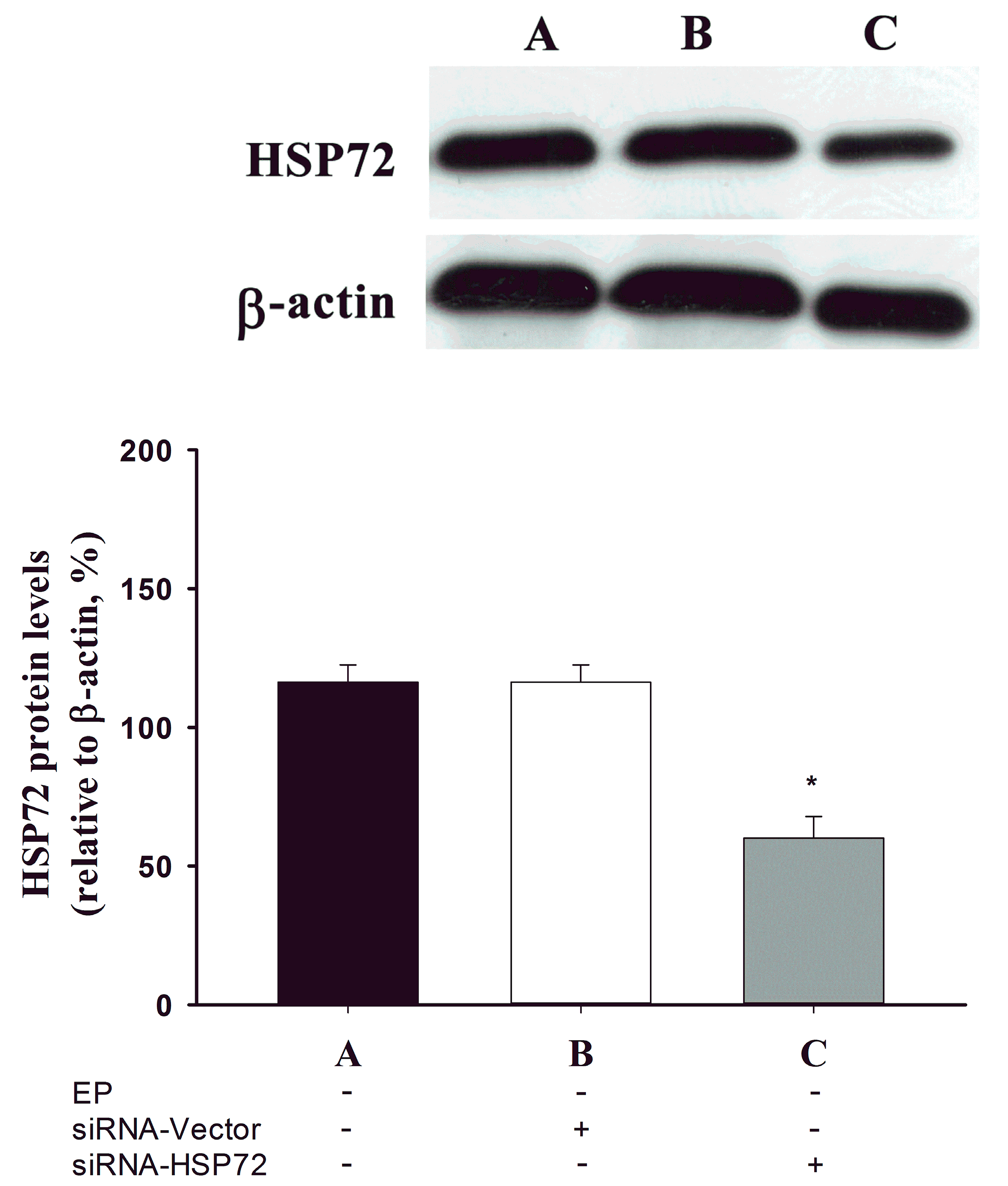
2.6. Post-Laminectomy Underexpression of Gray Matter Neuronal and Astroglial HSP 72

3. Discussion
4. Experimental Section
4.1. Ethics Statement
4.2. Animals and Surgery
4.3. Recombinant pSUPER Plasmid Expressing HSP 72 siRNA Construction
GAGAGTCGTTGGCGATGATCTCCTTTTTGGAAA-3' and
3'-GGGCCTCTAGTAGCGGTTGCTGAAGTTCTCTCAG
CAACCGCTACTAGAGGAAAAACCTTTTCGAA-5'
4.4. Exercise Training Protocol
4.5. Experimental Groups and Procedures
4.6. Assessing Hind-Limb Locomotor Function
4.7. Assessing the Volume of Spinal Cord Contusions 7 Days after SCI
4.8. Preparing the Tissue Samples
4.9. Protein Analysis and Quantification
| Antibody | Antigen | Host | Company | Catalog# | Dilution |
|---|---|---|---|---|---|
| Primary antibody | |||||
| HSP 72 | HSP 72 | Mouse | Enzo | SPA-810 | 1:1000 |
| β-actin | β-actin | Mouse | Santa Cruz | SC47778 | 1:1000 |
| Secondary antibody (conjugation) | |||||
| Mouse IgG | Mouse IgG | Sheep | GE Healthcare | NA931 | 1:10,000 |
| Rabbit IgG | Rabbit IgG | Donkey | GE Healthcare | NA934 | 1:40,000 |
4.10. Terminal Deoxynucleotidyl-Transferase-Mediated and dUTP-Biotin Nick End-Labeling (TUNEL) Staining and Immunostaining
| Antibody | Antigen | Host | Company | Catalog# | Dilution |
|---|---|---|---|---|---|
| Primary Antibody | |||||
| NeuN | Neuron | Mouse | Millipore | MAB377 | 1:400 |
| GFAP | Astrocyte | Mouse | Abcam | ab4648 | 1:200 |
| TUNEL | Apoptosis | Clontech | 630108 | 1:200 | |
| HSP 72 | HSP 72 | Rabbit | Abgent | AJ1380a | 1:200 |
| DAPI | Nucleic acids | Invitrogen | D1306 | 1:20,000 | |
| Secondary Antibody (conjugation) | |||||
| Mouse IgG (Alexa Fluor 568) | Mouse IgG | Goat | Invitrogen | A11031 | 1:200 |
| Rabbit IgG (Alexa Fluor 488) | Rabbit IgG | Goat | Invitrogen | A11034 | 1:200 |
4.11. Statistical Analysis
5. Conclusions
Abbreviations
| SCI | spinal cord injury |
| HSP | heat shock protein |
| EP | exercise preconditioning |
| siRNAs | small interfering RNAs |
| TTC | Triphenyltetrazolium chloride |
| TUNEL | terminal deoxynucleotidyl-transferase-mediated and dUTP-biotin nick-labeling |
| NeuN | neuronal nuclei |
| GFAP | glial fibrillary acidic protein |
| BBB | blood-brain-barrier |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Noble, E.G.; Milne, K.J.; Melling, C.W. Heat shock proteins and exercise: A primer. Appl. Physiol. Nutr. Metab. 2008, 33, 1050–1065. [Google Scholar] [CrossRef]
- Garrido, C.; Gurbuxani, S.; Ravagnan, L.; Kroemer, G. Heat shock proteins: Endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001, 286, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Homem de Bittencourt, P.I., Jr.; Lagranha, D.J.; Maslinkiewicz, A.; Senna, S.M.; Tavares, A.M.; Baldissera, L.P.; Janner, D.R.; Peralta, J.S.; Bock, P.M.; Gutierrez, L.L.; et al. LipoCardium: endothelium-directed cyclopentenone prostaglandin-based liposome formulation that completely reverses atherosclerotic lesions. Atherosclerosis 2007, 193, 245–258. [Google Scholar]
- Calderwood, S.K.; Mambula, S.S.; Gray, P.J., Jr.; Theriault, J.R. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007, 581, 3689–3694. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Fleshner, M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J. Leukoc. Biol. 2006, 79, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.C.; Koukoulas, I.; Garnham, A.; Moseley, P.L.; Hargreaves, M.; Febbraio, M.A. Exercise increases serum HSP72 in humans. Cell Stress Chaperones 2001, 6, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chen, S.H.; Chou, W.; Lo, Y.M.; Hung, C.H.; Lin, M.T. Exercise pretraining protects against cerebral ischemia induced by heat stroke in rats. Br. J. Sports Med. 2004, 41, 597–602. [Google Scholar]
- Hung, C.H.; Chang, N.C.; Cheng, B.C.; Lin, M.T. Progressive exercise preconditioning protects against circulatory shock during experimental heatstroke. Shock 2005, 23, 426–433. [Google Scholar] [PubMed]
- Liebelt, B.; Papapetrou, P.; Ali, A.; Guo, M.; Ji, X.; Peng, C.; Rogers, R.; Curry, A.; Jimenez, D.; Ding, Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience 2010, 166, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Buehner, J.J.; Forrest, G.F.; Schmidt-Read, M.; White, S.; Tansey, K.; Basso, D.M. Relationship between ASIA examination and functional outcomes in the NeuroRecovery Network Locomotor Training Program. Arch. Phys. Med. Rehabil. 2012, 93, 1530–1540. [Google Scholar] [PubMed]
- Lovely, R.G.; Gregor, R.J.; Roy, R.R.; Edgerton, V.R. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 1986, 92, 421–435. [Google Scholar] [PubMed]
- Sandrow-Feinberg, H.R.; Izzi, J.; Shumsky, J.S.; Zhukareva, V.; Houle, J.D. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J. Neurotrauma 2009, 26, 721–731. [Google Scholar] [PubMed]
- Edgerton, V.R.; Tillakaratne, N.J.; Bigbee, A.J.; de Leon, R.D.; Roy, R.R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004, 27, 145–167. [Google Scholar] [PubMed]
- Jean, A.; Conductier, G.; Manrique, C.; Bouras, C.; Berta, P.; Hen, R.; Charnay, Y.; Bockaert, J.; Compan, V. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc. Natl. Acad. Sci. USA 2007, 104, 16335–16340. [Google Scholar] [PubMed]
- Lingor, P.; Koeberle, P.; Kügler, S.; Bähr, M. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain 2005, 128, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Makimura, H.; Mizuno, T.M.; Mastaitis, J.W.; Agami, R.; Mobbs, C.V. Reducing hypothalamic AGRP by RNA interference increases metabolic rate and decreases body weight without influencing food intake. BMC Neurosci. 2002, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Compan, V.; Rosselet, C.; Duflo, S.G. Specific knock-down of GAD67 in the striatum using naked small interfering RNAs. J. Biotechnol. 2009, 142, 185–192. [Google Scholar] [PubMed]
- Brummelkamp, T.R.; Bernards, R.; Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 2002, 296, 550–553. [Google Scholar] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [PubMed]
- Wang, Y.; Lin, S.Z.; Chiou, A.L.; Williams, L.R.; Hoffer, B.J. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J. Neurosci. 1997, 17, 4341–4348. [Google Scholar] [PubMed]
- Chio, C.C.; Lin, J.W.; Chang, M.W.; Wang, C.C.; Kuo, J.R.; Yang, C.Z.; Chang, C.P. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J. Neurochem. 2010, 115, 921–929. [Google Scholar] [PubMed]
- Li, J.; Ding, Y.H.; Rafols, J.A.; Lai, Q.; McAllister, J.P., 2nd; Ding, Y. Increased astrocyte proliferation in rats after running exercise. Neurosci. Lett. 2005, 386, 160–164. [Google Scholar]
- Sun, Y.; Ouyang, Y.B.; Xu, L.; Chow, A.M.; Anderson, R.; Hecker, J.G.; Giffard, R.G. The carboxyl-terminal domain of inducible HSP70 protects from ischemic injury in vivo and in vitro. J. Cereb. Blood Flow Metab. 2006, 26, 937–950. [Google Scholar] [PubMed]
- Giffard, R.G.; Yenari, M.A. Many mechanisms for HSP70 protection from cerebral ischemia. J. Neurosurg. Anesthesiol. 2004, 16, 53–61. [Google Scholar] [PubMed]
- Lee, J.E.; Yenari, M.A.; Sun, G.H.; Xu, L.; Emond, M.R.; Cheng, D.; Steinberg, G.K.; Giffard, R.G. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp. Neurol. 2001, 170, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, D.; Tsuchiya, D.; Hong, S.; Matsumori, Y.; Kayama, T.; Swanson, R.A.; Dillman, W.H.; Liu, J.; Panter, S.S.; Weinstein, P.R. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery 2003, 53, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.D.; Vender, J.R.; Dhandapani, K.M. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals 2008, 16, 154–164. [Google Scholar] [PubMed]
- Myer, D.J.; Gurkoff, G.G.; Lee, S.M.; Hovda, D.A.; Sofroniew, M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 2006, 129, 2761–2772. [Google Scholar] [PubMed]
- Floyd, C.L.; Lyeth, B.G. Astroglia: Important mediators of traumatic brain injury. Prog. Brain Res. 2007, 161, 61–79. [Google Scholar] [PubMed]
- Chen, S.H.; Yeh, C.H.; Lin, M.Y.; Kang, C.Y.; Chu, C.C.; Chang, F.M.; Wang, J.J. Premarin improves outcomes of spinal cord injury in male rats through stimulating both angiogenesis and neurogenesis. Crit. Care Med. 2010, 38, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.H.; Chio, C.C.; Lin, M.T.; Yeh, C.H. Body cooling ameliorating spinal cord injury may be neurogenesis-, anti-inflammation- and angiogenesis-associated in rats. J. Trauma 2011, 70, 885–893. [Google Scholar] [PubMed]
- Tai, P.A.; Chang, C.K.; Niu, K.C.; Lin, M.T.; Chiu, W.T.; Lin, C.M. Attenuating experimental spinal cord injury by hyperbaric oxygen: stimulating production of vasculoendothelial and glial cell line-derived neurotrophic growth factors and interleukin-10. J. Neurotrauma 2010, 27, 1–8. [Google Scholar] [PubMed]
- Krause, M.; Rodrigues-Krause, J.D.C. Extracellular heat shock proteins (eHSP 70) in exercise: Possible target outside the immune system and their role for neurodegenerative disorders treatment. Med. Hypotheses 2011, 76, 286–290. [Google Scholar] [PubMed]
- Zhou, Y.; Xu, L.; Song, X.; Ding, L.; Chen, J.; Wang, C.; Gan, Y.; Zhu, X.; Yu, Y.; Liang, Q. The potential role of heat shock proteins in acute spinal cord injury. Eur. Spine J. 2014, 23, 1480–1490. [Google Scholar] [PubMed]
- Takahashi, J.L.; Giuliani, F.; Power, C.; Imai, Y.; Yong, V.W. Interleukin-1β promotes oligodendrocyte death through glutamate excitotoxicity. Ann. Neurol. 2003, 53, 588–595. [Google Scholar] [CrossRef] [PubMed]
- BLAST Assembled Genomes. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 1 October 2014).
- Limb Volumes Professional [LVP]. Available online: www.limbvolumes.org (accessed on 1 October 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-K.; Chou, W.; Lin, H.-J.; Huang, Y.-C.; Tang, L.-Y.; Lin, M.-T.; Chang, C.-P. Exercise Preconditioning Protects against Spinal Cord Injury in Rats by Upregulating Neuronal and Astroglial Heat Shock Protein 72. Int. J. Mol. Sci. 2014, 15, 19018-19036. https://doi.org/10.3390/ijms151019018
Chang C-K, Chou W, Lin H-J, Huang Y-C, Tang L-Y, Lin M-T, Chang C-P. Exercise Preconditioning Protects against Spinal Cord Injury in Rats by Upregulating Neuronal and Astroglial Heat Shock Protein 72. International Journal of Molecular Sciences. 2014; 15(10):19018-19036. https://doi.org/10.3390/ijms151019018
Chicago/Turabian StyleChang, Cheng-Kuei, Willy Chou, Hung-Jung Lin, Yi-Ching Huang, Ling-Yu Tang, Mao-Tsun Lin, and Ching-Ping Chang. 2014. "Exercise Preconditioning Protects against Spinal Cord Injury in Rats by Upregulating Neuronal and Astroglial Heat Shock Protein 72" International Journal of Molecular Sciences 15, no. 10: 19018-19036. https://doi.org/10.3390/ijms151019018





