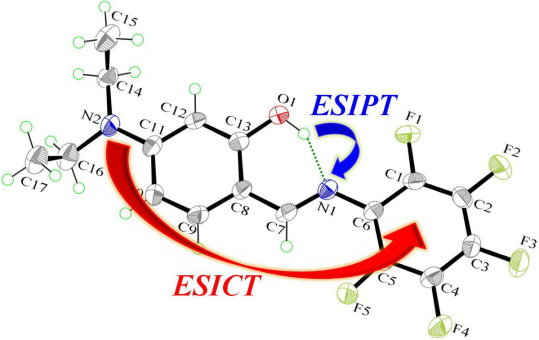
Synthesis, X-ray Structure, Spectroscopic Properties and DFT Studies of a Novel Schiff Base
Abstract
:1. Introduction
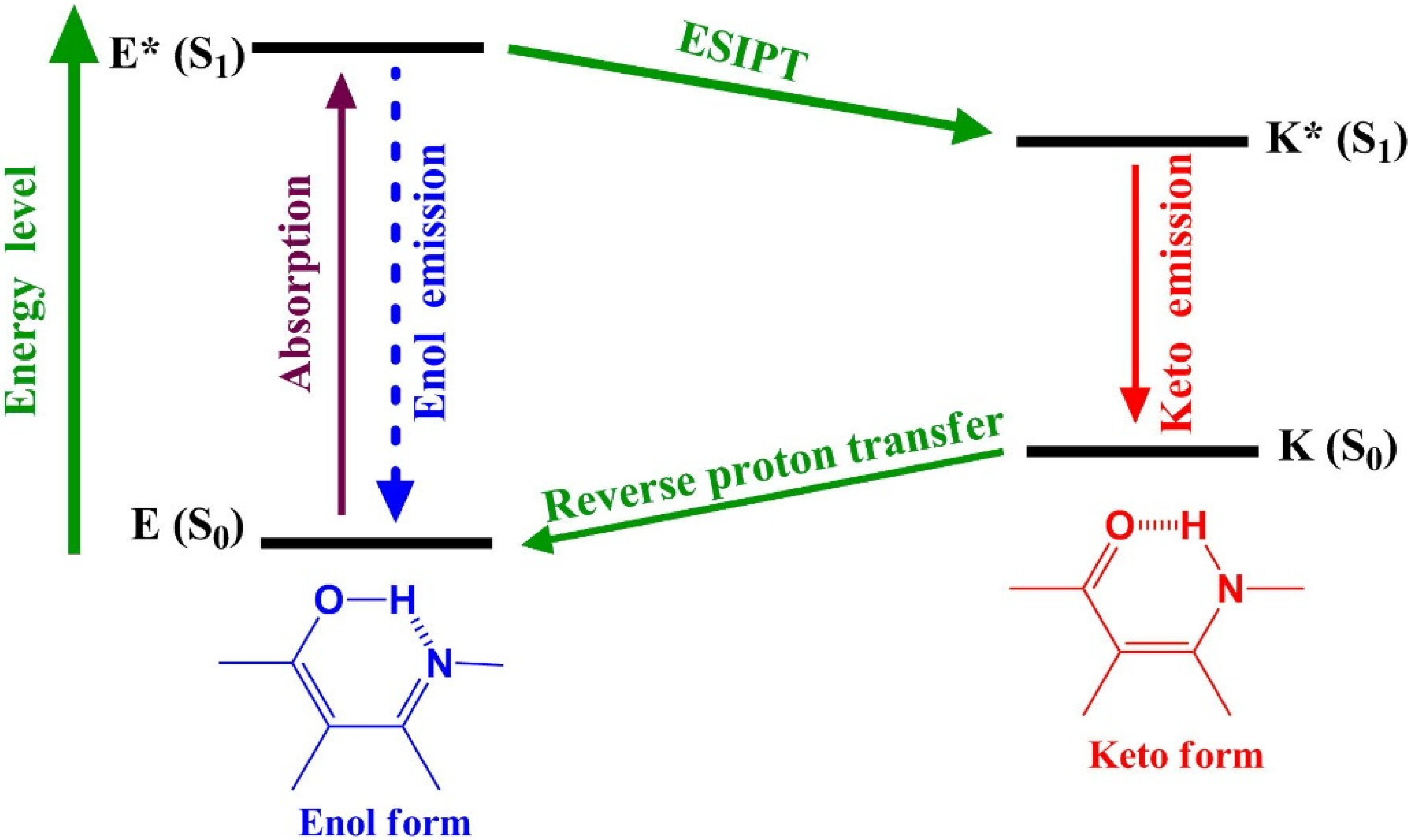
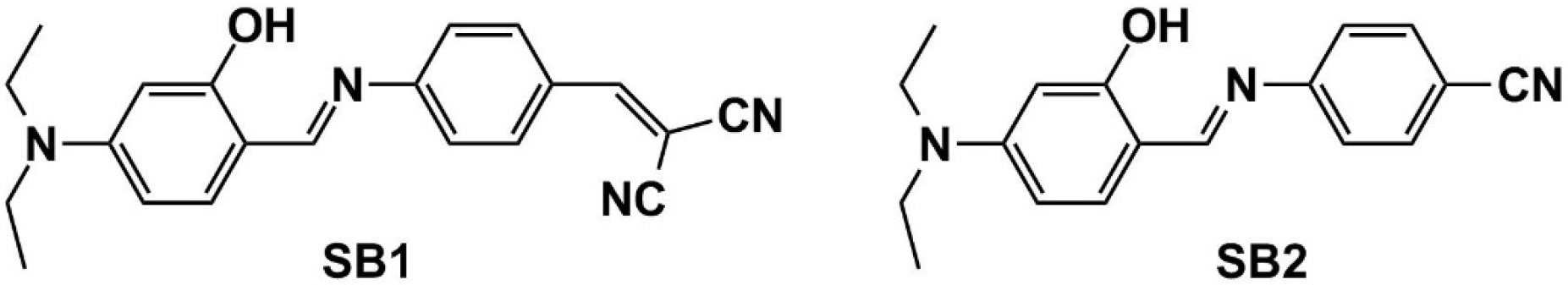
2. Results and Discussion
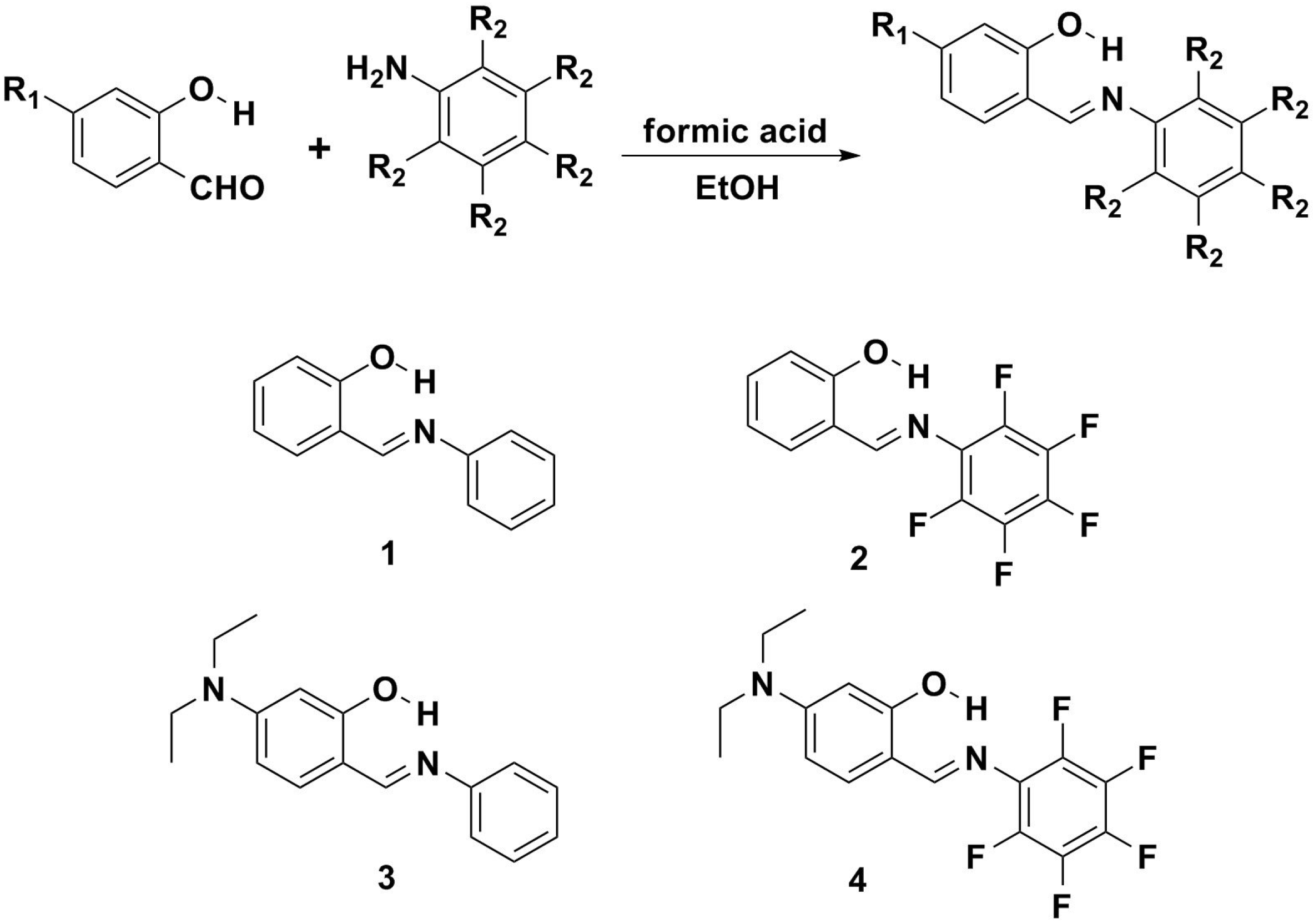
2.1. Synthesis
2.2. Hydrogen Bond Studies
| Compound | 1H NMR a | HB length b | EHB c | EHB d |
|---|---|---|---|---|
| 1 | 13.26 | 2.641 | 9.37 | 13.79 |
| 2 | 12.12 | 2.648 | 8.23 | 12.27 |
| 3 | 13.85 | 2.638 | 9.96 | 14.35 |
| 4 | 12.74 | 2.643 | 8.85 | 12.50 |
2.3. X-ray Structure
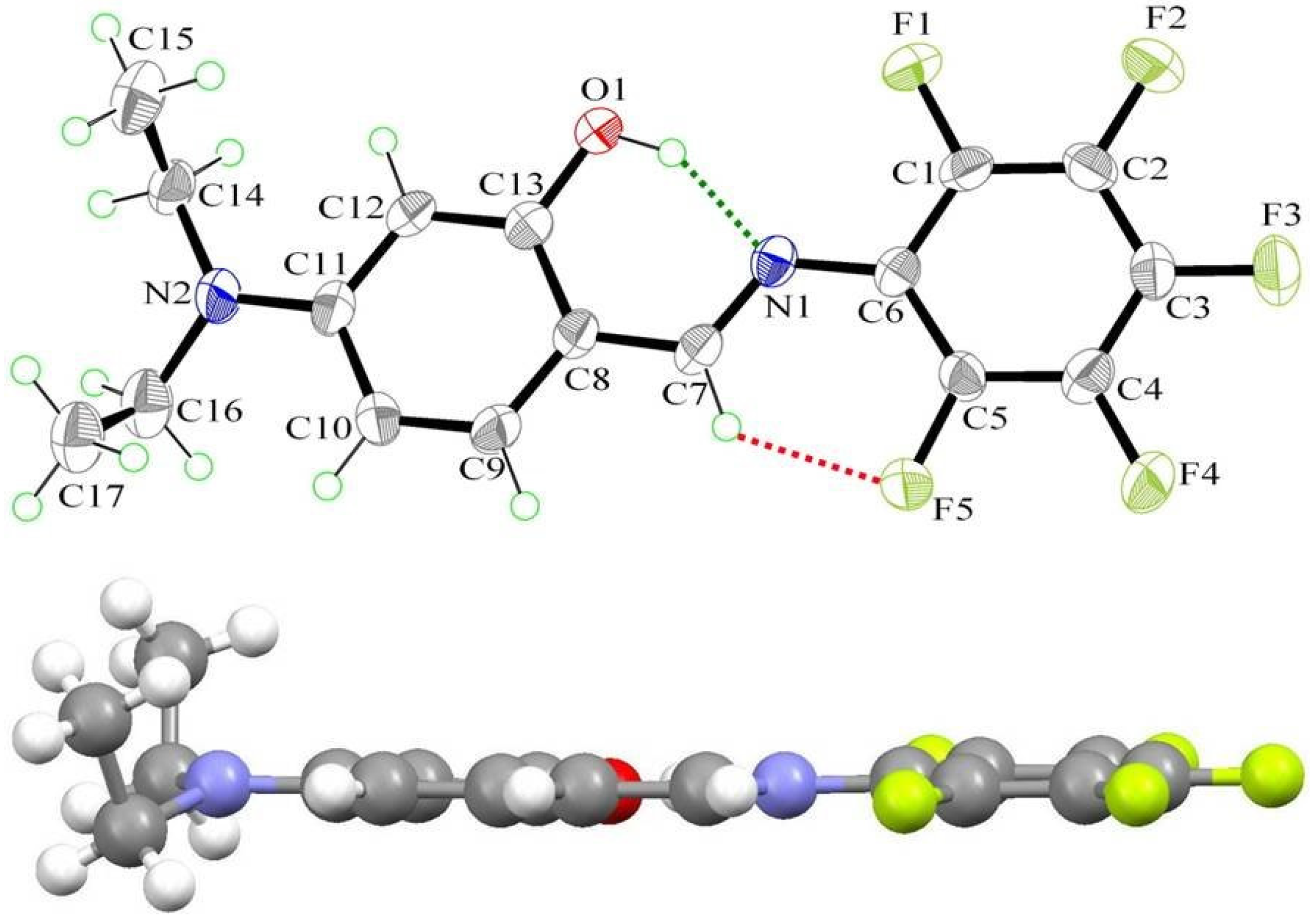
| X-ray | DFT | |
|---|---|---|
| Bond lengths (Å) | ||
| O–C(13) | 1.357(4) | 1.341 |
| C(1)–C(2) | 1.372(4) | 1.389 |
| C(5)–C(6) | 1.398(4) | 1.409 |
| N(1)–C(6) | 1.400(4) | 1.386 |
| N(1)–C(7) | 1.299(4) | 1.305 |
| N(2)–C(11) | 1.368(4) | 1.379 |
| C(12)–C(13) | 1.379(4) | 1.393 |
| C(5)–F(5) | 1.342(3) | 1.348 |
| Bond angles (°) | ||
| O(1)–C(13)–C(12) | 117.6(3) | 117.8 |
| C(2)–C(3)–C(4) | 118.2(3) | 119.2 |
| C(8)–C(7)–N(1) | 121.7(2) | 121.3 |
| C(1)–C(6)–N(1) | 117.4(1) | 116.3 |
| C(11)–C(12)–C(13) | 121.0(3) | 121.7 |
| C(4)–C(5)–F(5) | 116.5(3) | 117.0 |
| Torsion angles (°) | ||
| O(1)–C(13)–C(8)–C(7) | 2.8(2) | 0.3 |
| N(1)–C(6)–C(1)–C(2) | 178.8(2) | 178.6 |
| N(2)–C(11)–C(10)–C(9) | 179.7(2) | 178.1 |
| C(8)–C(7)–N(1)–C(6) | 179.2(2) | 179.5 |
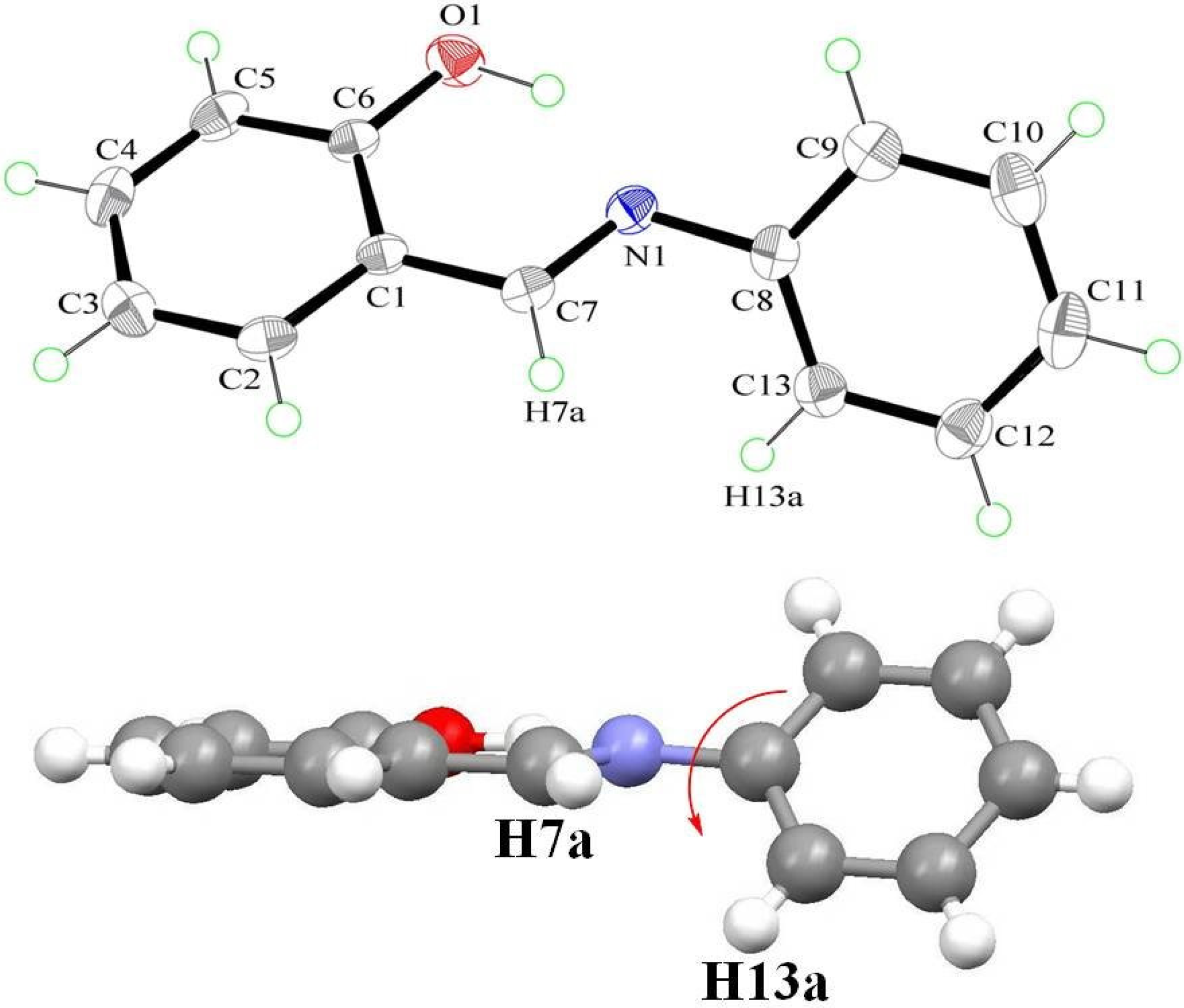
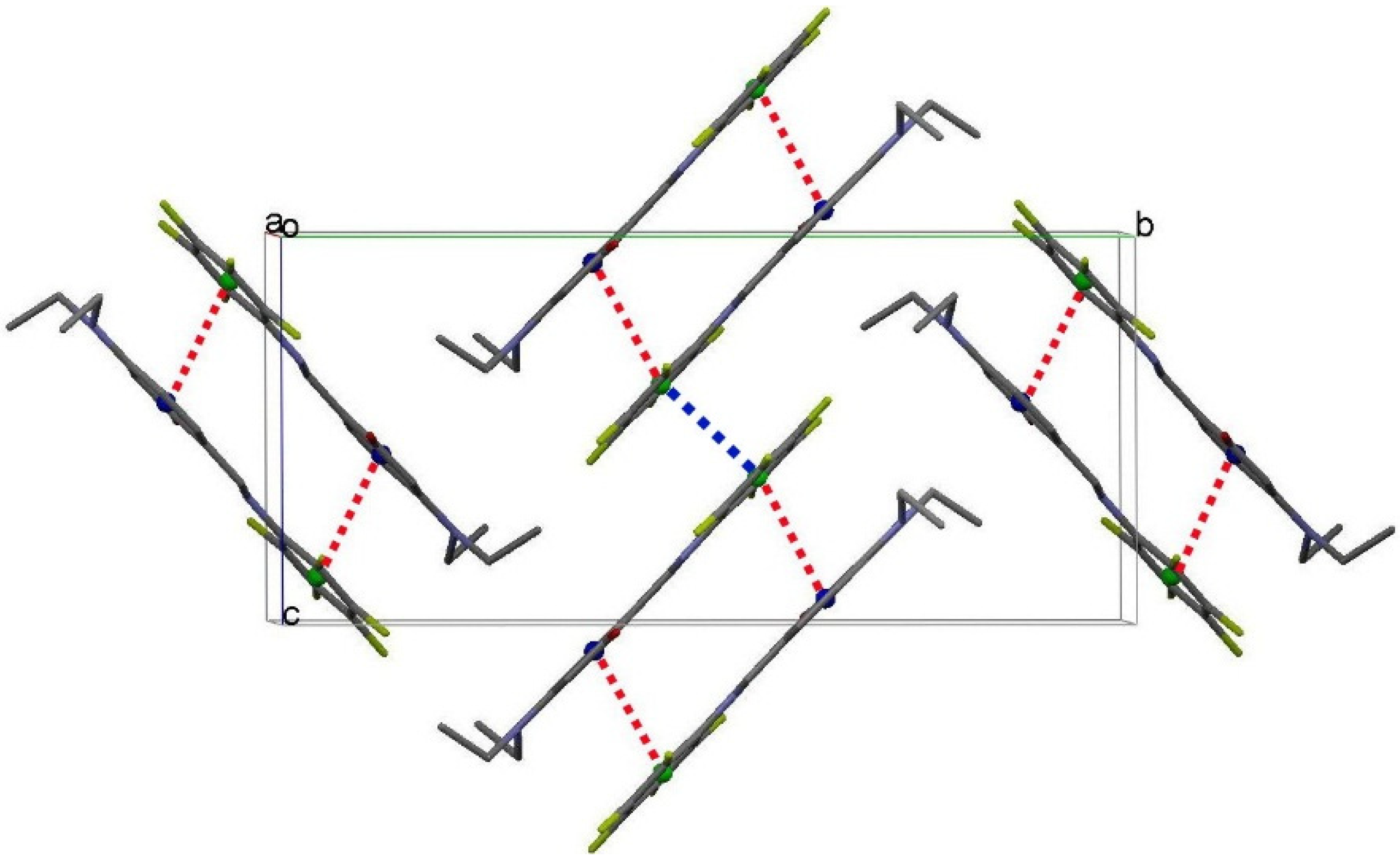
2.4. Optical Properties
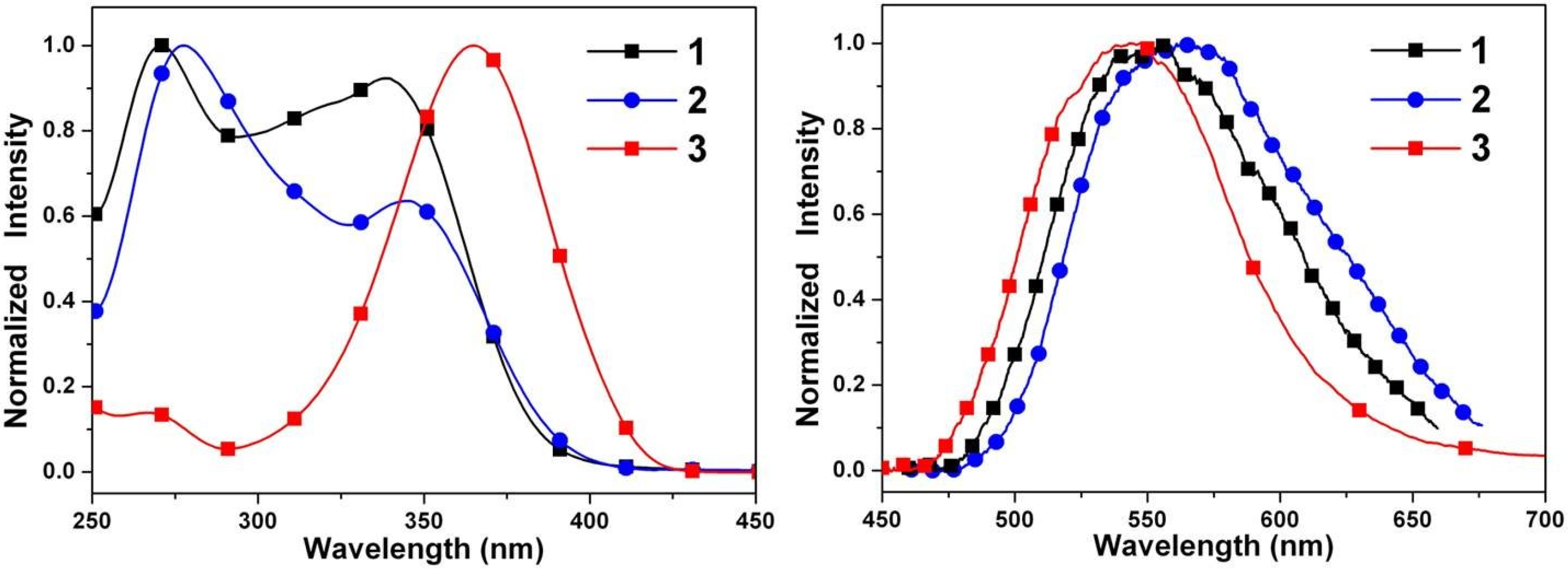
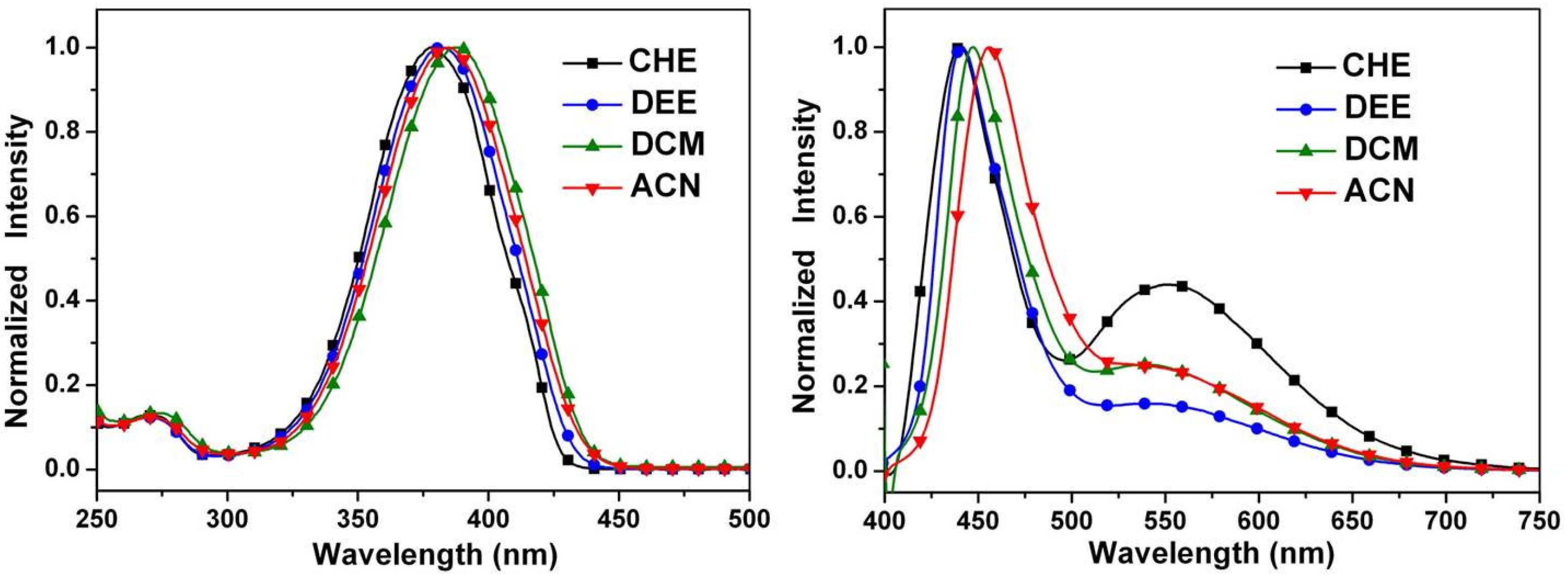
| Compound | λabs (nm) a | λem (nm) a | Stokes shift b | Φ c × 103 |
|---|---|---|---|---|
| 1 | 339 | 556 | 11,513 | 3.5 |
| 2 | 345 | 565 | 11,286 | 5.2 |
| 3 | 365 | 547 | 9116 | 8.5 |
| 4 | 379 | 436, 551 | 34,498,236 | 19.6 |

2.5. Quantum Chemistry Computation


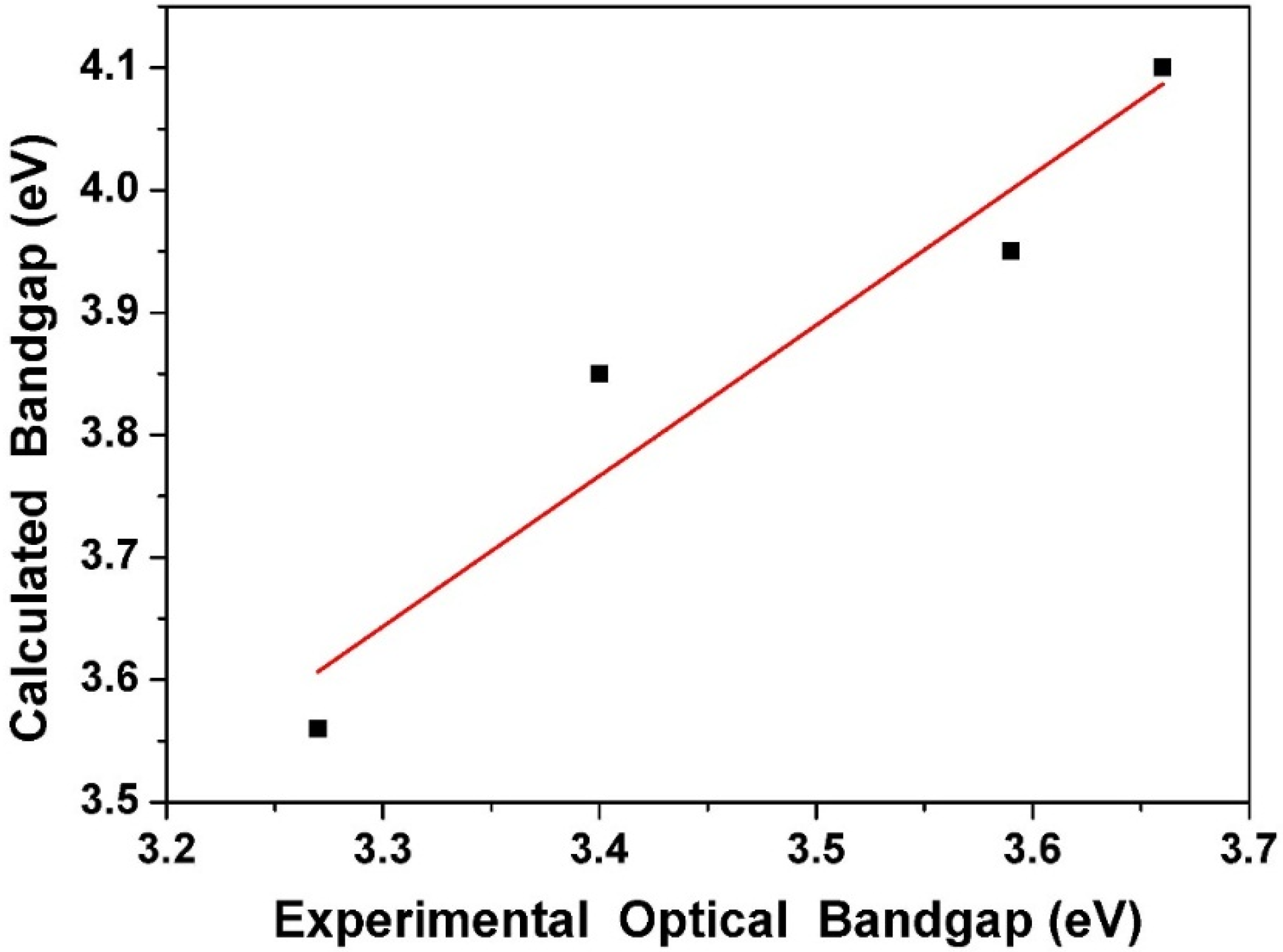
| Compound | HOMO a | LUMO a | Eg a | Eg b |
|---|---|---|---|---|
| 1 | −6.16 | −2.06 | 4.10 | 3.66 |
| 2 | −6.21 | −2.26 | 3.95 | 3.59 |
| 3 | −5.10 | −1.25 | 3.85 | 3.40 |
| 4 | −5.32 | −1.76 | 3.56 | 3.27 |

| Compound | Singlet | Electronic Transition | Energy | f | Composition b | CI c |
|---|---|---|---|---|---|---|
| 1 | UV–vis | S0→S1 | 3.67 eV/338 nm | 0.3302 | H→L | 0.68789 |
| FL | S1→S0 | 2.28 eV/542 nm | 0.1229 | H→L | 0.68020 | |
| 2 | UV–vis | S0→S1 | 3.59 eV/345 nm | 0.2603 | H→L | 0.68034 |
| FL | S1→S0 | 2.38 eV/521 nm | 0.1482 | H→L | 0.70535 | |
| 3 | UV–vis | S0→S1 | 3.44 eV/360 nm | 1.0334 | H→L | 0.69373 |
| FL | S1→S0 | 2.18 eV/569 nm | 0.0112 | H→L | 0.70469 | |
| 4 | UV–vis | S0→S1 | 3.39 eV/365 nm | 1.1279 | H→L | 0.70201 |
| FL (Enol) | S1→S0 | 2.92 eV/424 nm | 1.0981 | H→L | 0.70097 | |
| FL (Keto) | S1→S0 | 2.12 eV/583 nm | 0.0075 | H→L | 0.70576 |
| Compound | ∆E (kcal·mol−1) a | ∆E (kcal·mol−1) b |
|---|---|---|
| 1 | 4.4 | −11.6 |
| 2 | 5.9 | −11.8 |
| 3 | 6.1 | −12.5 |
| 4 | 5.5 | −12.0 |
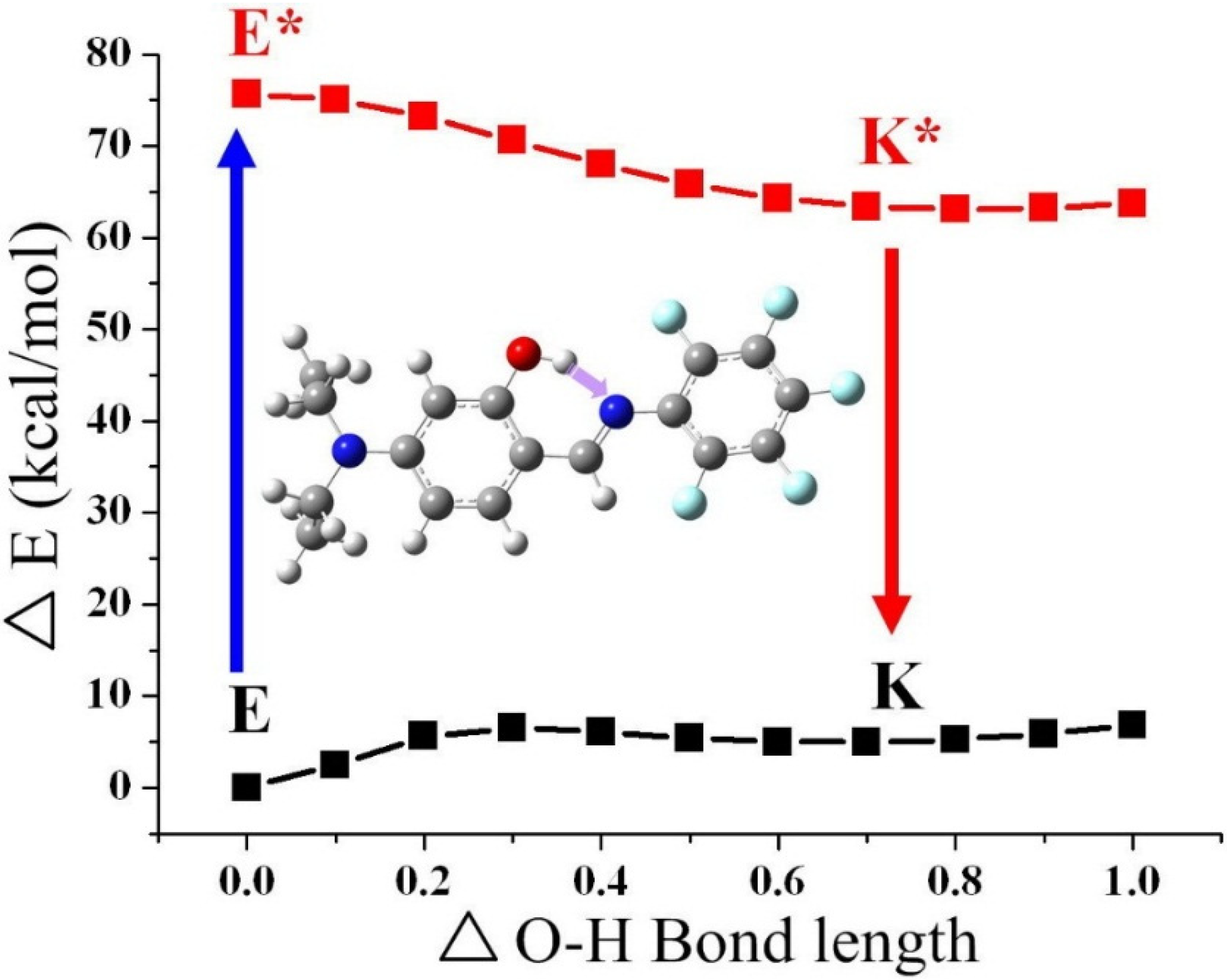
3. Experimental Section
3.1. General
3.2. Synthesis
3.3. Crystal Structural Determination
3.4. Computational Methods
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Schiff, H. Mittheilungen aus dem universitätslaboratorium in Pisa: Eine neue reihe organischer basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Stutzman, J. R.; McLuckey, S.A. Ion/ion reactions of MALDI-derived peptide ions: Increased sequence coverage via covalent and electrostatic modification upon charge inversion. Anal. Chem. 2012, 84, 10679–10685. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, L.; Yu, J.; Tong, S.L.; Yan, Y. Synthesis and spectroscopic characterization of meso-tetra (Schiff-base substituted phenyl) porphyrins and their zinc complexes. Dyes Pigm. 2013, 97, 423–428. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, Z.K.; Tang, H.; Tang, L.J.; Jiang, J.H. Activity-based DNA-gold nanoparticle probe as colorimetric biosensor for DNA methyltransferase/glycosylase assay. Anal. Chem. 2013, 85, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Loget, G.; Wood, J.B.; Cho, K.; Halpern, A.R.; Corn, R.M. Electrodeposition of polydopamine thin films for DNA patterning and microarrays. Anal. Chem. 2013, 85, 9991–9995. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, K.; Li, H.; Li, J.; He, J.; Sun, X. One pot synthesis of imines from aromatic nitro compounds with a novel Ni/SiO2 magnetic catalyst. Catal. Lett. 2009, 128, 465–474. [Google Scholar] [CrossRef]
- Taguchi, K.; Westheimer, F.H. Catalysis by molecular sieves in the preparation of ketimines and enamines. J. Org. Chem. 1971, 36, 1570–1572. [Google Scholar] [CrossRef]
- Love, B.E.; Ren, J. Synthesis of sterically hindered imines. J. Org. Chem. 1993, 58, 5556–5557. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Li, X.D.; Zhan, M.S. Synthesis and mesomorphic properties of bent-shaped molecule with low bent-angle central core and long alkylthio tail. Chin. Chem. Lett. 2011, 22, 1111–1114. [Google Scholar] [CrossRef]
- Fuh, A.Y.G.; Chen, Y.D.; Liu, C.K.; Cheng, K.T. Azo dye adsorption effect induced by elliptically polarized light in azo dye-doped liquid crystals. Dyes Pigm. 2012, 92, 949–953. [Google Scholar] [CrossRef]
- Ha, S.T.; Foo, K.L.; Subramaniam, R.T.; Ito, M.M.; Ong, S.T. Heterocyclic benzoxazole-based liquid crystals: Synthesis and mesomorphic properties. Chin. Chem. Lett. 2011, 22, 1191–1194. [Google Scholar] [CrossRef]
- Lee, W.; Yuk, S.B.; Choi, J.; Jung, D.H.; Choi, S.H.; Park, J.; Kim, J.P. Synthesis and characterization of solubility enhanced metal-free phthalocyanines for liquid crystal display black matrix of low dielectric constant. Dyes Pigm. 2012, 92, 942–948. [Google Scholar] [CrossRef]
- Menati, S.; Azadbakht, A.; Azadbakht, R.; Taeb, A.; Kakanejadifard, A. Synthesis, characterization, and electrochemical study of some novel, azo-containing Schiff bases and their Ni(II) complexes. Dyes Pigm. 2013, 98, 499–506. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, B.B.; Wei, T.B.; Zhang, Y.M.; Lin, Q.; Yao, H.; You, X.M. A naphtholic Schiff base for highly selective sensing of cyanide via different channels in aqueous solution. Dyes Pigm. 2013, 99, 857–862. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Ali, H.M.; Abdullah, M.A.; Hassandarvish, P. Acute toxicity and gastroprotective effect of the Schiff base ligand 1H-indole-3-ethylene-5-nitrosalicylaldimine and its nickel (II) complex on ethanol induced gastric lesions in rats. Molecules 2012, 17, 12449–12459. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Z.; Shan, G.G.; Li, P.; Zhou, Z.Y.; Su, Z.M. A novel class of Zn(II) Schiff base complexes with aggregation-induced emission enhancement (AIEE) properties: synthesis, characterization and photophysical/electrochemical properties. Dyes Pigm. 2013, 96, 467–474. [Google Scholar]
- Ahmadi, R.A.; Amani, S. Synthesis, spectroscopy, thermal analysis, magnetic properties and biological activity studies of Cu(II) and Co(II) complexes with Schiff base dye ligands. Molecules 2012, 17, 6434–6448. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Xu, F.; Chen, X.C.; Liu, Q.Q.; Huang, W.; You, X.Z. Comparisons between azo dyes and Schiff bases having the same benzothiazole/phenol skeleton: Syntheses, crystal structures and spectroscopic properties. Dyes Pigm. 2012, 92, 916–922. [Google Scholar] [CrossRef]
- Zabulica, A.; Balan, M.; Belei, D.; Sava, M.; Simionescu, B.C.; Marin, L. Novel luminescent phenothiazine-based Schiff bases with tuned morphology. Synthesis, structure, photophysical and thermotropic characterization. Dyes Pigm. 2013, 96, 686–698. [Google Scholar] [CrossRef]
- Ceyhan, G.; Tümer, M.; Köse, M.; McKee, V.; Akar, S. Structural characterization, luminescence and electrochemical properties of the Schiff base ligands. J. Lumin. 2012, 132, 2917–2928. [Google Scholar] [CrossRef]
- Seyedi, S.M.; Sandaroos, R.; Zohuri, G.H. Novel cobalt(II) complexes of amino acids-Schiff bases catalyzed aerobic oxidation of various alcohols to ketones and aldehyde. Chin. Chem. Lett. 2010, 21, 1303–1306. [Google Scholar] [CrossRef]
- Yin, L.; Jia, X.; Li, X.S. Simply air: Vanadium-catalyzed oxidative kinetic resolution of methyl o-chloromandelate by ambient air. Chin. Chem. Lett. 2010, 21, 774–777. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wan, N.N.; Wang, W.P.; Xie, Z.F.; Wang, J.D. Synthesis of bis(indolyl) methanes catalyzed by Schiff base-Cu(II) complex. Chin. Chem. Lett. 2011, 22, 1071–1074. [Google Scholar] [CrossRef]
- Chai, P.J.; Li, Y.S.; Tan, C.X. An efficient and convenient method for preparation of disulfides from thiols using air as oxidant catalyzed by Co-Salophen. Chin. Chem. Lett. 2011, 22, 1403–1406. [Google Scholar] [CrossRef]
- Al-Shaalan, N.H. Synthesis, characterization and biological activities of Cu(II), Co(II), Mn(II), Fe(II), and UO2(VI) complexes with a new Schiff base hydrazone: O-Hydroxyacetophenone-7-chloro-4-quinoline hydrazone. Molecules 2011, 16, 8629–8645. [Google Scholar] [CrossRef] [PubMed]
- Alwan, S.M. Synthesis and preliminary antimicrobial activities of new arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido cephalosporanic acids. Molecules 2012, 17, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.A.; Hamidi, R.M. Synthesis of new liquid crystalline diglycidyl ethers. Molecules 2012, 17, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, H.; Rabiei, K. Mild, convenient and efficient synthesis of novel 2,2-dichloro-1,3-diarylaziridines from Schiff bases by phase transfer CTAB catalysis under low concentration alkaline conditions. Chin. Chem. Lett. 2011, 22, 1273–1276. [Google Scholar] [CrossRef]
- Badrey, M.G.; Gomha, S.M. 3-Amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno [2,3-d]pyrimidine: An effecient key precursor for novel synthesis of some interesting triazines and triazepines as potential anti-tumor agents. Molecules 2012, 17, 11538–11553. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.; Köse, M.; Tümer, M.; Demirtaş, İ.; Yağlioğlu, A.Ş.; McKee, V. Structural characterization of some Schiff base compounds: Investigation of their electrochemical, photoluminescence, thermal and anticancer activity properties. J. Lumin. 2013, 143, 623–634. [Google Scholar] [CrossRef]
- Taha, Z.A.; Ajlouni, A.M.; Momani, W.A. Structural, luminescence and biological studies of trivalent lanthanide complexes with N,N'-bis(2-hydroxynaphthylmethylidene)-1,3-propanediamine Schiff base ligand. J. Lumin. 2012, 132, 2832–2841. [Google Scholar] [CrossRef]
- Fani, N.; Bordbar, A.K.; Ghayeb, Y. Spectroscopic, docking and molecular dynamics simulation studies on the interaction of two Schiff base complexes with human serum albumin. J. Lumin. 2013, 141, 166–172. [Google Scholar] [CrossRef]
- Ion, B.F.; Bushnell, E.A.C.; Luna, P.D.; Gauld, J.W. A molecular dynamics (MD) and quantum mechanics/molecular mechanics (QM/MM) study on ornithine cyclodeaminase (OCD): A tale of two iminiums. Int. J. Mol. Sci. 2012, 13, 12994–13011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Akhaja, T.N.; Raval, J.P. Design, synthesis, in vitro evaluation of tetrahydropyrimidine-isatin hybrids as potential antibacterial, antifungal and anti-tubercular agents. Chin. Chem. Lett. 2012, 23, 446–449. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.K.; Guchhait, N. 1-Hydroxy-2-naphthaldehyde: a prospective excited-state intramolecular proton transfer (ESIPT) probe with multi-faceted applications. J. Lumin. 2012, 132, 2194–2208. [Google Scholar] [CrossRef]
- Satam, M.A.; Raut, R.K.; Telore, R.D.; Sekar, N. Fuorescent acid azo dyes from 3-(1,3-benzothiazol-2-yl)naphthalen-2-ol and comparison with 2-naphthol analogs. Dyes Pigm. 2013, 97, 32–42. [Google Scholar] [CrossRef]
- Chen, K.Y.; Cheng, Y.M.; Lai, C.H.; Hsu, C.C.; Ho, M.L.; Lee, G.H.; Chou, P.T. Ortho green fluorescence protein synthetic chromophore; Excited-state intramolecular proton transfer via a seven-membered-ring hydrogen-bonding system. J. Am. Chem. Soc. 2007, 129, 4534–4535. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, A.K.; Maiti, K.; Sahoo, P.; Nandi, P.K. A new colorimetric and fluorescent bis(coumarin)methylene probe for fluoride ion detection based on the proton transfer signaling mode. J. Lumin. 2013, 143, 349–354. [Google Scholar] [CrossRef]
- Park, S.; Kwon, J.E.; Kim, S.H.; Seo, J.; Chung, K.; Park, S.Y.; Jang, D.J.; Medina, B.M.; Gierschner, J.; Park, S.Y. A white-light-emitting molecule: Frustrated energy transfer between constituent emitting centers. J. Am. Chem. Soc. 2009, 131, 14043–14049. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shao, Y.; Wu, F.; Liu, G.; Liu, L.; Peng, J.; Sun, Y. Targeting DNA abasic site by myricetin: Sequence-dependent ESIPT emission. J. Lumin. 2013, 136, 291–295. [Google Scholar] [CrossRef]
- Satam, M.A.; Raut, R.K.; Sekar, N. Fluorescent azo disperse dyes from 3-(1,3-benzothiazol-2-yl)naphthalen-2-ol and comparison with 2-naphthol analogs. Dyes Pigm. 2013, 96, 92–103. [Google Scholar] [CrossRef]
- Prabhu, S.; Saravanamoorthy, S.; Ashok, M.; Velmathi, S. Colorimetric and fluorescent sensing of multi metal ions and anions by salicylaldimine based receptors. J. Lumin. 2012, 132, 979–986. [Google Scholar] [CrossRef]
- Xu, H.; Yue, Y.; Wang, H.; Chen, I.; Hao, Y.; Xu, B. Single-crystal structure, photophysical characteristics and electroluminescent properties of bis(2-(4-trifluoromethyl-2-hydroxyphenyl)benzothiazolate)zinc. J. Lumin. 2012, 132, 919–923. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Chen, W.Q.; Duan, X.M. Seven-membered ring excited-state intramolecular proton-transfer in 2-benzamido-3-(pyridin-2-yl)acrylic acid. Dyes Pigm. 2011, 92, 619–625. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wang, L.; Li, Z.; Cui, Q.; Zhang, H.; Yang, H. Novel asymmetrical pyrene derivatives as light emitting materials: synthesis and photophysics. J. Lumin. 2012, 132, 1010–1014. [Google Scholar] [CrossRef]
- Patil, V.S.; Padalkar, V.S.; Tathe, A.B.; Sekar, N. ESIPT-inspired benzothiazole fluorescein: Photophysics of microenvironment pH and viscosity. Dyes Pigm. 2013, 98, 507–517. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Gryko, D.T. Deuterium isotope effects on 13C-NMR chemical shifts of 10-hydroxybenzo[h]quinolines. Molecules 2013, 18, 4544–4560. [Google Scholar] [CrossRef] [PubMed]
- Umape, P.G.; Patil, V.S.; Padalkar, V.S.; Phatangare, K.R.; Gupta, V.D.; Thate, A.B.; Sekar, N. Synthesis and characterization of novel yellow azo dyes from 2-morpholin-4-yl-1,3-thiazol-4(5H)-one and study of their azo–hydrazone tautomerism. Dyes Pigm. 2013, 99, 291–298. [Google Scholar] [CrossRef]
- Sobczyk, L.; Obrzud, M.; Filarowski, A. H/D isotope effects in hydrogen bonded systems. Molecules 2013, 18, 4467–4476. [Google Scholar] [CrossRef] [PubMed]
- Racané, L.; Mihalić, Z.; Cerić, H.; Popović, J.; Tralić-Kulenović, V. Synthesis, structure and tautomerism of two benzothiazolyl azo derivatives of 2-naphthol: A crystallographic, NMR and computational study. Dyes Pigm. 2013, 96, 672–678. [Google Scholar] [CrossRef]
- Li, C.; Yang, W.; Liu, H.; Li, M.; Zhou, W.; Xie, J. Crystal structures and antifungal activities of fluorine-containing thioureido complexes with nickel(II). Molecules 2013, 18, 15737–15749. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.L.; Gao, F.; Wang, Q.; Li, H.R.; Zhang, S.T. Excited state intramolecular proton transfer of novel conjugated derivatives containing hydroxy and imino groups. Chin. Chem. Lett. 2010, 21, 1195–1198. [Google Scholar] [CrossRef]
- Xie, L.; Chen, Y.; Wu, W.; Guo, H.; Zhao, J.; Yu, X. Fluorescent coumarin derivatives with large stokes shift, dual emission and solid state luminescent properties: An experimental and theoretical study. Dyes Pigm. 2012, 92, 1361–1369. [Google Scholar] [CrossRef]
- Lins, G.O.W.; Campo, L.F.; Rodembusch, F.S.; Stefani, V. Novel ESIPT fluorescent benzazolyl-4-quinolones: Synthesis, spectroscopic characterization and photophysical properties. Dyes Pigm. 2010, 84, 114–120. [Google Scholar] [CrossRef]
- Hong, W.H.; Lin, C.C.; Hsieh, T.S.; Chang, C.C. Preparation of fluoroionophores based on diamine-salicylaldehyde derivatives. Dyes Pigm. 2012, 94, 371–379. [Google Scholar] [CrossRef]
- Ito, Y.; Amimoto, K.; Kawato, T. Prototropic tautomerism and solid-state photochromism of N-phenyl-2-aminotropones. Dyes Pigm. 2011, 89, 319–323. [Google Scholar] [CrossRef]
- Lim, C.K.; Seo, J.; Kim, S.; Kwon, I.C.; Ahn, C.H.; Park, S.Y. Concentration and pH-modulated dual fluorescence in self-assembled nanoparticles of phototautomerizable biopolymeric amphiphile. Dyes Pigm. 2011, 90, 284–289. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, X.P.; Hu, M.; Li, Z.; Shi, X.X.; Chen, G.R. Highly optically selective and electrochemically active chemosensor for copper (II) based on triazole-linked glucosyl anthraquinone. Dyes Pigm. 2011, 88, 391–395. [Google Scholar] [CrossRef]
- Goswami, S.; Maity, S.; Das, A.K.; Maity, A.C.; Mandal, T.K.; Samanta, S. Remarkable ESIPT induced NIR emission by a selective colorimetric dibenzimidazolo diimine sensor for acetate. Tetrahedron Lett. 2013, 54, 5232–5235. [Google Scholar] [CrossRef]
- Li, T.; Yang, Z.; Li, Y.; Liu, Z.; Qi, G.; Wang, B. A novel fluorescein derivative as a colorimetric chemosensor for detecting copper(II) ion. Dyes Pigm. 2011, 88, 103–108. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, X.F.; Li, H. A ratiometric fluorescent probe for hydrogen sulfide based on an excited-state intramolecular proton transfer mechanism. Dyes Pigm. 2013, 99, 871–877. [Google Scholar] [CrossRef]
- Tang, K.C.; Chang, M.J.; Lin, T.Y.; Pan, H.A.; Fang, T.C.; Chen, K.Y.; Hung, W.Y.; Hsu, Y.H.; Chou, P.T. Fine tuning the energetics of excited-state intramolecular proton transfer (ESIPT): White light generation in a single ESIPT system. J. Am. Chem. Soc. 2011, 133, 17738–17745. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.T.; Hsieh, C.C.; Lai, C.H.; Lai, C.H.; Shih, C.W.; Chen, K.Y.; Hung, W.Y.; Hsu, Y.H.; Chou, P.T. Excited-state intramolecular proton transfer molecules bearing ortho-hydroxy analogues of green fluorescent protein chromophore. J. Org. Chem. 2011, 76, 8189–8202. [Google Scholar]
- Fang, S.K.; Tsai, H.Y.; Hu, J.W.; Chen, K.Y. A white-light-emitting small molecule: Synthesis, crystal structure and optical properties. Int. J. Photoenergy 2014. [Google Scholar] [CrossRef]
- Chou, P.T.; Pu, S.C.; Cheng, Y.M.; Yu, W.S.; Yu, Y.C.; Hung, F.T.; Hu, W.P. Femtosecond dynamics on excited-state proton/charge-transfer reaction in 4‘-N,N-diethylamino-3-hydroxyflavone. J. Phys. Chem. A 2005, 109, 3777–3787. [Google Scholar] [CrossRef]
- Lin, W.C.; Fang, S.K.; Hu, J.W.; Tsai, H.Y.; Chen, K.Y. Ratiometric fluorescent/colorimetric cyanide-selective sensor based on excited-state intramolecular charge transfer-excited-state intramolecular proton transfer switching. Anal. Chem. 2014, 86, 4648–4652. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.C.; Tsai, H.Y.; Luo, M.H.; Chang, C.W.; Chen, K.Y. Excited-state charge coupled proton transfer reaction via the dipolar functionality of salicylideneaniline. Chin. Chem. Lett. 2013, 24, 145–148. [Google Scholar] [CrossRef]
- Luo, M.H.; Tsai, H.Y.; Lin, H.Y.; Fang, S.K.; Chen, K.Y. Extensive spectral tuning of the proton transfer emission from green to red via a rational derivatization of salicylideneaniline. Chin. Chem. Lett. 2012, 23, 1279–1282. [Google Scholar] [CrossRef]
- Destro, R.; Gavezzotti, A.; Simonetta, M. Salicylideneaniline. Acta Cryst. 1978, 34, 2867–2869. [Google Scholar] [CrossRef]
- Moloney, G.P.; Gable, R.W.; Iskander, M.N.; Craik, D.J.; Mackay, M.F. Anomalies in the reduction of the Schiff bases 5-(Diethylamino)-2-(phenyliminomethyl)phenol and 2-[(4-diethylaminophenyl)iminomethyl]-phenol and their crystal structures. Aust. J. Chem. 1990, 43, 99–107. [Google Scholar] [CrossRef]
- Schaefer, T. Relation between hydroxyl proton chemical shifts and torsional frequencies in some ortho-substituted phenol derivatives. J. Phys. Chem. 1975, 79, 1888–1890. [Google Scholar] [CrossRef]
- Arod, F.; Gardon, M.; Pattison, P.; Chapuis, G. The α2-polymorph of salicylideneaniline. Acta Cryst. 2005, 61, 317–320. [Google Scholar] [CrossRef]
- Arod, F.; Pattison, P.; Schenk, K.T.; Chapuis, G. Polymorphism in N-salicylideneaniline reconsidered. Cryst. Growth Des. 2007, 7, 1679–1685. [Google Scholar] [CrossRef]
- Chen, K.Y.; Hsieh, C.C.; Cheng, Y.M.; Lai, C.H.; Chou, P.T. Extensive spectral tuning of the proton transfer emission from 550 to 675 nm via a rational derivatization of 10-hydroxybenzo[h]quinoline. Chem. Commun. 2006, 42, 4395–4397. [Google Scholar] [CrossRef]
- Chen, K.Y.; Tsai, H.Y.; Lin, W.C.; Chu, H.H.; Weng, Y.C.; Chan, C.C. ESIPT fluorescent dyes with adjustable optical properties: Substituent and conjugation effects. J. Lumin. 2014, 154, 168–177. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Chen, K.Y. 1,7-Diaminoperylene bisimides: Synthesis, optical and electrochemical properties. Dyes Pigm. 2013, 96, 319–327. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Plenum: Berlin, Germany, 1999. [Google Scholar]
- Luo, M.H.; Chen, K.Y. Asymmetric perylene bisimide dyes with strong solvatofluorism. Dyes Pigm. 2013, 99, 456–464. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS97, A Program for Automatic Solution of Crystal Structure; University of Göttingen: Wilhelmsplatz, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELX97, A Program for Crystal Structure Refinement; University of Göttingen: Wilhelmsplatz, Germany, 1997. [Google Scholar]
- Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44 1223 336 033; E-Mail: [email protected]).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Koné, M.; Illien, B.; Graton, J.; Laurenc, C. B3LYP and MP2 calculations of the enthalpies of hydrogen-bonded complexes of methanol with neutral bases and anions: Comparison with experimental data. J. Phys. Chem. A 2005, 109, 11907–11913. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.-Y.; Tsai, H.-Y. Synthesis, X-ray Structure, Spectroscopic Properties and DFT Studies of a Novel Schiff Base. Int. J. Mol. Sci. 2014, 15, 18706-18724. https://doi.org/10.3390/ijms151018706
Chen K-Y, Tsai H-Y. Synthesis, X-ray Structure, Spectroscopic Properties and DFT Studies of a Novel Schiff Base. International Journal of Molecular Sciences. 2014; 15(10):18706-18724. https://doi.org/10.3390/ijms151018706
Chicago/Turabian StyleChen, Kew-Yu, and Hsing-Yang Tsai. 2014. "Synthesis, X-ray Structure, Spectroscopic Properties and DFT Studies of a Novel Schiff Base" International Journal of Molecular Sciences 15, no. 10: 18706-18724. https://doi.org/10.3390/ijms151018706