Beyond the Role of Dietary Protein and Amino Acids in the Prevention of Diet-Induced Obesity
Abstract
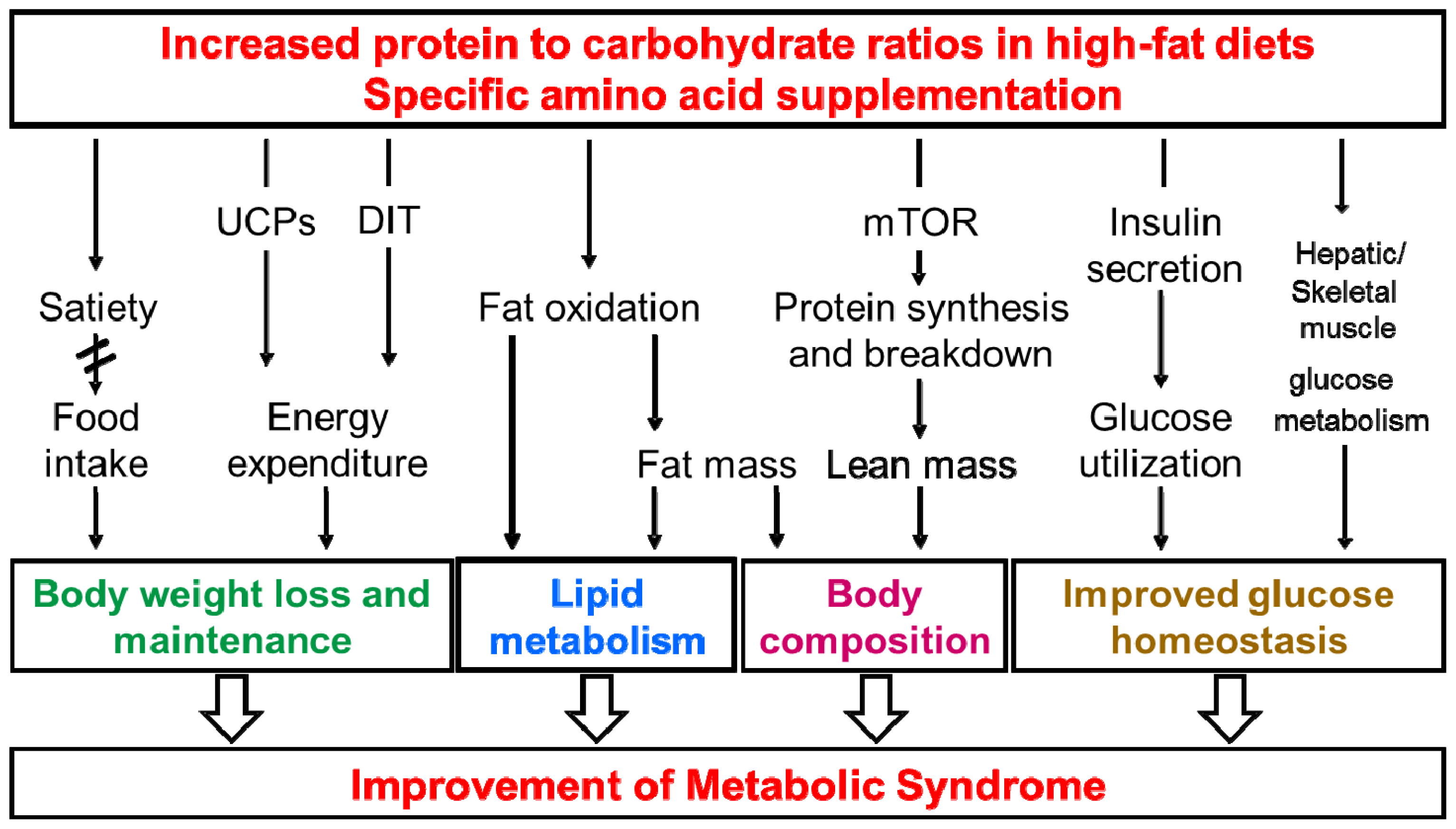
:1. Introduction
2. Amino Acids and Insulin Signaling
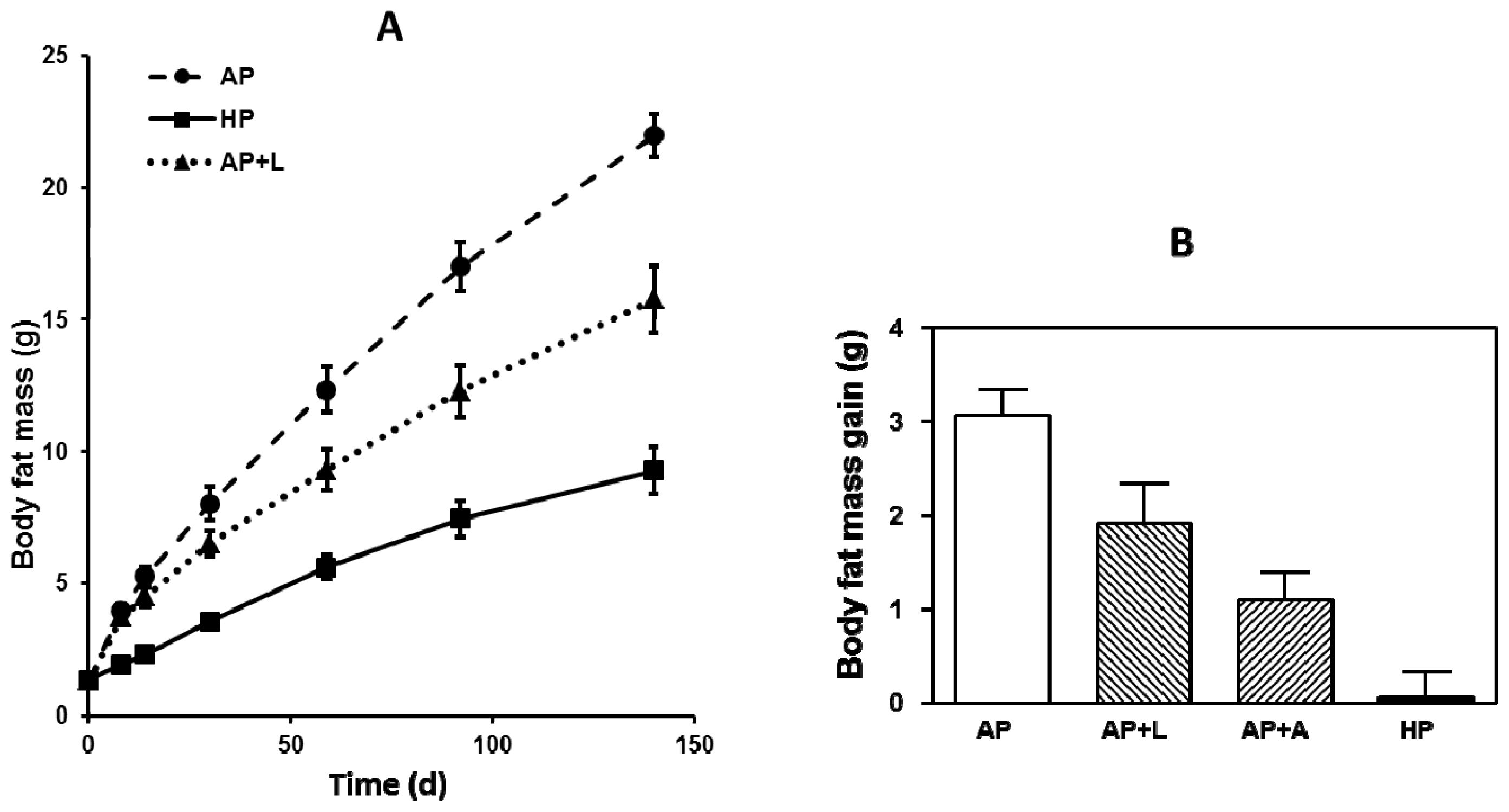
3. Body Weight and Body Composition
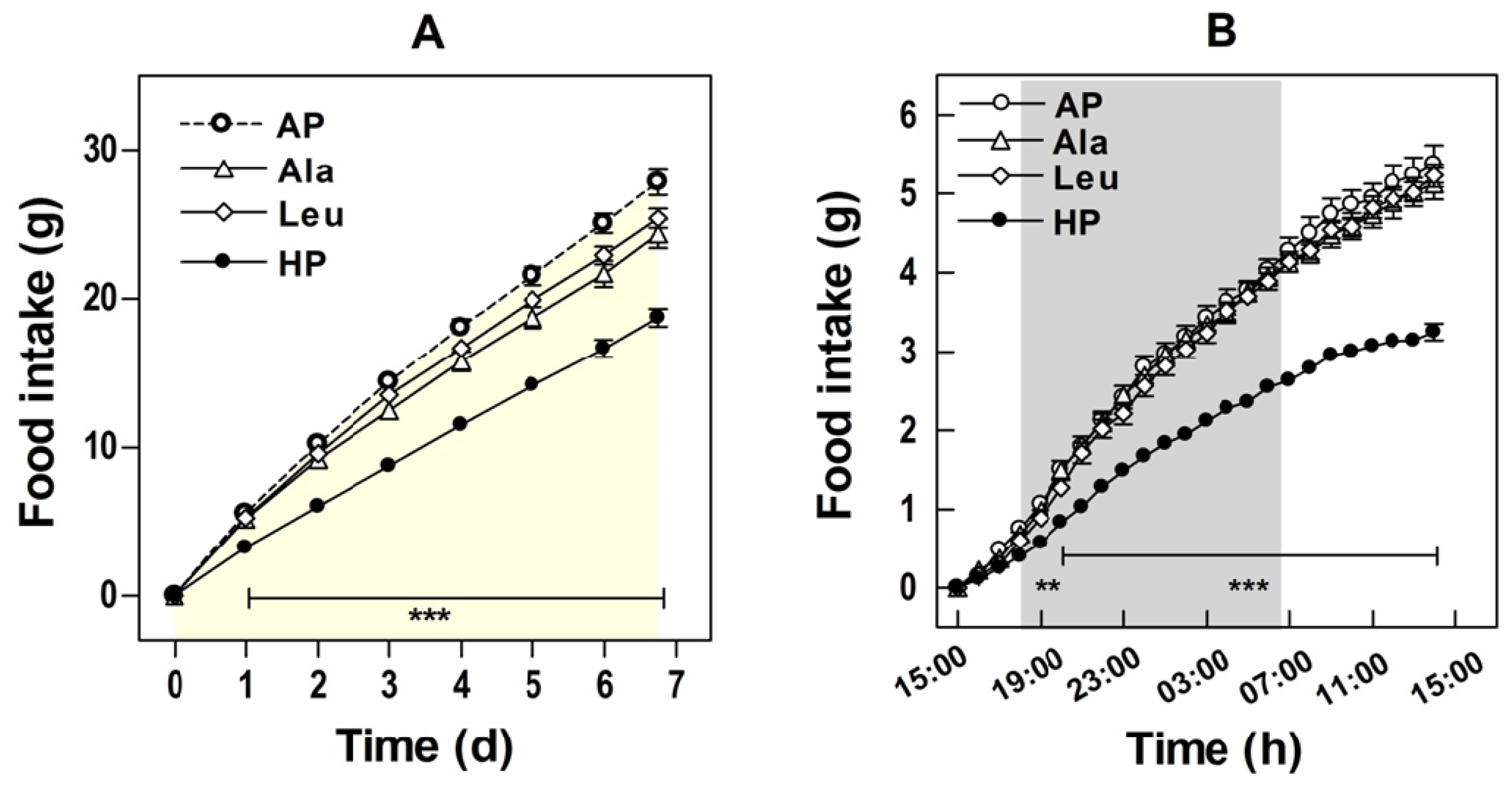
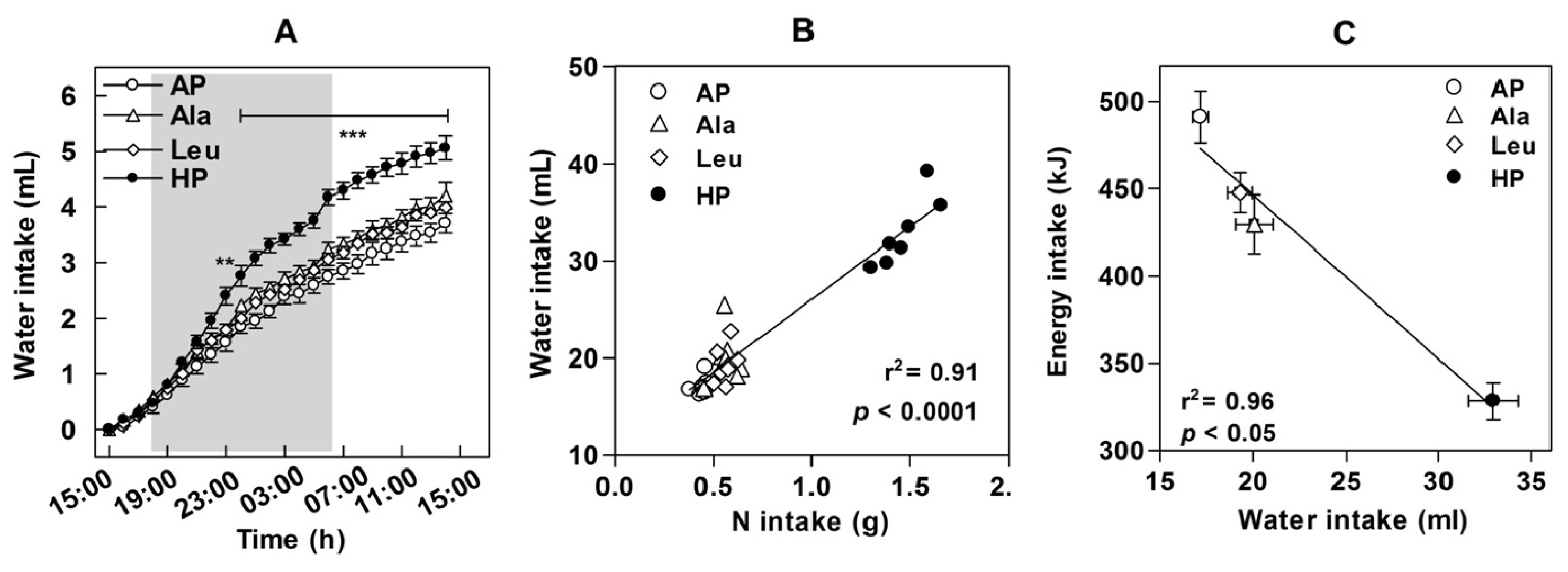
4. Energy and Water Intake
5. Energy Expenditure (EE)
6. Lipid Metabolism and NAFLD
7. Specificity of Observed Leucine Effects
8. Metabolic Consequences of Increased Water Intake in Response to High-Protein Diets
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/# (accessed on 4 November 2013).
- Hodgson, J.M.; Burke, V.; Beilin, L.J.; Puddey, I.B. Partial substitution of carbohydrate intake with protein intake from lean red meat lowers blood pressure in hypertensive persons. Am. J. Clin. Nutr 2006, 83, 780–787. [Google Scholar]
- Johnstone, A.M. Safety and efficacy of high-protein diets for weight loss. Proc. Nutr. Soc 2012, 71, 339–349. [Google Scholar]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression 1. Am. J. Clin. Nutr 2006, 83, 260–274. [Google Scholar]
- Layman, D.K.; Evans, E.M.; Erickson, D.; Seyler, J.; Weber, J.; Bagshaw, D.; Griel, A.; Psota, T.; Kris-Etherton, P. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J. Nutr 2009, 139, 514–521. [Google Scholar]
- Westerterp-Plantenga, M.S. Protein intake and energy balance. Regul. Pept 2008, 149, 67–69. [Google Scholar]
- Westerterp-Plantenga, M.S.; Nieuwenhuizen, A.; Tomé, D.; Soenen, S.; Westerterp, K.R. Dietary protein, weight loss, and weight maintenance. Annu. Rev. Nutr 2009, 29, 21–41. [Google Scholar]
- Freudenberg, A.; Petzke, K.J.; Klaus, S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J. Nutr. Biochem 2012, 23, 1524–1530. [Google Scholar]
- Klaus, S. Increasing the protein: Carbohydrate ratio in a high-fat diet delays the development of adiposity and improves glucose homeostasis in mice. J. Nutr 2005, 135, 1854–1858. [Google Scholar]
- Shertzer, H.G.; Woods, S.E.; Krishan, M.; Genter, M.B.; Pearson, K.J. Dietary whey protein lowers the risk for metabolic disease in mice fed a high-fat diet. J. Nutr 2011, 141, 582–587. [Google Scholar]
- Sorensen, A.; Mayntz, D.; Raubenheimer, D.; Simpson, S.J. Protein-leverage in mice: The geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 2008, 16, 566–571. [Google Scholar]
- Alam, M.A.; Kauter, K.; Withers, K.; Sernia, C.; Brown, L. Chronic l-arginine treatment improves metabolic, cardiovascular and liver complications in diet-induced obesity in rats. Food Funct 2013, 4, 83–91. [Google Scholar]
- Alvarado-Vasquez, N.; Zamudio, P.; Ceron, E.; Vanda, B.; Zenteno, E.; Carvajal-Sandoval, G. Effect of glycine in streptozotocin-induced diabetic rats. Comp. Biochem. Physiol. C 2003, 134, 521–527. [Google Scholar]
- El Hafidi, M.; Perez, I.; Zamora, J.; Soto, V.; Carvajal-Sandoval, G.; Banos, G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 2004, 287, R1387–R1393. [Google Scholar]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.E.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary l-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J. Nutr 2005, 135, 714–721. [Google Scholar]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary l-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr 2009, 139, 230–237. [Google Scholar]
- Opara, E.C.; Petro, A.; Tevrizian, A.; Feinglos, M.N.; Surwit, R.S. l-glutamine supplementation of a high fat diet reduces body weight and attenuates hyperglycemia and hyperinsulinemia in C57BL/6J mice. J. Nutr 1996, 126, 273–279. [Google Scholar]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar]
- Freudenberg, A.; Petzke, K.J.; Klaus, S. Dietary l-leucine and l-alanine supplementation have similar acute effects in the prevention of high-fat diet-induced obesity. Amino Acids 2013, 44, 519–528. [Google Scholar]
- Noatsch, A.; Petzke, K.J.; Millrose, M.K.; Klaus, S. Body weight and energy homeostasis was not affected in C57BL/6 mice fed high whey protein or leucine-supplemented low-fat diets. Eur. J. Nutr 2011, 50, 479–488. [Google Scholar]
- Arakawa, M.; Masaki, T.; Nishimura, J.; Seike, M.; Yoshimatsu, H. The effects of branched-chain amino acid granules on the accumulation of tissue triglycerides and uncoupling proteins in diet-induced obese mice. Endocr. J 2011, 58, 161–170. [Google Scholar]
- Eller, L.K.; Saha, D.C.; Shearer, J.; Reimer, R.A. Dietary leucine improves whole-body insulin sensitivity independent of body fat in diet-induced obese Sprague-Dawley rats. J. Nutr. Biochem 2013, 24, 1285–1294. [Google Scholar]
- Guo, K.; Yu, Y.H.; Hou, J.; Zhang, Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr. Metab. (Lond.) 2010, 7, 57. [Google Scholar]
- Macotela, Y.; Emanuelli, B.; Bang, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary leucine—An environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One 2011, 6, e21187. [Google Scholar]
- Cota, D. Mammalian target of rapamycin complex 1 (mTORC1) signaling in energy balance and obesity. Physiol. Behav 2009, 97, 520–524. [Google Scholar]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mTOR signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar]
- Drummond, M.J.; Rasmussen, B.B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar]
- Potier, M.; Darcel, N.; Tome, D. Protein, amino acids and the control of food intake. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 54–58. [Google Scholar]
- Ropelle, E.R.; Pauli, J.R.; Fernandes, M.F.; Rocco, S.A.; Marin, R.M.; Morari, J.; Souza, K.K.; Dias, M.M.; Gomes-Marcondes, M.C.; Gontijo, J.A.; et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 2008, 57, 594–605. [Google Scholar]
- Escobar, J.; Frank, J.W.; Suryawan, A.; Nguyen, H.V.; Van Horn, C.G.; Hutson, S.M.; Davis, T.A. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J. Nutr 2010, 140, 1418–1424. [Google Scholar]
- Balage, M.; Dardevet, D. Long-term effects of leucine supplementation on body composition. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 265–270. [Google Scholar]
- Tremblay, F.; Krebs, M.; Dombrowski, L.; Brehm, A.; Bernroider, E.; Roth, E.; Nowotny, P.; Waldhausl, W.; Marette, A.; Roden, M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005, 54, 2674–2684. [Google Scholar]
- Um, S.H.; D'Alessio, D.; Thomas, G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 2006, 3, 393–402. [Google Scholar]
- Bernard, J.R.; Liao, Y.H.; Ding, Z.; Hara, D.; Kleinert, M.; Nelson, J.L.; Ivy, J.L. An amino acid mixture improves glucose tolerance and lowers insulin resistance in the obese Zucker rat. Amino Acids 2013, 45, 191–203. [Google Scholar]
- Bernard, J.R.; Liao, Y.H.; Hara, D.; Ding, Z.; Chen, C.Y.; Nelson, J.L.; Ivy, J.L. An amino acid mixture improves glucose tolerance and insulin signaling in Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab 2011, 300, E752–E760. [Google Scholar]
- Balage, M.; Dupont, J.; Mothe-Satney, I.; Tesseraud, S.; Mosoni, L.; Dardevet, D. Leucine supplementation in rats induced a delay in muscle IR/PI3K signaling pathway associated with overall impaired glucose tolerance. J. Nutr. Biochem 2011, 22, 219–226. [Google Scholar]
- Krebs, M.; Roden, M. Nutrient-induced insulin resistance in human skeletal muscle. Curr. Med. Chem 2004, 11, 901–908. [Google Scholar]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009, 9, 311–326. [Google Scholar]
- Promintzer, M.; Krebs, M. Effects of dietary protein on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 463–468. [Google Scholar]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr 2007, 27, 293–310. [Google Scholar]
- Lynch, C.J.; Hutson, S.M.; Patson, B.J.; Vaval, A.; Vary, T.C. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am. J. Physiol. Endocrinol. Metab 2002, 283, E824–E835. [Google Scholar]
- Nairizi, A.; She, P.; Vary, T.C.; Lynch, C.J. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J. Nutr 2009, 139, 715–719. [Google Scholar]
- Torres-Leal, F.L.; Fonseca-Alaniz, M.H.; Teodoro, G.F.; de Capitani, M.D.; Vianna, D.; Pantaleao, L.C.; Matos-Neto, E.M.; Rogero, M.M.; Donato, J., Jr.; Tirapegui, J. Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutr. Metab. (Lond.) 2011, 8, 62. [Google Scholar]
- Zeanandin, G.; Balage, M.; Schneider, S.M.; Dupont, J.; Hebuterne, X.; Mothe-Satney, I.; Dardevet, D. Differential effect of long-term leucine supplementation on skeletal muscle and adipose tissue in old rats: An insulin signaling pathway approach. Age (Dordr.) 2012, 34, 371–387. [Google Scholar]
- Zanchi, N.E.; Guimaraes-Ferreira, L.; de Siqueira-Filho, M.A.; Felitti, V.; Nicastro, H.; Bueno, C.; Lira, F.S.; Naimo, M.A.; Campos-Ferraz, P.; Nunes, M.T.; et al. Dose and latency effects of leucine supplementation in modulating glucose homeostasis: Opposite effects in healthy and glucocorticoid-induced insulin-resistance states. Nutrients 2012, 4, 1851–1867. [Google Scholar]
- Kalogeropoulou, D.; Lafave, L.; Schweim, K.; Gannon, M.C.; Nuttall, F.Q. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism 2008, 57, 1747–1752. [Google Scholar]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhausl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar]
- Tremblay, F.; Marette, A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J. Biol. Chem 2001, 276, 38052–38060. [Google Scholar]
- Devkota, S.; Layman, D.K. Protein metabolic roles in treatment of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 403–407. [Google Scholar]
- Layman, D.K.; Boileau, R.A.; Erickson, D.J.; Painter, J.E.; Shiue, H.; Sather, C.; Christou, D.D. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J. Nutr 2003, 133, 411–417. [Google Scholar]
- Layman, D.K.; Walker, D.A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J. Nutr 2006, 136, 319S–323S. [Google Scholar]
- Bruckbauer, A.; Zemel, M.B. Effects of dairy consumption on SIRT1 and mitochondrial biogenesis in adipocytes and muscle cells. Nutr. Metab. (Lond.) 2011, 8, 91. [Google Scholar]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. (Lond.) 2012, 9, 77. [Google Scholar]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab 2012, 303, E1234–E1244. [Google Scholar]
- Metges, C.C.; Barth, C.A. Metabolic consequences of a high dietary-protein intake in adulthood: Assessment of the available evidence. J. Nutr 2000, 130, 886–889. [Google Scholar]
- Stepien, M.; Gaudichon, C.; Fromentin, G.; Even, P.; Tome, D.; Azzout-Marniche, D. Increasing protein at the expense of carbohydrate in the diet down-regulates glucose utilization as glucose sparing effect in rats. PLoS One 2011, 6, e14664. [Google Scholar]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; Blaut, M.; Alpert, C.; Gogebakan, O.; Bumke-Vogt, C.; et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am. J. Clin. Nutr 2011, 94, 459–471. [Google Scholar]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr 2011, 2, 445–456. [Google Scholar]
- Murgas Torrazza, R.; Suryawan, A.; Gazzaneo, M.C.; Orellana, R.A.; Frank, J.W.; Nguyen, H.V.; Fiorotto, M.L.; El-Kadi, S.; Davis, T.A. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J. Nutr 2010, 140, 2145–2152. [Google Scholar]
- Lang, C.H.; Frost, R.A.; Bronson, S.K.; Lynch, C.J.; Vary, T.C. Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine. Am. J. Physiol. Endocrinol. Metab 2010, 298, E1283–E1294. [Google Scholar]
- Wilson, F.A.; Suryawan, A.; Gazzaneo, M.C.; Orellana, R.A.; Nguyen, H.V.; Davis, T.A. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J. Nutr 2010, 140, 264–270. [Google Scholar]
- Buse, M.G.; Reid, S.S. Leucine. A possible regulator of protein turnover in muscle. J. Clin. Invest 1975, 56, 1250–1261. [Google Scholar]
- Matthews, D.E. Observations of branched-chain amino acid administration in humans. J. Nutr 2005, 135, 1580S–1584S. [Google Scholar]
- May, M.E.; Buse, M.G. Effects of branched-chain amino acids on protein turnover. Diabetes Metab. Rev 1989, 5, 227–245. [Google Scholar]
- Zanchi, N.E.; Nicastro, H.; Lancha, A.H., Jr. Potential antiproteolytic effects of l-leucine: Observations of in vitro and in vivo studies. Nutr. Metab. (Lond.) 2008, 5, 20. [Google Scholar]
- Hamel, F.G.; Upward, J.L.; Siford, G.L.; Duckworth, W.C. Inhibition of proteasome activity by selected amino acids. Metabolism 2003, 52, 810–814. [Google Scholar]
- Nakashima, K.; Ishida, A.; Yamazaki, M.; Abe, H. Leucine suppresses myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem. Biophys. Res. Commun 2005, 336, 660–666. [Google Scholar]
- Sugawara, T.; Ito, Y.; Nishizawa, N.; Nagasawa, T. Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids 2009, 37, 609–616. [Google Scholar]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr 2004, 23, 373–385. [Google Scholar]
- Gannon, M.C.; Nuttall, F.Q.; Saeed, A.; Jordan, K.; Hoover, H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am. J. Clin. Nutr 2003, 78, 734–741. [Google Scholar]
- Luscombe, N.D.; Clifton, P.M.; Noakes, M.; Parker, B.; Wittert, G. Effects of energy-restricted diets containing increased protein on weight loss, resting energy expenditure, and the thermic effect of feeding in type 2 diabetes. Diabetes Care 2002, 25, 652–665. [Google Scholar]
- Sacks, F.M.; Bray, G.A.; Carey, V.J.; Smith, S.R.; Ryan, D.H.; Anton, S.D.; McManus, K.; Champagne, C.M.; Bishop, L.M.; Laranjo, N.; et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med 2009, 360, 859–873. [Google Scholar]
- Sargrad, K.R.; Homko, C.; Mozzoli, M.; Boden, G. Effect of high protein vs high carbohydrate intake on insulin sensitivity, body weight, hemoglobin A1c, and blood pressure in patients with type 2 diabetes mellitus. J. Am. Diet. Assoc 2005, 105, 573–580. [Google Scholar]
- Golay, A.; Allaz, A.F.; Morel, Y.; de Tonnac, N.; Tankova, S.; Reaven, G. Similar weight loss with low- or high-carbohydrate diets. Am. J. Clin. Nutr 1996, 63, 174–178. [Google Scholar]
- Lean, M.E.; Han, T.S.; Prvan, T.; Richmond, P.R.; Avenell, A. Weight loss with high and low carbohydrate 1200 kcal diets in free living women. Eur. J. Clin. Nutr 1997, 51, 243–248. [Google Scholar]
- Clifton, P. High-protein and low-glycaemic diets improve dietary compliance and maintenance of weight loss in overweight adults who have lost weight on a low-calorie diet. Evid. Based Med 2011, 16, 112–113. [Google Scholar]
- Fromentin, G.; Darcel, N.; Chaumontet, C.; Marsset-Baglieri, A.; Nadkarni, N.; Tome, D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev 2012, 25, 29–39. [Google Scholar]
- Dennis, E.A.; Flack, K.D.; Davy, B.M. Beverage consumption and adult weight management: A review. Eat. Behav 2009, 10, 237–246. [Google Scholar]
- Davy, B.M.; Dennis, E.A.; Dengo, A.L.; Wilson, K.L.; Davy, K.P. Water consumption reduces energy intake at a breakfast meal in obese older adults. J. Am. Diet. Assoc 2008, 108, 1236–1239. [Google Scholar]
- Popkin, B.M.; Armstrong, L.E.; Bray, G.M.; Caballero, B.; Frei, B.; Willett, W.C. A new proposed guidance system for beverage consumption in the United States. Am. J. Clin. Nutr 2006, 83, 529–542. [Google Scholar]
- Stookey, J.D.; Constant, F.; Popkin, B.M.; Gardner, C.D. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008, 16, 2481–2488. [Google Scholar]
- Forslund, A.H.; Hambraeus, L.; Olsson, R.M.; El-Khoury, A.E.; Yu, Y.M.; Young, V.R. The 24-h whole body leucine and urea kinetics at normal and high protein intakes with exercise in healthy adults. Am. J. Physiol 1998, 275, E310–E320. [Google Scholar]
- Young, V.R.; El-Khoury, A.E.; Raguso, C.A.; Forslund, A.H.; Hambraeus, L. Rates of urea production and hydrolysis and leucine oxidation change linearly over widely varying protein intakes in healthy adults. J. Nutr 2000, 130, 761–766. [Google Scholar]
- Speakman, J.R.; Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc 2003, 62, 621–634. [Google Scholar]
- Weyer, C.; Walford, R.L.; Harper, I.T.; Milner, M.; MacCallum, T.; Tataranni, P.A.; Ravussin, E. Energy metabolism after 2 y of energy restriction: The biosphere 2 experiment. Am. J. Clin. Nutr 2000, 72, 946–953. [Google Scholar]
- Zhang, K.; Sun, M.; Werner, P.; Kovera, A.J.; Albu, J.; Pi-Sunyer, F.X.; Boozer, C.N. Sleeping metabolic rate in relation to body mass index and body composition. Int. J. Obes. Relat. Metab. Disord 2002, 26, 376–383. [Google Scholar]
- Boschmann, M.; Steiniger, J.; Hille, U.; Tank, J.; Adams, F.; Sharma, A.M.; Klaus, S.; Luft, F.C.; Jordan, J. Water-induced thermogenesis. J. Clin. Endocrinol. Metab 2003, 88, 6015–6019. [Google Scholar]
- Boschmann, M.; Steiniger, J.; Franke, G.; Birkenfeld, A.L.; Luft, F.C.; Jordan, J. Water drinking induces thermogenesis through osmosensitive mechanisms. J. Clin. Endocrinol. Metab 2007, 92, 3334–3337. [Google Scholar]
- Petzke, K.J.; Friedrich, M.; Metges, C.C.; Klaus, S. Long-term dietary high protein intake up-regulates tissue specific gene expression of uncoupling proteins 1 and 2 in rats. Eur. J. Nutr 2005, 44, 414–421. [Google Scholar]
- Petzke, K.J.; Riese, C.; Klaus, S. Short-term, increasing dietary protein and fat moderately affect energy expenditure, substrate oxidation and uncoupling protein gene expression in rats. J. Nutr. Biochem 2007, 18, 400–407. [Google Scholar]
- Schwarz, J.; Tome, D.; Baars, A.; Hooiveld, G.J.; Muller, M. Dietary protein affects gene expression and prevents lipid accumulation in the liver in mice. PLoS One 2012, 7, e47303. [Google Scholar]
- Sommerfeld, A.; Krones-Herzig, A.; Herzig, S. Transcriptional co-factors and hepatic energy metabolism. Mol. Cell. Endocrinol 2011, 332, 21–31. [Google Scholar]
- Zhu, L.; Baker, S.S.; Liu, W.; Tao, M.H.; Patel, R.; Nowak, N.J.; Baker, R.D. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: Combined effects of pathways on steatosis. Metabolism 2011, 60, 1001–1011. [Google Scholar]
- Pfeiffer, A.; Henkel, H.; Verstegen, M.W.A.; Philipczyk, I. The influence of protein intake on water balance, flow rate and apparent digestibilty of nutrients at the distal ileum in growing pigs. Livestock Prod. Sci 1995, 44, 179–187. [Google Scholar]
- Daniels, M.C.; Popkin, B.M. Impact of water intake on energy intake and weight status: A systematic review. Nutr. Rev 2010, 68, 505–521. [Google Scholar]
- Muckelbauer, R.; Sarganas, G.; Gruneis, A.; Muller-Nordhorn, J. Association between water consumption and body weight outcomes: A systematic review. Am. J. Clin. Nutr 2013, 98, 282–299. [Google Scholar]
- Dennis, E.A.; Dengo, A.L.; Comber, D.L.; Flack, K.D.; Savla, J.; Davy, K.P.; Davy, B.M. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity (Silver Spring) 2010, 18, 300–307. [Google Scholar]
- Muckelbauer, R.; Libuda, L.; Clausen, K.; Reinehr, T.; Kersting, M. A simple dietary intervention in the school setting decreased incidence of overweight in children. Obes. Facts 2009, 2, 282–285. [Google Scholar]
- May, M.; Gueler, F.; Barg-Hock, H.; Heiringhoff, K.H.; Engeli, S.; Heusser, K.; Diedrich, A.; Brandt, A.; Strassburg, C.P.; Tank, J.; et al. Liver afferents contribute to water drinking-induced sympathetic activation in human subjects: A clinical trial. PLoS One 2011, 6, e25898. [Google Scholar]
- Frey, I.M.; Rubio-Aliaga, I.; Klempt, M.; Wolf, E.; Daniel, H. Phenotype analysis of mice deficient in the peptide transporter PEPT2 in response to alterations in dietary protein intake. Pflugers Arch 2006, 452, 300–306. [Google Scholar]
- Bouby, N.; Trinh-Trang-Tan, M.M.; Coutaud, C.; Bankir, L. Vasopressin is involved in renal effects of high-protein diet: Study in homozygous Brattleboro rats. Am. J. Physiol 1991, 260, F96–F100. [Google Scholar]
- Chan, K.C.; Lou, P.P.; Hargrove, J.L. High casein-lactalbumin diet accelerates blood coagulation in rats. J. Nutr 1993, 123, 1010–1016. [Google Scholar]
- Jorda, A.; Zaragoza, R.; Portoles, M.; Baguena-Cervellera, R.; Renau-Piqueras, J. Long-term high-protein diet induces biochemical and ultrastructural changes in rat liver mitochondria. Arch. Biochem. Biophys 1988, 265, 241–248. [Google Scholar]
- Haussinger, D.; Reinehr, R.; Schliess, F. The hepatocyte integrin system and cell volume sensing. Acta Physiol. (Oxf.) 2006, 187, 249–255. [Google Scholar]
- Lang, F. Effect of cell hydration on metabolism. Nestle Nutr. Inst. Workshop Ser 2011, 69, 115–126, discussion 126–130. [Google Scholar]
- Mathai, M.L.; Naik, S.; Sinclair, A.J.; Weisinger, H.S.; Weisinger, R.S. Selective reduction in body fat mass and plasma leptin induced by angiotensin-converting enzyme inhibition in rats. Int. J. Obes. (Lond.) 2008, 32, 1576–1584. [Google Scholar]
- Thornton, S.N.; Even, P.C.; van Dijk, G. Hydration increases cell metabolism. Int. J. Obes. (Lond.) 2009, 33, 385, author reply 386. [Google Scholar]
- Schliess, F.; Richter, L.; vom Dahl, S.; Haussinger, D. Cell hydration and mTOR-dependent signalling. Acta Physiol. (Oxf.) 2006, 187, 223–229. [Google Scholar]
- Kusudo, T.; Wang, Z.; Mizuno, A.; Suzuki, M.; Yamashita, H. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. J. Appl. Physiol 2012, 112, 1223–1232. [Google Scholar]
- Bilz, S.; Ninnis, R.; Keller, U. Effects of hypoosmolality on whole-body lipolysis in man. Metabolism 1999, 48, 472–476. [Google Scholar]
- Keller, U.; Szinnai, G.; Bilz, S.; Berneis, K. Effects of changes in hydration on protein, glucose and lipid metabolism in man: Impact on health. Eur. J. Clin. Nutr 2003, 57, S69–S74. [Google Scholar]

| AP | AP + A | AP + L | HP | p < | |
|---|---|---|---|---|---|
| Liver | |||||
| ACCα | 1.00 ± 0.09 a | 0.78 ± 0.12 a | 0.67 ± 0.09 a | 0.35 ± 0.03 b | 0.01 |
| CD36 | 1.00 ± 0.08 | 0.73 ± 0.10 | 0.99 ± 0.10 | 1.04 ± 0.16 | NS |
| FAS | 1.00 ± 0.26 a | 0.55 ± 0.10 b | 0.37 ± 0.05 b | 0.18 ± 0.03 b | 0.05 |
| L-FABP | 1.00 ± 0.14 a | 0.77 ± 0.05 b | 0.59 ± 0.05 b,c | 0.48 ± 0.03 c | 0.05 |
| Epididymal white fat | |||||
| ATGL | 1.00 ± 0.07 a | 1.28 ± 0.15 a | 1.38 ± 0.13 a | 1.86 ± 0.25 b | 0.01 |
| HSL | 1.00 ± 0.05 | 1.10 ± 0.10 | 0.89 ± 0.15 | 1.21 ± 0.08 | NS |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Petzke, K.J.; Freudenberg, A.; Klaus, S. Beyond the Role of Dietary Protein and Amino Acids in the Prevention of Diet-Induced Obesity. Int. J. Mol. Sci. 2014, 15, 1374-1391. https://doi.org/10.3390/ijms15011374
Petzke KJ, Freudenberg A, Klaus S. Beyond the Role of Dietary Protein and Amino Acids in the Prevention of Diet-Induced Obesity. International Journal of Molecular Sciences. 2014; 15(1):1374-1391. https://doi.org/10.3390/ijms15011374
Chicago/Turabian StylePetzke, Klaus J., Anne Freudenberg, and Susanne Klaus. 2014. "Beyond the Role of Dietary Protein and Amino Acids in the Prevention of Diet-Induced Obesity" International Journal of Molecular Sciences 15, no. 1: 1374-1391. https://doi.org/10.3390/ijms15011374




