A Proteomic Analysis of Human Follicular Fluid: Comparison between Younger and Older Women with Normal FSH Levels
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of Patients
2.2. Number of Retrieved Mature Oocytes
| Characteristics | Younger | Older |
|---|---|---|
| Mean age (year) | 25.6 ± 3.2 | 39.6 ± 2.1 * |
| BMI (kg/m2) | 26.8 ± 2.6 | 28.6 ± 1.5 |
| Basal FSH (mIU/mL) | 6.1 ± 1.2 | 6.9 ± 1.9 |
| No. of MII oocytes | 9 ± 4.4 | 3.3 ± 4.1 * |
| Serum Transferrin (mg/dL) | 302.5 ± 38.1 | 251.5 ± 38.7 * |
2.3. Albumin/IgG Depletion
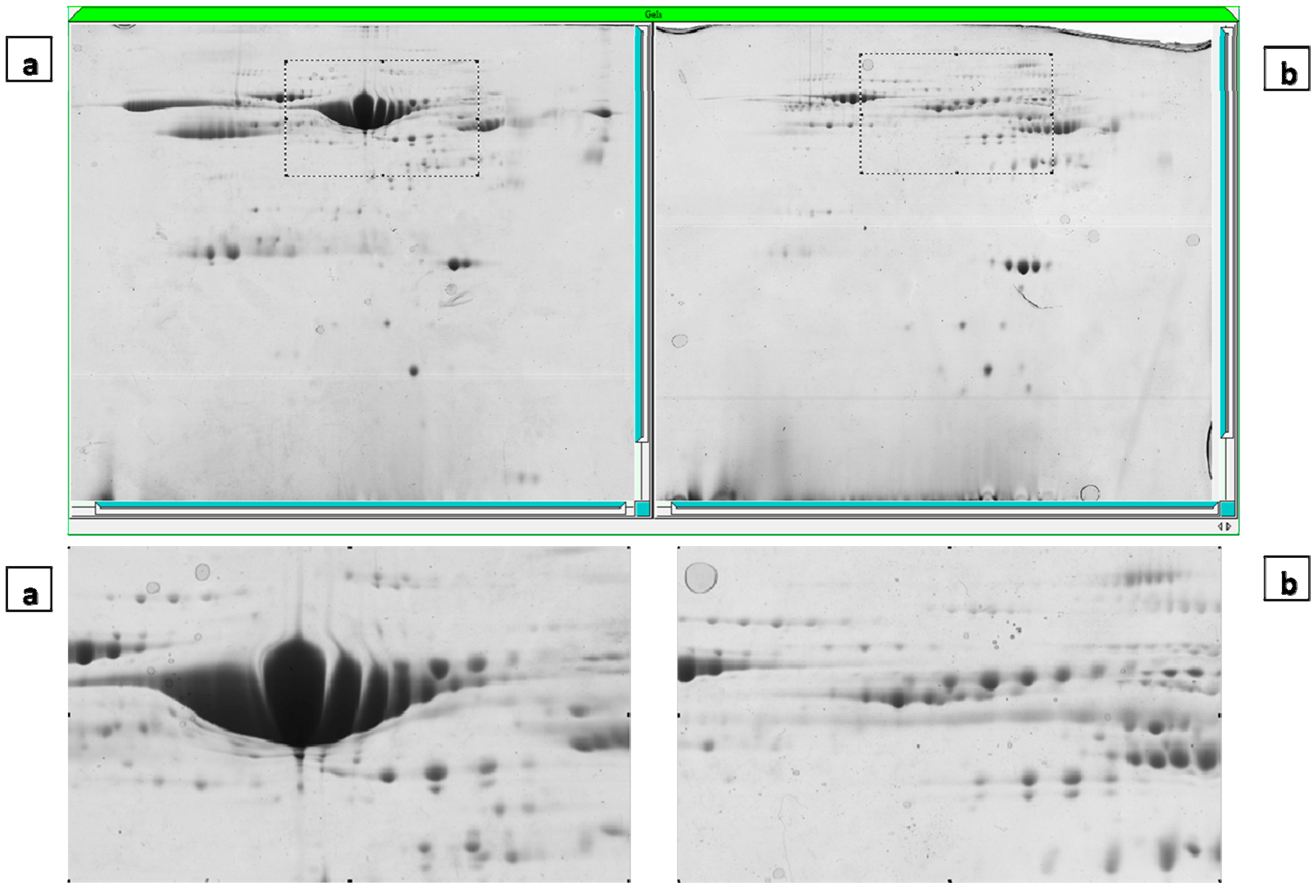
2.4. Gel Imaging
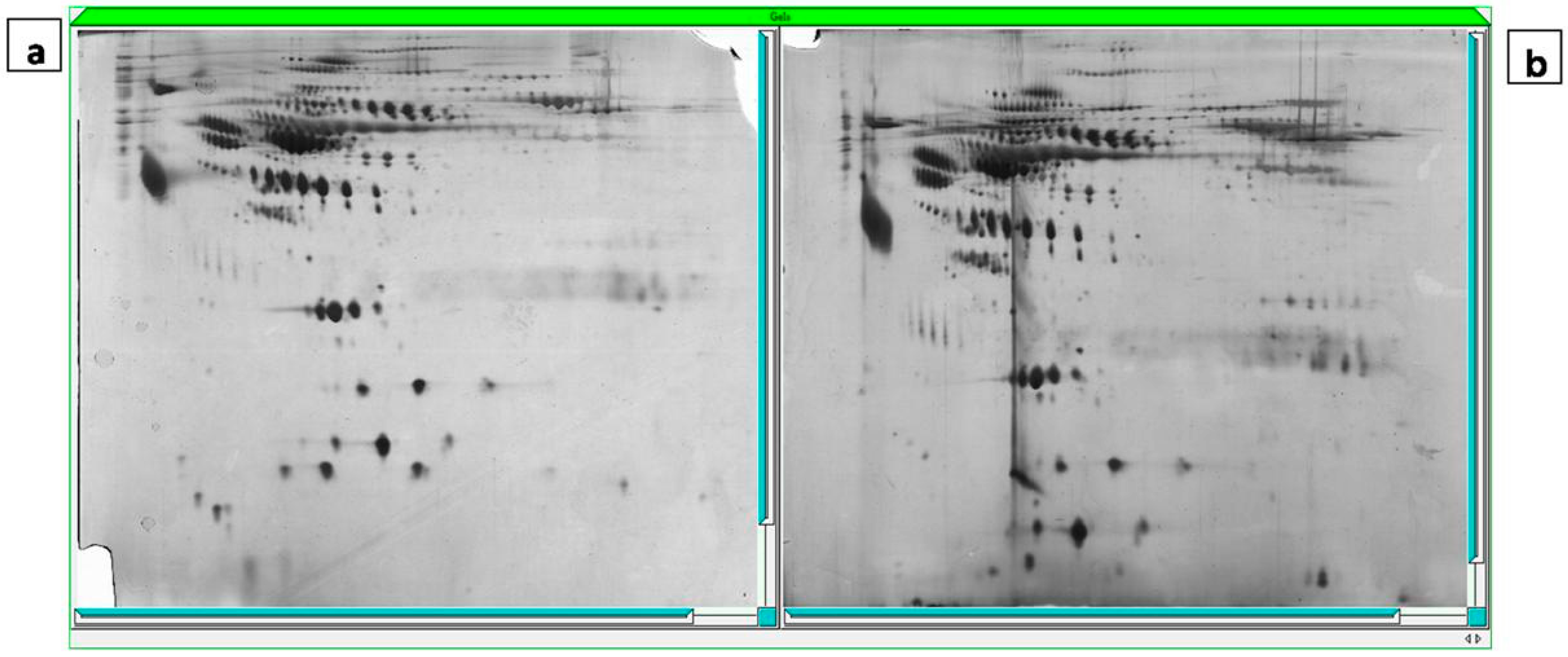
2.5. The Mass Spectrometry Assessment and MASCOT Report
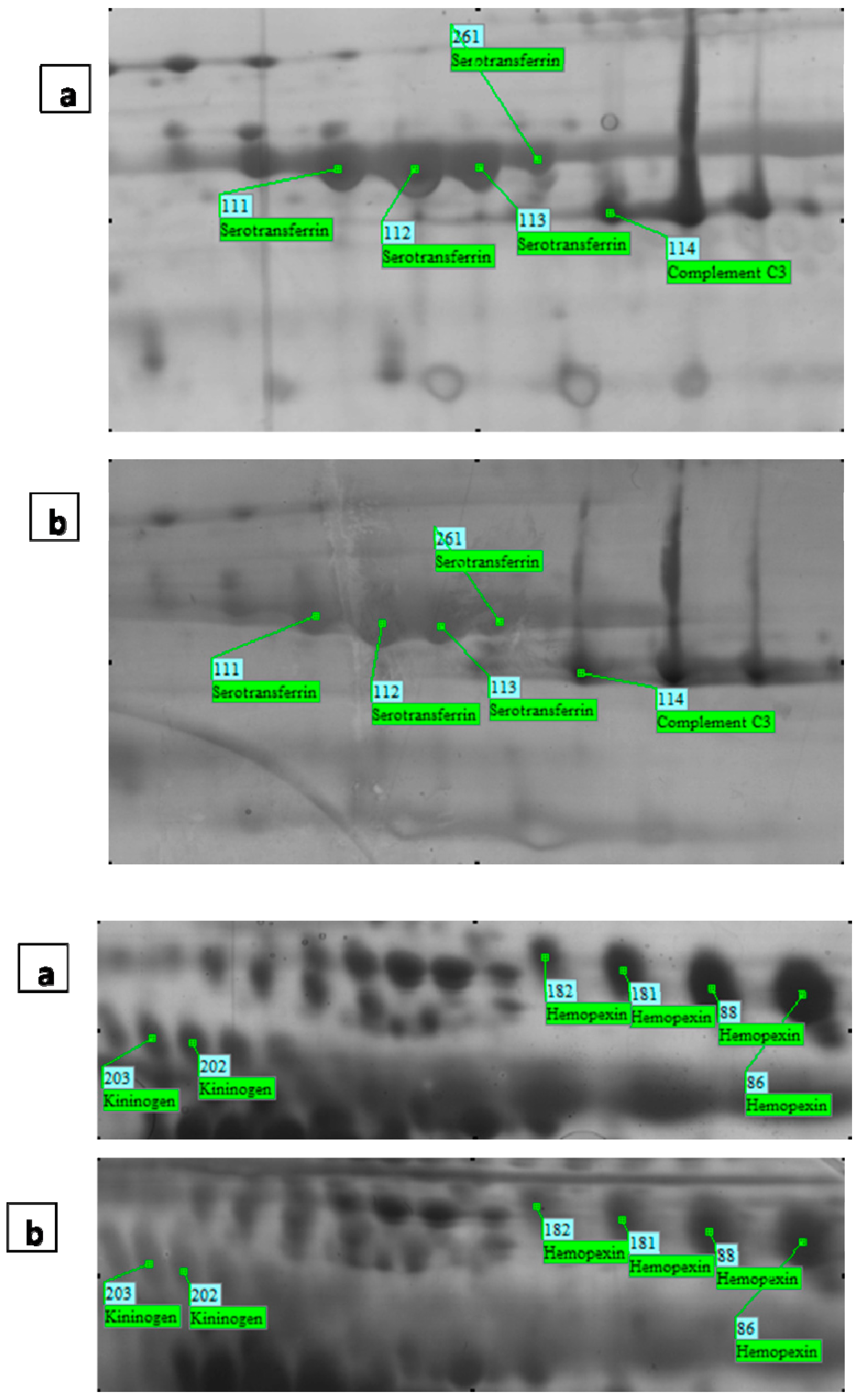
| Spot ID a | MASCOT Search Results | Accession Number (SWISS-PROT) | Protein Name | Fold Change f | Peptide | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Mass (kDa) | pI b | No. of Matched Peptides | Sequence Coverage c (%) | Score d | Accession Number e | |||||
| 75 | 52,385 | 6.55 | 6 | 18 | 396 | IPI00022488 | P02790 | Hemopexin | −1.59 | K.GSFPWQAK.M R.VGYVSGWGR.N R.HYEGSTVPEK.K K.SCAVAEYGVYVK.V K.YVMLPVADQDQCIR.H K.YVMLPVADQDQCIR.H |
| 86 | 52,385 | 6.55 | 5 | 17 | 539 | IPI00022488 | P02790 | Hemopexin | −1.51 | K.NFPSPVDAAFR.Q R.YYCFQGNQFLR.F R.GECQAEGVLFFQGDR.E K.LLQDEFPGIPSPLDAAVECHR.G K.EVGTPHGIILDSVDAAFICPGSSR.L |
| 88 | 52,385 | 6.55 | 3 | 8 | 256 | IPI00022488 | P02790 | Hemopexin | −1.53 | K.NFPSPVDAAFR.Q R.GECQAEGVLFFQGDR.E R.YYCFQGNQFLR.F |
| 90 | 52,385 | 6.55 | 5 | 14 | 473 | IPI00022488 | P02790 | Hemopexin | −1.67 | K.NFPSPVDAAFR.Q R.FDPVRGEVPPR.Y R.YYCFQGNQFLR.F R.GECQAEGVLFFQGDR.E K.LLQDEFPGIPSPLDAAVECHR.G |
| 91 | 52,385 | 6.55 | 4 | 12 | 358 | IPI00022488 | P02790 | Hemopexin | −1.51 | K.NFPSPVDAAFR.Q K.LLQDEFPGIPSPLDAAVECHR.G R.GECQAEGVLFFQGDR.E R.YYCFQGNQFLR.F |
| 181 | 52,385 | 6.55 | 4 | 12 | 382 | IPI00022488 | P02790 | Hemopexin | −1.51 | K.NFPSPVDAAFR.Q K.LLQDEFPGIPSPLDAAVECHR.G R.GECQAEGVLFFQGDR.E R.YYCFQGNQFLR.F |
| 182 | 52,385 | 6.55 | 3 | 8 | 181 | IPI00022488 | P02790 | Hemopexin | 1.67 | K.NFPSPVDAAFR.Q R.GECQAEGVLFFQGDR.E R.YYCFQGNQFLR.F |
| 202 | 48,936 | 6.29 | 3 | 7 | 224 | IPI00215894 | P01042 | Kininogen-1 | −2.2 | K.YNSQNQSNNQFVLYR.I R.QVVAGLNFR.I K.EETTSHLR.S |
| 203 | 48,936 | 6.29 | 7 | 14 | 427 | IPI00215894 | P01042 | Kininogen-1 | −1.8 | K.YNSQNQSNNQFVLYR.I K.TVGSDTFYSFK.Y R.QVVAGLNFR.I K.KYFIDFVAR.E K.YFIDFVAR.E K.RPPGFSPFR.S K.EETTSHLR.S |
| 108 | 79,294 | 6.81 | 10 | 16 | 731 | IPI00022463 | P02787 | Serotransferrin | −2.03 | R.APNHAVVTR.K K.ASYLDCIR.A K.WCALSHHER.L K.EGYYGYTGAFR.C K.DYELLCLDGTR.K K.MYLGYEYVTAIR.N K.CSTSSLLEACTFR.R R.DQYELLCLDNTR.K R.KPVEEYANCHLAR.A K.DCHLAQVPSHTVVAR.S |
| 111 | 79,294 | 6.81 | 10 | 15 | 846 | IPI00022463 | P02787 | Serotransferrin | −2.1 | K.DSGFQMNQLR.G R.DQYELLCLDNTR.K K.DCHLAQVPSHTVVAR.S K.MYLGYEYVTAIR.N K.EGYYGYTGAFR.C K.NLNEKDYELLCLDGTR.K K.DYELLCLDGTR.K R.KPVEEYANCHLAR.A R.APNHAVVTR.K K.CSTSSLLEACTFR.R |
| 112 | 79,294 | 6.81 | 10 | 16 | 752 | IPI00022463 | P02787 | Serotransferrin | −2.01 | R.APNHAVVTR.K K.ASYLDCIR.A K.WCALSHHER.L K.EGYYGYTGAFR.C K.DYELLCLDGTR.K K.MYLGYEYVTAIR.N K.CSTSSLLEACTFR.R R.DQYELLCLDNTR.K R.KPVEEYANCHLAR.A K.DCHLAQVPSHTVVAR.S |
| 113 | 79,294 | 6.81 | 10 | 16 | 787 | IPI00022463 | P02787 | Serotransferrin | −1.51 | K.ASYLDCIR.A R.DQYELLCLDNTR.K K.DCHLAQVPSHTVVAR.S K.MYLGYEYVTAIR.N K.WCALSHHER.L K.EGYYGYTGAFR.C K.DYELLCLDGTR.K R.KPVEEYANCHLAR.A R.APNHAVVTR.K K.CSTSSLLEACTFR.R |
| 261 | 79,294 | 6.81 | 9 | 14 | 689 | IPI00022463 | P02787 | Serotransferrin | −1.52 | K.ASYLDCIR.A R.DQYELLCLDNTR.K K.DCHLAQVPSHTVVAR.S K.MYLGYEYVTAIR.N K.WCALSHHER.L K.EGYYGYTGAFR.C K.DYELLCLDGTR.K R.KPVEEYANCHLAR.A K.CSTSSLLEACTFR.R |
| 254 | 79,294 | 6.81 | 10 | 16 | 724 | IPI00022463 | P02787 | Serotransferrin | −2.06 | K.ASYLDCIR.A R.DQYELLCLDNTR.K K.DCHLAQVPSHTVVAR.S K.MYLGYEYVTAIR.N K.WCALSHHER.L K.EGYYGYTGAFR.C K.DYELLCLDGTR.K R.KPVEEYANCHLAR.A R.APNHAVVTR.K K.CSTSSLLEACTFR.R |
| 114 | 188,569 | 6.02 | 3 | 2 | 222 | IPI00783987 | P01024 | Complement C3 | −1.6 | K.TIYTPGSTVLYR.I K.KVEGTAFVIFGIQDGEQR.I R.IPIEDGSGEVVLSR.K |
| 199 | 194,247 | 6.65 | 2 | 1 | 91 | IPI00032258 | P01028 | Complement C4-A | −2.2 | R.EELVYELNPLDHR.G R.QGSFQGGFR.S |
| 200 | 194,170 | 6.89 | 4 | 2 | 219 | IPI00418163 | P0C0L5.2 | Complement C4-B preproprotein | −2.19 | R.EFHLHLR.L R.EELVYELNPLDHR.G K.AEMADQAAAWLTR.Q R.QGSFQGGFR.S |
| 285 | 194,247 | 6.65 | 2 | 1 | 80 | IPI00032258 | P01028 | Complement C4-A | −1.78 | K.GLCVATPVQLR.V R.EELVYELNPLDHR.G |
2.6. Serum Transferrin Level
3. Discussion
4. Experimental Section
4.1. Patients
4.2. Ovarian Stimulation, FF Sampling and Oocyte Collection
4.3. Assessment of Oocytes Maturation (Metaphase II Oocytes) and Fertilization
4.4. Determination of FSH Concentrations in Serum
4.5. Sample Preparation and Two-Dimensional Gel Electrophoresis
4.5.1. Depletion of Albumin/IgG from FF Using Qproteome Albumin/IgG Depletion Plates
4.5.2. Protein Assay
4.6. Two-Dimensional Gel Electrophoresis
4.7. Silver and Coomassie Blue Staining
4.8. Imaging and Statistical Analysis
4.9. Protein Identification by Mass Spectrometry and Sequence Database Searching
4.9.1. MALDI-TOF-TOF MS
4.9.2. Data Processing and Protein Identification
4.9.3. Determination of Transferrin Concentrations in Serum
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Von Wald, T.; Monisova, Y.; Hacker, M.R.; Yoo, S.W.; Penzias, A.S.; Reindollar, R.R.; Usheva, A. Age-related variations in follicular apolipoproteins may influence human oocyte maturation and fertility potential. Fertil. Steril. 2010, 93, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Hanrieder, J.; Nyakas, A.; Naessen, T.; Bergquist, J. Proteomic analysis of human follicular fluid using an alternative bottom-up approach. Proteome Res. 2008, 7, 443–449. [Google Scholar] [CrossRef]
- Appasamy, M.; Jauniaux, E.; Serhal, P.; Al-Qahtani, A.; Groome, N.P.; Muttukrishna, S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil. Steril. 2008, 89, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Fahiminiya, S.; Reynaud, K.; Labas, V.; Batard, S.; Chastant-Maillard, S.; Gérard, N. Steroid hormones content and proteomic analysis of canine follicular fluid during the preovulatory period. Reprod. Biol. Endocrinol. 2010, 8. [Google Scholar] [CrossRef]
- Mason, H.D.; Willis, D.S.; Beard, R.W.; Winston, R.M.; Margara, R.; Franks, S. Estradiol production by granulosa cells of normal and polycystic ovaries: Relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J. Clin. Endocrinol. Metab. 1994, 79, 1355–1360. [Google Scholar] [PubMed]
- Revelli, A.; Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7. [Google Scholar] [CrossRef]
- Monteleone, P.; Giovanni, A.P.; Simi, G.; Casarosa, E.; Cela, V.; Genazzani, A. Follicular fluid VEGF levels directly correlate with perifollicular blood flow in normoresponder patients undergoing IVF. J. Assist. Reprod. Genet. 2008, 25, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ocal, P.; Aydin, S.; Cepni, I.; Idil, S.; Idil, M.; Uzun, H.; Benian, A. Follicular fluid concentrations of vascular endothelial growth factor, inhibin A and inhibin B in IVF cycles: Are they markers for ovarian response and pregnancy outcome? Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 194–199. [Google Scholar] [CrossRef]
- Barroso, G.; Barrionuevo, M.; Rao, P.; Graham, L.; Danforth, D.; Huey, S.; Abuhamad, A.; Oehninger, S. Vascular endothelial growth factor, nitric oxide, and leptin follicular fluid levels correlate negatively with embryo quality in IVF patients. Fertil. Steril. 1999, 72, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Mashiach, R.; Amit, A.; Hasson, J.; Amzalzg, S.; Almog, B.; Ben-Yosef, D.; Lessing, J.B.; Limor, R.; Azem, F. Follicular fluid levels of anti-Mullerian hormone as a predictor of oocyte maturation, fertilization rate, and embryonic development in patients with polycystic ovary syndrome. Fertil. Steril. 2010, 93, 2299–2302. [Google Scholar] [CrossRef]
- Yanaihara, A.; Mitsukawa, K.; Iwasaki, S.; Otsuki, K.; Kawamura, T.; Okai, T. High concentrations of lactoferrin in the follicular fluid correlate with embryo quality during in vitro fertilization cycles. Fertil. Steril. 2007, 87, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Chang, C.L.; Wu, H.M.; Chiu, Y.M.; Chen, C.K.; Wang, H.S. Insulin-like growth factor-II (IGF-II), IGF-binding protein-3 (IGFBP-3), and IGFBP-4 in follicular fluid are associated with oocyte maturation and embryo development. Fertil. Steril. 2006, 86, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Saito, T.; Kaneko, T.; Sasagawa, I.; Kuramoto, T.; Hiroi, M. Relatively poor oocyte quality is an indication for intracytoplasmic sperm injection. Fertil. Steril. 2000, 73, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Wu, M.Y.; Chen, M.J.; Chao, K.H.; Ho, H.N.; Yang, Y.S. Nitric oxide is associated with poor embryo quality and pregnancy outcome in in vitro fertilization cycles. Fertil. Steril. 2004, 82, 126–131. [Google Scholar] [CrossRef] [PubMed]
- De Placido, G.; Alviggi, C.; Clarizia, R.; Mollo, A.; Alviggi, E.; Strina, I.; Fiore, E.; Wilding, M.; Pagano, T.; Matarese, G. Intra-follicular leptin concentration as a predictive factor for in vitro oocyte fertilization in assisted reproductive techniques. J. Endocrinol. Investig. 2006, 29, 719–726. [Google Scholar] [CrossRef]
- Gürbüz, B.; Yalti, S.; Ficicioglu, C.; Taşdemir, S. The relation of serum and follicular fluid leptin and ovarian steroid levels in response to induction of ovulation in in vitro fertilization cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.M.; Dafopoulos, K.; Messini, C.I.; Chalvatzas, N.; Liakos, N.; Pournaras, S.; Messinis, I.E. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod. Biol. Endocrinol. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Wang, T.-T.; Chen, X.-J.; Zhu, X.-M.; Dong, M.-Y.; Sheng, J.-Z.; Xu, C.-M.; Huang, H.-F. Bone morphogenetic protein-15 in follicle fluid combined with age may differentiate between successful and unsuccessful poor ovarian responders. Reprod. Biol. Endocrinol. 2012, 10. [Google Scholar] [CrossRef]
- Vireque, A.A.; Reis, R.M.; e Silva, A.A.M.R.; Resende, L.O.T.; Ferreira, E.M.; e Silva, A.R.; Ferriani, R.A. Involvement of Bone Morphogenetic Proteins (BMPs) in Ovarian Function and Infertility. Open Reprod. Sci. J. 2008, 1, 11–15. [Google Scholar] [CrossRef]
- Malizia, B.A.; Wook, Y.S.; Penzias, A.S.; Usheva, A. The human ovarian follicular fluid level of interleukin-8 is associated with follicular size and patient age. Fertil. Steril. 2010, 93, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Polak, G.; Wertel, I.; Barczyński, B.; Kwaśniewski, W.; Bednarek, W.; Kotarski, J. Increased levels of oxidative stress markers in the peritoneal fluid of women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.J.; Jung, Y.S.; Yoon, B.H.; Choi, S.H.; Choi, Y.S.; Lee, B.S. Follicular fluid interleukin-6 and -8 concentrations in infertile patients with endometriosis. Fertil. Steril. 2013, 100, S325. [Google Scholar] [CrossRef]
- Turco, E.G.L.; Cordeiro, F.B.; de Carvalho Lopes, P.H.; Gozzo, F.C.; Pilau, E.J.; Soler, T.B.; da Silva, B.F.; del Giudice, P.T.; Bertolla, R.P.; Fraietta, R.; et al. Proteomic analysis of follicular fluid from women with and without endometriosis: New therapeutic targets and biomarkers. Mol. Reprod. Dev. 2013, 80, 441–450. [Google Scholar] [PubMed]
- Dai, G.; Lu, G. Different protein expression patterns associated with polycystic ovary syndrome in human follicular fluid during controlled ovarian hyperstimulation. Reprod. Fertil. Dev. 2012, 24, 893–904. [Google Scholar] [CrossRef]
- Diez-Fraile, A.; Lammens, T.; Tilleman, K.; Witkowski, W.; Verhasselt, B.; de Sutter, P.; Benoit, Y.; Espeel, M.; D’Herde, K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum. Fertil. 2014, 17, 90–98. [Google Scholar] [CrossRef]
- Tatone C., C.M.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.M.; Amicarelli, F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.C.; Tatone, C.; delle Monache, S.; Marci, R.; Caserta, D.; Colonna, R.; Amicarelli, F. Antioxidant enzymatic defences in human follicular fluid: Characterization and age-dependent changes. Mol. Hum. Reprod. 2003, 9, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Roest, J.; Van Heusden, A.M.; Mous, H.; Zeilmaker, G.H.; Verhoeff, A. The ovarian response as a predictor for successful in vitro fertilization treatment after the age of 40 years. Fertil. Steril. 1996, 66, 969–973. [Google Scholar]
- Karimzadeh, M.A.; Ghandi, S. Age and basal FSH as a predictor of ART outcome. Iran. J.Reprod. Med. 2009, 7, 65–71. [Google Scholar]
- Lau, W.N.; So, W.W.; Yeung, W.S.; Ho, P.C. The effect of ageing on female fertility in an assisted. Hong Kong Med. J. 2000, 6, 147–152. [Google Scholar] [PubMed]
- Ito, M.; Miyado, K.; Nakagawa, K.; Muraki, M.; Imai, M.; Yamakawa, N.; Qin, J.; Hosoi, Y.; Saito, H.; Takahashi, Y. Age-associated changes in the subcellular localization of phosphorylated p38 MAPK in human granulosa cells. Mol. Hum. Reprod. 2010, 16, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Han M., P.S.; Park, B.J. Lower growth factor expression in follicular fluid undergone in-vitro fertilization. Clin. Exp. Reprod. Med. 2011, 38, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.A.; Houmard, B.S.; Hansen, K.R.; Woodruff, T.K.; Sluss, P.M.; Bremner, W.J.; Soules, M.R. Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J. Clin. Endocrinol. Metab. 2004, 89, 2977–2981. [Google Scholar] [CrossRef] [PubMed]
- Iwase, A.; Kobayashi, H.; Goto, M.; Nakahara, T.; Nakamura, T.; Kondo, M.; Nagatomo, Y.; Kotani, T.; Kikkawa, F. A proteomic analysis of human follicular fluid: Comparison between fertilized oocytes and non-fertilized oocytes in the same patient. J. Assist. Reprod. Genet. 2013, 30, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, D.; Murach, K.F.; Lottspeich, F.; Staudach, A.; Illmensee, K. Different protein patterns derived from follicular fluid of mature and immature human follicles. Hum. Reprod. 1996, 11, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Anahory, T.; Dechaud, H.; Bennes, R.; Marin, P.; Lamb, N.J.; Laoudj, D. Identification of new proteins in follicular fluid of mature human follicles. Electrophoresis 2002, 23, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Lee K.W., L.S.; Lee, S.W.; Cha, K.Y.; Kim, K.H.; Lee, S. Identification of new proteins in follicular fluid from mature human follicles by direct sample rehydration method of two-dimensional polyacrylamide gel electrophoresis. J. Korean Med. Sci. 2005, 20, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, S.; Ciavardelli, D.; di Giuseppe, F.; Eleuterio, E.; Sulpizio, M.; Tiboni, G.M.; Giampietro, F.; Palumbo, P.; di Ilio, C. Proteome analysis of human follicular fluid. Biochimi. Biophys. Acta(BBA)-Proteins Proteomics 2006, 1764, 1775–1785. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, M.-S.; Lee, S.-H.; Choi, B.-C.; Lim, J.-M.; Cha, K.Y.; Baek, K.-H. Proteomic analysis of recurrent spontaneous abortion: Identification of an inadequately expressed set of proteins in human follicular fluid. Proteomics 2006, 6, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Jarkovska, K.; Skalnikova, H.K.; Halada, P.; Hrabakova, R.; Moos, J.; Rezabek, K.; Gadher, S.J.; Kovarova, H. Development of ovarian hyperstimulation syndrome: Interrogation of key proteins and biological processes in human follicular fluid of women undergoing in vitro fertilization. Mol. Hum. Reprod. 2011, 17, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Estes, S.J.; Ye, B.; Qiu, W.; Cramer, D.; Hornstein, M.D.; Missmer, S.A. A proteomic analysis of IVF follicular fluid in women ≤32 years old. Fertil. Steril. 2009, 92, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.J.M. Follicle pool depletion: Factors involved and implications. Fertil. Steril. 2000, 74, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Kwee, J.; Hendriks, D.J.; Mol, B.W.; Lambalk, C.B. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum. Reprod. Update 2006, 12, 685–718. [Google Scholar] [CrossRef] [PubMed]
- Bancsi, L.; Broekmans, F.J.M.; Mol, B.W.J.; Habbema, J.D.F.; te Velde, E.R. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: A meta-analysis. Fertil. Steril. 2003, 79, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Jirge, P.R. Ovarian reserve tests. J. Hum. Reprod. Sci. 2011, 4, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Jarkovska, K.; Martinkova, J.; Liskova, L.; Halada, P.; Moos, J.; Rezabek, K.; Gadher, S.J.; Kovarova, H. Proteome mining of human follicular fluid reveals a crucial role of complement cascade and key biological pathways in women undergoing in vitro fertilization. J. Proteome Res. 2010, 9, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.; Lesourd, S.; van Dreden, P.; Richard, P.; Lefebvre, G.; Brouzes, D.V. Protein composition of follicular fluid and oocyte cleavage occurrence in in vitro fertilization (IVF). J. Assist. Reprod. Genet. 1992, 9, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Manoni, F.; Businaro, V.; Donadel, C.; Indino, M.; Scandellari, C. Possible significance of transferrin levels in seminal plasma of fertile and infertile men. J. Androl. 1986, 7, 77–82. [Google Scholar] [PubMed]
- Twigt, J.; Steegers-Theunissen, R.P.; Bezstarosti, K.; Demmers, J.A.A. Proteomic analysis of the microenvironment of developing oocytes. Proteomics 2012, 12, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.A.; Sharp, D.J.; Miller, D.; Gosden, R.G. Transferrin in the developing ovarian follicle: Evidence for de-novo expression by granulosa cells. Mol. Hum. Reprod. 1999, 5, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L. Molecular and genetic basis of normal and toxicant-induced apoptosis in female germ cells. Toxicol. Lett. 1998, 102, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Aleshire, S.L.; Osteen, K.G.; Maxson, W.S.; Entman, S.S.; Bradley, C.A.; Parl, F.F. Localization of transferrin and its receptor in ovarian follicular cells: Morphologic studies in relation to follicular development. Fertil. Steril. 1989, 51, 444. [Google Scholar] [PubMed]
- Entman, S.S.; Maxson, W.S.; Bradley, C.A.; Osteen, K.; Webster, B.W.; Vaughn, W.K.; Wentz, A.C. Follicular fluid transferrin levels in preovulatory human follicles. J. Vitro Fertil. Embryo Transf. 1987, 4, 98–102. [Google Scholar] [CrossRef]
- Mantzavinos, T.; Dalamanga, N.; Hassiakos, D.; Dimitriadou, F.; Gregoriou, O.; Zourlas, P.A. Immunoglobulins IgG, IgA, IgM, complement C3, C4 and ferritin and transferrin levels in serum and follicular fluid in IVF patients. Clin. Exp. Obstet. Gynecol. 1993, 20, 32–36. [Google Scholar] [PubMed]
- Al-Rumaih, H.M.; Gillott, D.J.; Price, K.M.; Grudzinskas, G.J. Proteomic analysis of uterine flushings from infertile women in the proliferative phase of the menstrual cycle with respect to estrogen level. Middle East Fertil. Soc. J. 2006, 11, 183–190. [Google Scholar]
- Tolosano, E.; Fagoonee, S.; Morello, N.; Vinchi, F.; Fiorito, V. Heme scavenging and the other facets of hemopexin. Antioxid. Redox Signal. 2010, 12, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.I.; Smith, A.; Morgan, W.T.; Shaklai, N. Role of hemopexin in protection of low-density lipoprotein against hemoglobin-induced oxidation. Biochemistry 1996, 35, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Greenbaum, L.M.; Mahesh, V.B.; Brann, D.W. Characterization of the kinin system in the ovary during ovulation in the rat. Biol. Reprod. 1992, 47, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Espey, L.L.; Miller, D.H.; Margolius, H.S. Ovarian increase in kinin-generating capacity in PMSG/hCG-primed immature rat. Am. J. Physiol. Endocrinol. Metab. 1986, 251, E362–E365. [Google Scholar]
- Clements, J.A.; Mukhtar, A.; Holland, A.M.; Ehrlich, A.R.; Fuller, P.J. Kallikrein gene family expression in the rat ovary: Localization to the granulosa cell. Endocrinology 1995, 136, 1137–1144. [Google Scholar] [PubMed]
- Guo, Y.L.; Colman, R.W. Two faces of high-molecular-weight kininogen (HK) in angiogenesis: Bradykinin turns it on and cleaved HK (HKa) turns it off. J. Thromb. Haemost. 2005, 3, 670–676. [Google Scholar] [CrossRef]
- Hayashi, I.; Amano, H.; Yoshida, S.; Kamata, K.; Kamata, M.; Inukai, M.; Fujita, T.; Kumagai, Y.; Furudate, S.-I.; Majima, M. Suppressed angiogenesis in kininogen-deficiencies. Lab. Investig. 2002, 82, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Oh, D.S.; Jeong, J.H.; Shin, B.S.; Joo, B.S.; Lee, K.S. Follicular blood flow is a better predictor of the outcome of in vitro fertilization-embryo transfer than follicular fluid vascular endothelial growth factor and nitric oxide concentrations. Fertil. Steril. 2004, 82, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.B.; Matos, M.H.T.; Chaves, R.N.; Celestino, J.J.H.; Saraiva, M.V.A.; Lima-Verde, I.B.; Araújo, V.R.; Figueiredo, J.R. Angiogenic factors and ovarian follicle development. Anim. Reprod. 2009, 6, 371–379. [Google Scholar]
- Tatone, C.; Amicarelli, F.; Carbone, M.C.; Monteleone, P.; Caserta, D.; Marci, R.; Artini, P.G.; Piomboni, P.; Focarelli, R. Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, J.M.; Jindal, S.; Greenseid, K.; Berger, D.; Brooks, A.; Santoro, N.; Pal, L. Reproductive aging is associated with altered gene expression in human luteinized granulosa cells. Reprod. Sci. 2010, 17, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Lainas, T.G.; Sfontouris, I.A.; Papanikolaou, E.G.; Zorzovilis, J.Z.; Petsas, G.K.; Lainas, G.T.; Kolibianakis, E.M. Flexible GnRH antagonist versus flare-up GnRH agonist protocol in poor responders treated by IVF: A randomized controlled trial. Hum. Reprod. 2008, 23, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Gulekli, B.; Dogan, E.; Korhan, P.; Dogan, S.; Bige, O.; Cimrin, D.; Atabey, N. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil. Steril. 2011, 95, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Mostafaie, A.; Yari, K.; Kiani, S. A comparative evaluation of rehydration and cuploading sample application for modified twodimensional gel electrophoresis of human serum proteins using immobilized pH gradient. Afr. J. Biotechnol. 2013, 10, 11711–11715. [Google Scholar]
- Sun, Y.L.; Ping, Z.G.; Li, C.J.; Sun, Y.F.; Yi, K.L.; Chen, L.; Li, X.Y.; Wang, X.L.; Zhou, X. Comparative proteomic analysis of follicular fluids from normal and cystic follicles in sows. Reprod. Domest. Anim. 2011, 46, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Görg, A.; Klaus, A.; Lück, C.; Weiland, F.; Weiss, W. Two-Dimensional Electrophoresis with Immobilized pH Gradients for Proteome Analysis; Technische Universität München: Munich, Germany, 2003; Volume 53. [Google Scholar]
- Gromova, I.; Celis, J.E. Protein detection in gels by silver staining: A procedure compatible with mass-spectrometry. Cell Biol.: Lab. Handb. 2006, 4, 421–429. [Google Scholar]
- Corporeau, C.; Vanderplancke, G.; Boulais, M.; Suquet, M.; Quéré, C.; Boudry, P.; Huvet, A.; Madec, S. Proteomic identification of quality factors for oocytes in the Pacific oyster Crassostrea gigas. J. Proteomics 2012, 75, 5554–5563. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemitabar, M.; Bahmanzadeh, M.; Mostafaie, A.; Orazizadeh, M.; Farimani, M.; Nikbakht, R. A Proteomic Analysis of Human Follicular Fluid: Comparison between Younger and Older Women with Normal FSH Levels. Int. J. Mol. Sci. 2014, 15, 17518-17540. https://doi.org/10.3390/ijms151017518
Hashemitabar M, Bahmanzadeh M, Mostafaie A, Orazizadeh M, Farimani M, Nikbakht R. A Proteomic Analysis of Human Follicular Fluid: Comparison between Younger and Older Women with Normal FSH Levels. International Journal of Molecular Sciences. 2014; 15(10):17518-17540. https://doi.org/10.3390/ijms151017518
Chicago/Turabian StyleHashemitabar, Mahmoud, Maryam Bahmanzadeh, Ali Mostafaie, Mahmoud Orazizadeh, Marzieh Farimani, and Roshan Nikbakht. 2014. "A Proteomic Analysis of Human Follicular Fluid: Comparison between Younger and Older Women with Normal FSH Levels" International Journal of Molecular Sciences 15, no. 10: 17518-17540. https://doi.org/10.3390/ijms151017518




