Lactate Transporters in the Context of Prostate Cancer Metabolism: What Do We Know?
Abstract
:1. Introduction
2. Reprogramming of Energy Metabolism as an Emerging Hallmark of Cancer
3. Role of Monocarboxylate Transporters (MCTs) in Cellular Metabolism
4. Role of MCTs in the Context of Cancer
Role of MCTs as Therapeutic Targets in Cancer
5. Diagnostic and Prognostic Value of MCTs in Prostate Cancer
5.1. Clinico-Pathological Significance of MCTs and CD147 Expressions in Prostate Carcinoma
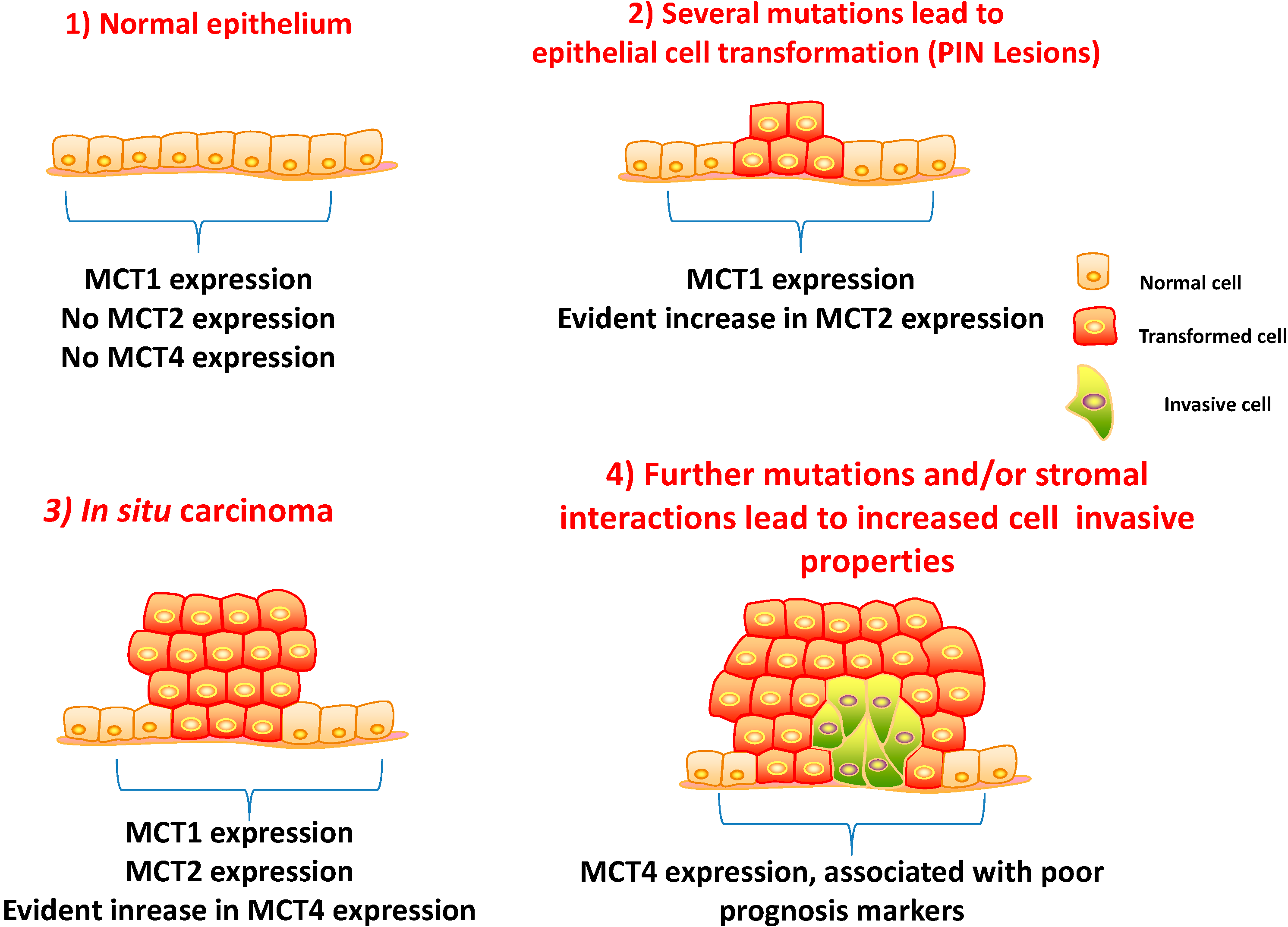
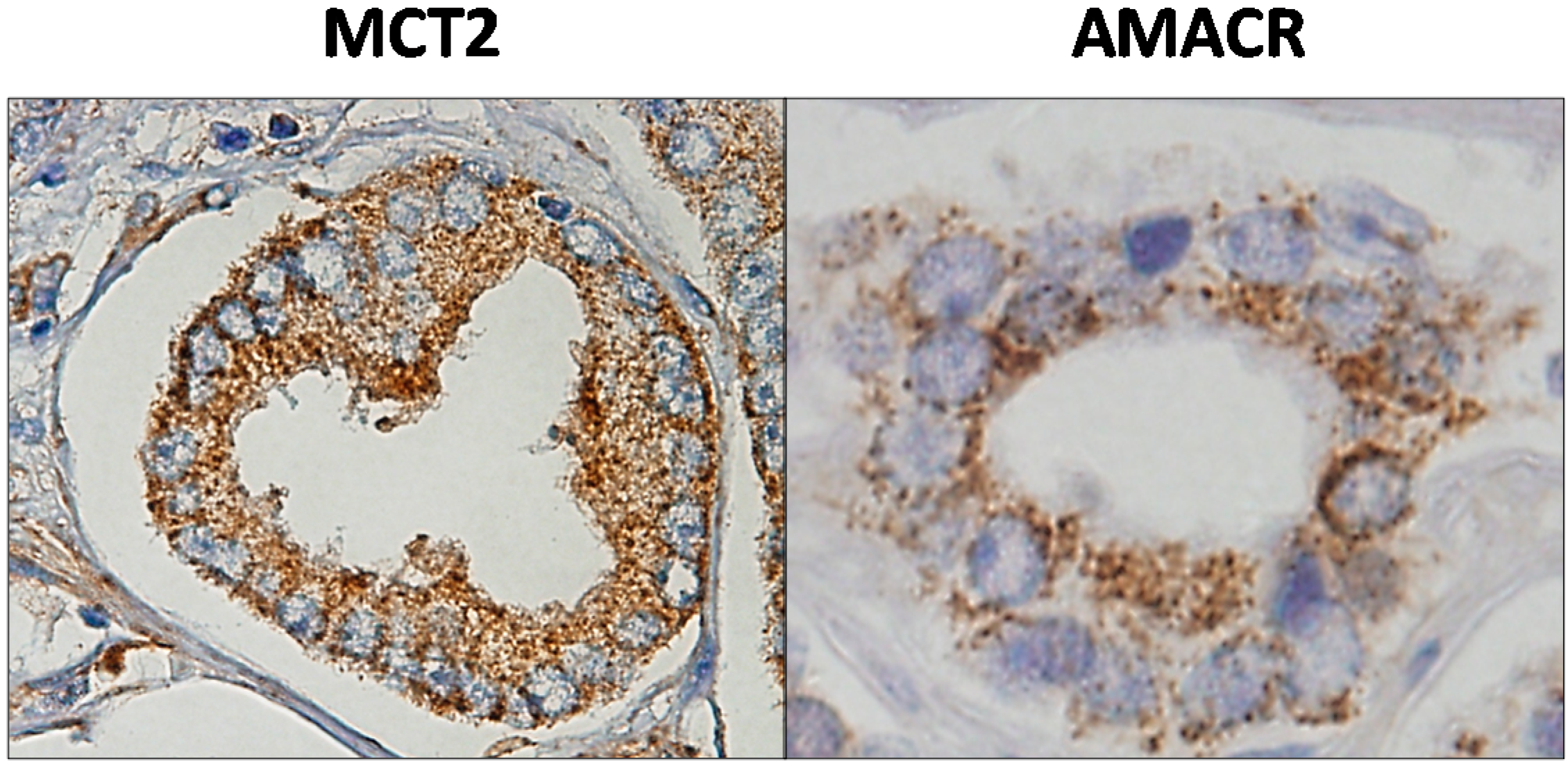
5.2. MCT2 as a Putative Prostate Cancer Biomarker
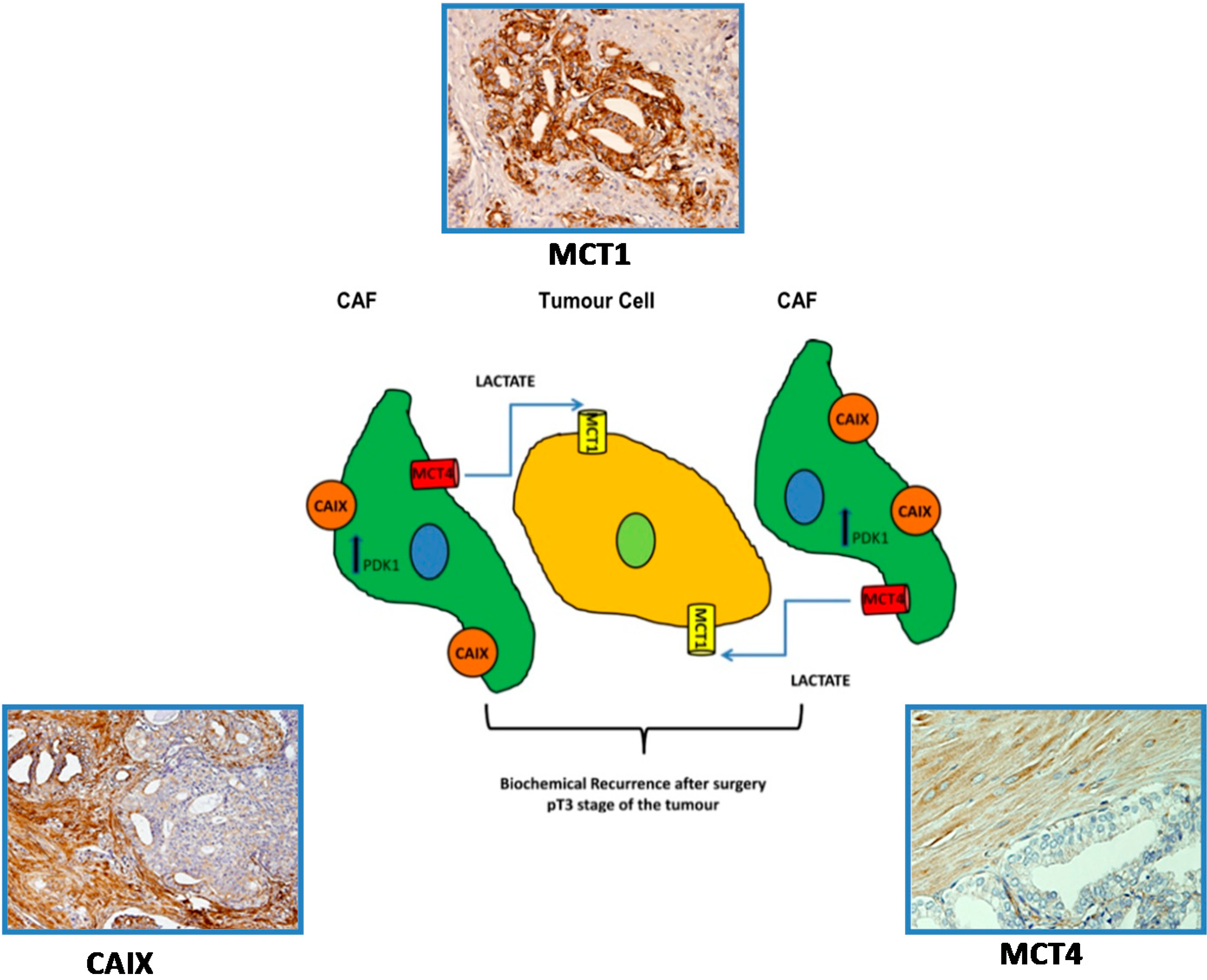
5.3. A Prognostic Value for a Lactate Shuttle Established between Prostate Cancer Cells and Cancer Associated Fibroblasts
6. Targeting Metabolism in Prostate Cancer: Is There a Therapeutic Window?
7. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: A Cancer J. Clin. 2011, 61, 212–236. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2010; American Cancer Society Inc.: Atlanta, GA, USA, 2010. [Google Scholar]
- Hsing, A.W.; Tsao, L.; Devesa, S.S. International trends and patterns of prostate cancer incidence and mortality. Int. J. Cancer 2000, 85, 60–67. [Google Scholar]
- Potosky, A.L.; Kessler, L.; Gridley, G.; Brown, C.C.; Horm, J.W. Rise in prostatic cancer incidence associated with increased use of transurethral resection. J. Natl. Cancer Inst. 1990, 82, 1624–1628. [Google Scholar]
- Delongchamps, N.B.; Singh, A.; Haas, G.P. The role of prevalence in the diagnosis of prostate cancer. Cancer Control 2006, 13, 158–168. [Google Scholar]
- Welch, H.G.; Albertsen, P.C. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J. Natl. Cancer Inst. 2009, 101, 1325–1329. [Google Scholar]
- Boyle, P.; Maisonneuve, P.; Napalkov, P. Geographical and temporal patterns of incidence and mortality from prostate cancer. Urology 1995, 46, 47–55. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar]
- De Berardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar]
- Jones, R.G.; Thompson, C.B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009, 23, 537–548. [Google Scholar]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar]
- Phelps, M.E. PET: The merging of biology and imaging into molecular imaging. J. Nucl. Med. 2000, 41, 661–681. [Google Scholar]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [Google Scholar]
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell. 2008, 13, 472–482. [Google Scholar]
- Ertel, A.; Tsirigos, A.; Whitaker-Menezes, D.; Birbe, R.C.; Pavlides, S.; Martinez-Outschoorn, U.E.; Pestell, R.G.; Howell, A.; Sotgia, F.; Lisanti, M.P. Is cancer a metabolic rebellion against host aging? In the quest for immortality, tumor cells try to save themselves by boosting mitochondrial metabolism. Cell Cycle 2012, 11, 253–263. [Google Scholar]
- Dakubo, G.D.; Parr, R.L.; Costello, L.C.; Franklin, R.B.; Thayer, R.E. Altered metabolism and mitochondrial genome in prostate cancer. J. Clin. Pathol. 2006, 59, 10–16. [Google Scholar]
- Costello, L.C.; Franklin, R.B.; Feng, P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion 2005, 5, 143–153. [Google Scholar]
- Costello, L.C.; Liu, Y.; Franklin, R.B.; Kennedy, M.C. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J. Biol. Chem. 1997, 272, 28875–28881. [Google Scholar]
- Liu, Y.; Costello, L.C.; Franklin, R.B. Prolactin specifically regulates citrate oxidation and m-aconitase of rat prostate epithelial cells. Metabolism 1996, 45, 442–449. [Google Scholar]
- Cooper, J.F.; Farid, I. The role of citric acid in the physiology of the prostate. 3. lactate/citrate ratios in benign and malignant prostatic homogenates as an index of prostatic malignancy. J. Urol. 1964, 92, 533–536. [Google Scholar]
- Costello, L.C.; Franklin, R.B. The intermediary metabolism of the prostate: A key to understanding the pathogenesis and progression of prostate malignancy. Oncology 2000, 59, 269–282. [Google Scholar]
- Costello, L.C.; Feng, P.; Milon, B.; Tan, M.; Franklin, R.B. Role of zinc in the pathogenesis and treatment of prostate cancer: Critical issues to resolve. Prostate Cancer Prostatic Dis. 2004, 7, 111–117. [Google Scholar]
- Kurhanewicz, J.; Vigneron, D.B.; Hricak, H.; Narayan, P.; Carroll, P.; Nelson, S.J. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7-cm3) spatial resolution. Radiology 1996, 198, 795–805. [Google Scholar]
- Zha, S.; Ferdinandusse, S.; Hicks, J.L.; Denis, S.; Dunn, T.A.; Wanders, R.J.; Luo, J.; de Marzo, A.M.; Isaacs, W.B. Peroxisomal branched chain fatty acid β-oxidation pathway is up-regulated in prostate cancer. Prostate 2005, 63, 316–323. [Google Scholar]
- Liu, Y.; Zuckier, L.S.; Ghesani, N.V. Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer Res. 2010, 30, 369–374. [Google Scholar]
- Quennet, V.; Yaromina, A.; Zips, D.; Rosner, A.; Walenta, S.; Baumann, M. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother. Oncol. 2006, 81, 130–135. [Google Scholar]
- Brizel, D.M.; Schroeder, T.; Scher, R.L.; Walenta, S.; Clough, R.W.; Dewhirst, M.W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 349–353. [Google Scholar]
- Paschen, W.; Djuricic, B.; Mies, G.; Schmidt-Kastner, R.; Linn, F. Lactate and pH in the brain: Association and dissociation in different pathophysiological states. J. Neurochem. 1987, 48, 154–159. [Google Scholar]
- Juel, C.; Halestrap, A.P. Lactate transport in skeletal muscle—Role and regulation of the monocarboxylate transporter. J. Physiol. 1999, 517, 633–642. [Google Scholar]
- Halestrap, A.P.; Denton, R.M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by α-cyano-4-hydroxycinnamate. Biochem. J. 1974, 138, 313–316. [Google Scholar]
- Carpenter, L.; Poole, R.C.; Halestrap, A.P. Cloning and sequencing of the monocarboxylate transporter from mouse Ehrlich Lettre tumour cell confirms its identity as MCT1 and demonstrates that glycosylation is not required for MCT1 function. Biochim. Biophys. Acta 1996, 1279, 157–163. [Google Scholar]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar]
- Juel, C. Lactate-proton cotransport in skeletal muscle. Physiol. Rev. 1997, 77, 321–358. [Google Scholar]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004, 447, 619–628. [Google Scholar]
- Price, N.T.; Jackson, V.N.; Halestrap, A.P. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem. J. 1998, 329, 321–328. [Google Scholar]
- Yoon, H.; Fanelli, A.; Grollman, E.F.; Philp, N.J. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochem. Biophys. Res. Commun. 1997, 234, 90–94. [Google Scholar]
- Yoon, H.; Philp, N.J. Genomic structure and developmental expression of the chicken nonocarboxylate transporter MCT3 gene. Exp. Eye Res. 1998, 67, 417–424. [Google Scholar]
- Garcia, C.K.; Brown, M.S.; Pathak, R.K.; Goldstein, J.L. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J. Biol. Chem. 1995, 270, 1843–1849. [Google Scholar]
- Jackson, V.N.; Price, N.T.; Carpenter, L.; Halestrap, A.P. Cloning of the monocarboxylate transporter isoform MCT2 from rat testis provides evidence that expression in tissues is species-specific and may involve post-transcriptional regulation. Biochem. J. 1997, 324, 447–453. [Google Scholar]
- Dimmer, K.S.; Friedrich, B.; Lang, F.; Deitmer, J.W.; Broer, S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J. 2000, 350, 219–227. [Google Scholar]
- Manning Fox, J.E.; Meredith, D.; Halestrap, A.P. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J. Physiol. 2000, 529, 285–293. [Google Scholar]
- Zhang, F.; Vannucci, S.J.; Philp, N.J.; Simpson, I.A. Monocarboxylate transporter expression in the spontaneous hypertensive rat: Effect of stroke. J. Neurosci. Res. 2005, 79, 139–145. [Google Scholar]
- Ullah, M.S.; Davies, A.J.; Halestrap, A.P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanis. J. Biol. Chem. 2006, 281, 9030–9037. [Google Scholar]
- Kay, H.H.; Zhu, S.; Tsoi, S. Hypoxia and lactate production in trophoblast cells. Placenta 2007, 28, 854–860. [Google Scholar]
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; de Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar]
- De Heredia, P.F.; Wood, I.S.; Trayhurn, P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocyte. Pflugers Arch. 2010, 459, 509–518. [Google Scholar]
- Wilson, M.C.; Meredith, D.; Fox, J.E.; Manoharan, C.; Davies, A.J.; Halestrap, A.P. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: The ancillary protein for the insensitive MCT2 is EMBIGIN (gp70). J. Biol. Chem. 2005, 280, 27213–27221. [Google Scholar]
- Deora, A.A.; Philp, N.; Hu, J.; Bok, D.; Rodriguez-Boulan, E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc. Natl. Acad. Sci. USA 2005, 102, 16245–16250. [Google Scholar]
- Biswas, C.; Zhang, Y.; de Castro, R.; Guo, H.; Nakamura, T.; Kataoka, H.; Nabeshima, K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995, 55, 434–439. [Google Scholar]
- Wilson, M.C.; Meredith, D.; Halestrap, A.P. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J. Biol. Chem. 2002, 277, 3666–3672. [Google Scholar]
- Pinheiro, C.; Longatto-Filho, A.; Scapulatempo, C.; Ferreira, L.; Martins, S.; Pellerin, L.; Rodrigues, M.; Alves, V.A.F.; Schmitt, F.; Baltazar, F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008, 452, 139–146. [Google Scholar]
- Pinheiro, C.; Longatto-Filho, A.; Ferreira, L.; Pereira, S.M.; Etlinger, D.; Moreira, M.A.; Jubé, L.F.; Queiroz, G.S.; Schmitt, F.; Baltazar, F. Increasing expression of monocarboxylate transporters 1 and 4 along progression to invasive cervical carcinoma. Int. J. Gynecol. Pathol. 2008, 27, 568–574. [Google Scholar]
- Miranda-Goncalves, V.; Honavar, M.; Pinheiro, C.; Martinho, O.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; Costa, P.; Palmeirim, I.; et al. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro-Oncol. 2013, 15, 172–188. [Google Scholar]
- Pinheiro, C.; Albergaria, A.; Paredes, J.; Sousa, B.; Dufloth, R.; Vieira, D.; Schmitt, F.; Baltazar, F. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology 2010, 56, 860–867. [Google Scholar]
- Koukourakis, M.I.; Giatromanolaki, A.; Bougioukas, G.; Sivridis, E. Lung cancer: A comparative study of metabolism related protein expression in cancer cells and tumor associated stroma. Cancer Biol. Ther. 2007, 6, 1476–1479. [Google Scholar]
- Chen, H.; Wang, L.; Beretov, J.; Hao, J.; Xiao, W.; Li, Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin. Exp. Metastasis 2010, 27, 557–569. [Google Scholar]
- Porporato, P.E.; Dhup, S.; Dadhich, R.K.; Copetti, T.; Sonveaux, P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front. Pharmacol. 2011, 2, 49. [Google Scholar]
- Colen, C.B.; Shen, Y.; Ghoddoussi, F.; Yu, P.; Francis, T.B.; Koch, B.J.; Monterey, M.D.; Galloway, M.P.; Sloan, A.E.; Mathupala, S.P. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: An in vivo study. Neoplasia 2011, 13, 620–632. [Google Scholar]
- Wahl, M.L.; Owen, J.A.; Burd, R.; Herlands, R.A.; Nogami, S.S.; Rodeck, U.; Berd, D.; Leeper, D.B.; Owen, C.S. Regulation of intracellular pH in human melanoma: Potential therapeutic implications. Mol. Cancer Ther. 2002, 1, 617–628. [Google Scholar]
- Morais-Santos, F.; Miranda-Goncalves, V.; Pinheiro, S.; Vieira, A.F.; Paredes, J.; Schmitt, F.C.; Baltazar, F.; Pinheiro, C. Differential sensitivities to lactate transport inhibitors of breast cancer cell lines. Endocr.-Relat. Cancer 2014, 21, 27–38. [Google Scholar]
- Le Floch, R.; Chiche, J.; Marchiq, I.; Naiken, T.; Ilc, K.; Murray, C.M.; Ilk, K; Murray, C.M.; Critchlow, S.E.; Roux, D.; et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl Acad. Sci. USA 2011, 108, 16663–16668. [Google Scholar]
- Mathupala, S.P.; Parajuli, P.; Sloan, A.E. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: An in vitro study. Neurosurgery 2004, 55, 1410–1419. [Google Scholar]
- Gallagher, S.M.; Castorino, J.J.; Philp, N.J. Interaction of monocarboxylate transporter 4 with β1-integrin and its role in cell migration. Am. J. Physiol. Cell Physiol. 2009, 296, C414–C421. [Google Scholar]
- Gallagher, S.M.; Castorino, J.J.; Wang, D.; Philp, N.J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007, 67, 4182–4189. [Google Scholar]
- Kim, H.S.; Masko, E.M.; Poulton, S.L.; Kennedy, K.M.; Pizzo, S.V.; Dewhirst, M.W.; Freedland, S.J. Carbohydrate restriction and lactate transporter inhibition in a mouse xenograft model of human prostate cancer. BJU Int. 2012, 110, 1062–1069. [Google Scholar]
- Hao, J.; Chen, H.; Madigan, M.C.; Cozzi, P.J.; Beretov, J.; Xiao, W.; Delprado, W.J.; Russell, P.J.; Li, Y. Co-expression of CD147 (EMMPRIN), CD44v3–10, MDR1 and monocarboxylate transporters is associated with prostate cancer drug resistance and progression. Br. J. Cancer 2010, 103, 1008–1018. [Google Scholar]
- Pertega-Gomes, N.; Vizcaino, J.R.; Miranda-Goncalves, V.; Pinheiro, C.; Silva, J.; Pereira, H.; Monteiro, P.; Henrique, R.M.; Reis, R.M.; Lopes, C.; et al. Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer 2011, 11, 312. [Google Scholar] [Green Version]
- Pertega-Gomes, N.; Vizcaino, J.R.; Gouveia, C.; Jeronimo, C.; Henrique, R.M.; Lopes, C.; Baltazar, F. Monocarboxylate transporter 2 (MCT2) as putative biomarker in prostate cancer. Prostate 2013, 73, 763–769. [Google Scholar]
- Martinez-Outschoorn, U.E.; Lisanti, M.P.; Sotgia, F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin. Cancer Biol. 2014, 25, 47–60. [Google Scholar]
- Lisanti, M.P.; Martinez-Outschoorn, U.E.; Sotgia, F. Oncogenes induce the cancer-associated fibroblast phenotype: Metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle 2013, 12, 2723–2732. [Google Scholar]
- Martinez-Outschoorn, U.; Sotgia, F.; Lisanti, M.P. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin. Oncol. 2014, 41, 195–216. [Google Scholar]
- Martinez-Outschoorn, U.E.; Whitaker-Menezes, D.; Valsecchi, M.; Martinez-Cantarin, M.P.; Dulau-Florea, A.; Gong, J.; Howell, A.; Flomenberg, N.; Pestell, R.G.; Wagner, J.; et al. Reverse Warburg effect in a patient with aggressive B-cell lymphoma: Is lactic acidosis a paraneoplastic syndrome? Semin. Oncol. 2013, 40, 403–418. [Google Scholar]
- Fiaschi, T.; Marini, A.; Giannoni, E.; Taddei, M.L.; Gandellini, P.; de Donatis, A.; Lanciotti, M.; Serni, S.; Cirri, P.; Chiarugi, P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012, 72, 5130–5140. [Google Scholar]
- Sanita, P.; Capulli, M.; Teti, A.; Galatioto, G.P.; Vicentini, C.; Chiarugi, P.; Bologna, M.; Angelucci, A. Tumor-stroma metabolic relationship based on lactate shuttle can sustain prostate cancer progression. BMC Cancer 2014, 14, 154. [Google Scholar]
- Pertega-Gomes, N.; Vizcaino, J.R.; Attig, J.; Jurmeister, S.; Lopes, C.; Baltazar, F. A lactate shuttle system between tumour and stromal cells is associated with poor prognosis in prostate cancer. BMC Cancer 2014, 14, 352. [Google Scholar] [Green Version]
- Czernin, J.; Benz, M.R.; Allen-Auerbach, M.S. PET imaging of prostate cancer using C-acetate. PET Clin. 2009, 4, 163–172. [Google Scholar]
- Flavin, R.; Zadra, G.; Loda, M. Metabolic alterations and targeted therapies in prostate cancer. J. Pathol. 2011, 223, 283–294. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pértega-Gomes, N.; Baltazar, F. Lactate Transporters in the Context of Prostate Cancer Metabolism: What Do We Know? Int. J. Mol. Sci. 2014, 15, 18333-18348. https://doi.org/10.3390/ijms151018333
Pértega-Gomes N, Baltazar F. Lactate Transporters in the Context of Prostate Cancer Metabolism: What Do We Know? International Journal of Molecular Sciences. 2014; 15(10):18333-18348. https://doi.org/10.3390/ijms151018333
Chicago/Turabian StylePértega-Gomes, Nelma, and Fátima Baltazar. 2014. "Lactate Transporters in the Context of Prostate Cancer Metabolism: What Do We Know?" International Journal of Molecular Sciences 15, no. 10: 18333-18348. https://doi.org/10.3390/ijms151018333



