Protein Structures among Bio-Ethanol Co-Products and Its Relationships with Ruminal and Intestinal Availability of Protein in Dairy Cattle
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protein Molecular Structures, Amide I and Amide II and Their Ratio among Different Types and Batches of DDGS
2.2. Protein Secondary Structure Profile and Ratio among Different Types and Batches of DDGS
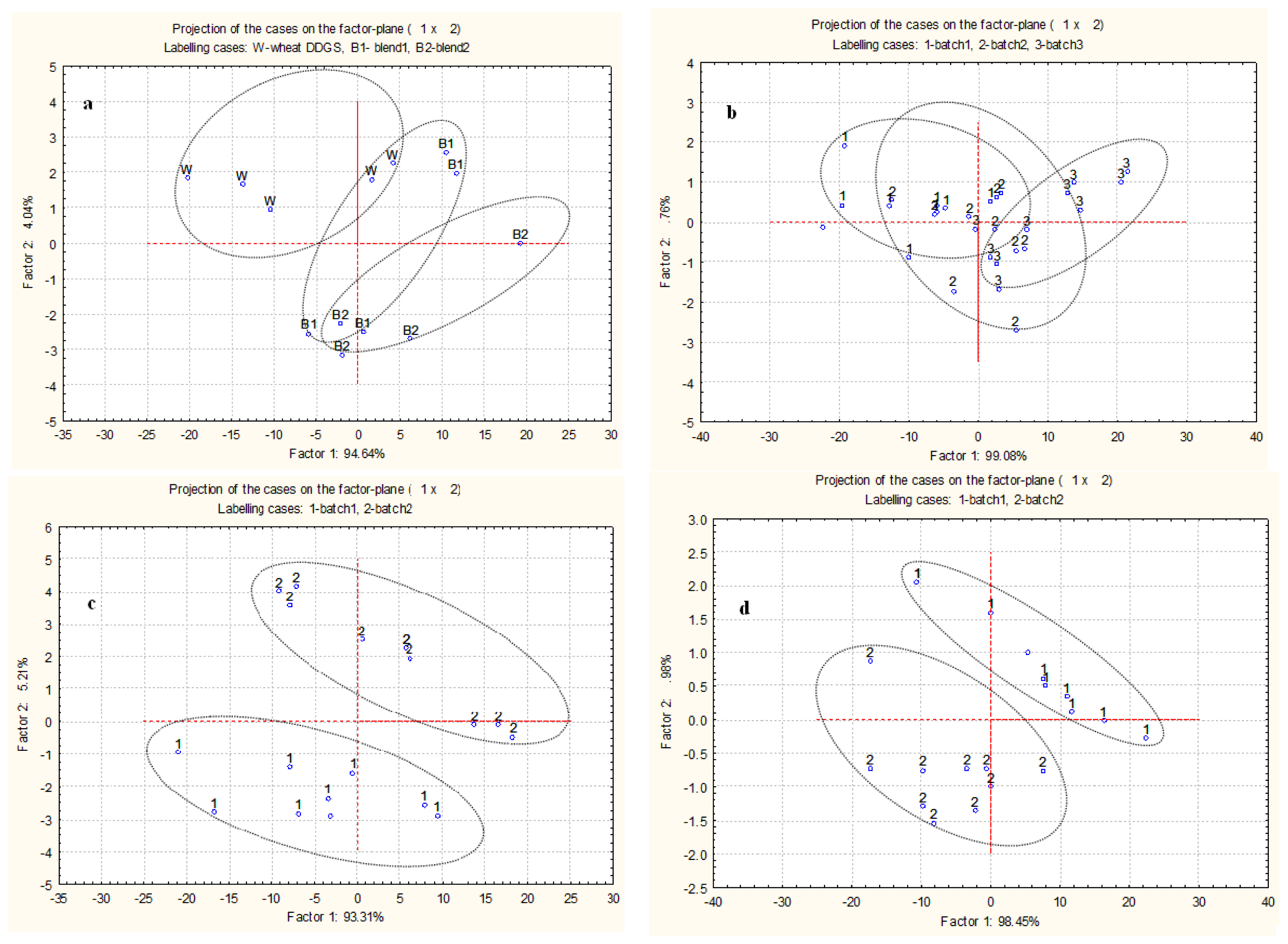
2.3. Multivariate Analysis of Spectra from Protein Internal Structures among Different Types and Batches of DDGS
2.4. Correlation between α-Helix to β-Sheet Ratios and Nutrient Profile
3. Experimental Section
3.1. Sample Collection and Preparation
3.2. Diffused Reflectance Fourier Transformed Infrared Spectroscopy (Drift)
3.3. Amide I, Amide II and α-Helix and β-Sheet Ratio Identification
3.4. Chemical Analysis and Protein Partitioning
3.5. Rumen Incubation Procedure
3.6. Rumen Degradation Kinetics
3.7. Intestinal Digestion of Rumen Undegraded Protein
3.8. The DVE/OEB Protein Evaluation System
3.9. Statistical Analysis
3.10. Multivariate Analysis of Drift Protein Molecular Spectra
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CFat | crude fat |
| CP | crude protein |
| DM | dry matter |
| DVE | true protein digested and absorbed in the small intestine |
| ESC | ethanol soluble carbohydrates |
| PA | non-protein nitrogen |
| PB1 | soluble true protein |
| PB2 | intermediately degradable true protein |
| PB3 | slowly degradable true protein |
| PC | indigestible protein |
| RUP | rumen undegraded protein |
| OEB | rumen degraded protein balance |
| OM | organic matter |
References
- Doiron, K.J.; Yu, P.; McKinnon, J.J.; Christensen, D.A. Heat induced protein structures and protein sub-fractions in realtion to protein degradation kinetics and intestinal availability in dairy cattle. J. Dairy Sci 2009, 92, 3319–3330. [Google Scholar]
- Damiran, D.; Yu, P. Structural makeup, biopolymer conformation, and biodegradation characteristics of a newly developed super genotype of oats (CDS SO-I versus conventional varieties): A novel approach. J. Agric. Food Chem 2010, 58, 2337–2387. [Google Scholar]
- Yu, P.; Niu, Z.; Damiran, D. Protein molecular structures and protein fraction profiles of new coproducts from bioethanol production: A novel approach. J. Agric. Food Chem 2010, 58, 3460–3464. [Google Scholar]
- Nuez-Ortín, W.G.; Yu, P. Effects of bioethanol plant and coproduct type on the metabolic characteristics of the proteins in dairy cattle. J. Dairy Sci 2010, 93, 3775–3783. [Google Scholar]
- Azarfar, A.; Jonker, A.; Yu, P. Assessing protein availability of different bioethanol coproducts in dairy cattle. Animal 2013, 7, 255–264. [Google Scholar]
- Spiehs, M.J.; Whitney, M.H.; Shurson, G.C. Nutrient database for distiller’s dried grains with solubles produced from new ethanol plants in Minnesota and South Dakota. J. Anim. Sci 2002, 80, 2639–2645. [Google Scholar]
- Van Duinkerken, G.; Blok, M.C.; Bannink, A.; Cone, J.W.; Dijkstra, J.; van Vuuren, A.M.; Tamminga, S. Update of the Dutch protein evaluation system for ruminants: The DVE/OEB2010 system. J. Agric. Sci 2010, 149, 351–367. [Google Scholar]
- Yu, P.; Blok, H.C.; Doiron, K.J. Understanding the differences in molecular conformation of carbohydrate and protein in endosperm tissues of grains with different biodegradation kinetics using advanced synchrotron technology. Spectrochim. Acta 2009, 71, 1837–1844. [Google Scholar]
- Jackson, M.; Mantsch, H.H. Ex Vivo Tissue Analysis by Infrared Spectroscopy. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley & Sons: New York, NY, USA, 2000; Volume 1, pp. 131–156. [Google Scholar]
- Yu, P. Molecular chemistry of plant protein structure at a cellular level by synchrotron-based FTIR spectroscopy: Comaprison of yellow (Brassica rapa) and brown (Brassica napus) canola seed tissues. Infrared Phys. Technol 2008, 51, 473–481. [Google Scholar]
- Yu, P. Synchrotron IR microspectroscopy for protein structure analysis: Potential and questions. Spectroscopy 2006, 20, 229–251. [Google Scholar]
- Doiron, K.J.; Yu, P.; Christensen, D.A.; McKinnon, J.J. Detecting molecular changes in vimy falxseed protein structure using synchrotron FTIRM and DRIFT spectroscopic techniques: Structural and biochemical charaterization. Spectroscopy 2009, 23, 307–322. [Google Scholar]
- Yu, P. Protein secondary structures (α-Heilx and β-Sheet) at a cellular level and protein fractions in relation to rumen degradation behaviours of protein: A new approach. Br. J. Nutr 2005, 94, 655–665. [Google Scholar]
- Yu, P. Applications of hierarchical cluster analysis (CLA) and principal component analysis (PCA) in feed structure and feed molecular chemistry reserach, using synchrotron-based feed Fourier transform infrared (FTIR) microspectroscopy. J. Agric. Food Chem 2005, 53, 7115–7127. [Google Scholar]
- Liu, B.; Thacker, P.; McKinnon, J.; Yu, P. In-depth study of the protein molecular structures of different types of dried distillers grains with solubles and their relationship to digestive characteristics. J. Sci. Food Agric 2013, 93, 1438–1448. [Google Scholar]
- Gamage, I.H.; Jonker, A.; Christensen, D.A.; Yu, P. Metabolic characteristics of proteins and biomolecular spectroscopic profiles in different batches of feedstock (wheat) and their co-products (wheat distillers dried grains with solubles) from the same bioethanol processing plant. J. Dairy Sci 2012, 95, 6695–6715. [Google Scholar]
- Yu, P.; Nuez-Ortín, W.G. Relationship of protein molecular structure to metabolisable proteins in different types of dried distillers grains with solubles: A novel approach. Br. J. Nutr 2010, 104, 1429–1437. [Google Scholar]
- Wetzel, D.L.; Srivarin, P.; Finney, J.R. Revealing protein infrared spectral detail in a heterogeneous matrix dominated by starch. Vib. Spectrosc 2003, 31, 109–114. [Google Scholar]
- Miller, L.M.; Dumas, P. Chemical imaging of biological tissue with synchrotron infrared light. Biochem. Biophys. Acta 2006, 1757, 846–857. [Google Scholar]
- Jackson, M.; Mantsch, H.H. Biomedical Infrared Spectroscopy. In Infrared Spectroscopy of Biomolecules; Mantsch, H.H., Chapman, D., Eds.; Wiley-Liss: New York, NY, USA, 1996; pp. 311–340. [Google Scholar]
- Azarfar, A.; Jonker, A.; Hettiarachchi-Gamage, I.K.; Yu, P. Nutrient profile and availability of co-products from bioethanol processing. J. Anim. Physiol. Anim. Nutr 2012, 96, 450–458. [Google Scholar]
- CCAC, Guide to the Care and Useof Experimental Animals; Olfert, E.D.; Cross, B.M.; McWilliam, A.A. (Eds.) Canadian Council on Animal Care: Ottawa, Canada, 1993; Volume 1.
- Yu, P.; Hart, K.; Du, L. An investigation of carbohydrate and protein degradation ratios, nitrogen to energy synchronization and hourly effective rumen digestion of barley: Effect of variety and growth year. J. Anim. Physiol. Anim. Nutr 2009, 93, 555–567. [Google Scholar]
- Yu, P.; McKinnon, J.J.; Christensen, D.A. The ratios of degradation characteristics of forages in the rumen of dairy cows: Effect of variety and stage of maturity. J. Sci. Food. Agric 2004, 84, 179–189. [Google Scholar]
- Azarfar, A.; Williams, B.A.; Boer, H.; Tamminga, S. In vitro gas production profile and the formation of end products from non-washable, insoluble washable and soluble washable fractions in some concentrate ingredients. J. Sci. Food. Agric 2007, 87, 1345–1375. [Google Scholar]
- Robinson, P.H.; Fadel, J.G.; Tamminga, S. Evaluation of mathematical models to describe neutral detergent residue in terms of its susceptibility to degradation in the rumen. Anim. Feed Sci. Technol 1986, 15, 249–271. [Google Scholar]
- Tamminga, S.; van Straalen, W.M.; Subnel, A.P.J.; Meijer, R.G.M.; Steg, A.; Wever, C.J.G.; Blok, M.C. The Dutch protein evaluation system: DVE/OEB system. Livest. Prod. Sci 1994, 40, 139–155. [Google Scholar]
- SAS/STAT Package, version 9.2; SAS Institute Inc: Cary, NC, USA, 2002–2007.
- Calsamiglia, S.; Stern, M.D. A three-step in vitro procedure for estimating intestinal digestion of protein in ruminants. J. Anim. Sci 1995, 73, 1459–1465. [Google Scholar]
- Saxton, A.M. A macro for converting mean separation output to letter groupings in proc mixed. Available online: http://www2.sas.com/proceedings/sugi23/Stats/p230.pdf (accessed on 13 August 2013).
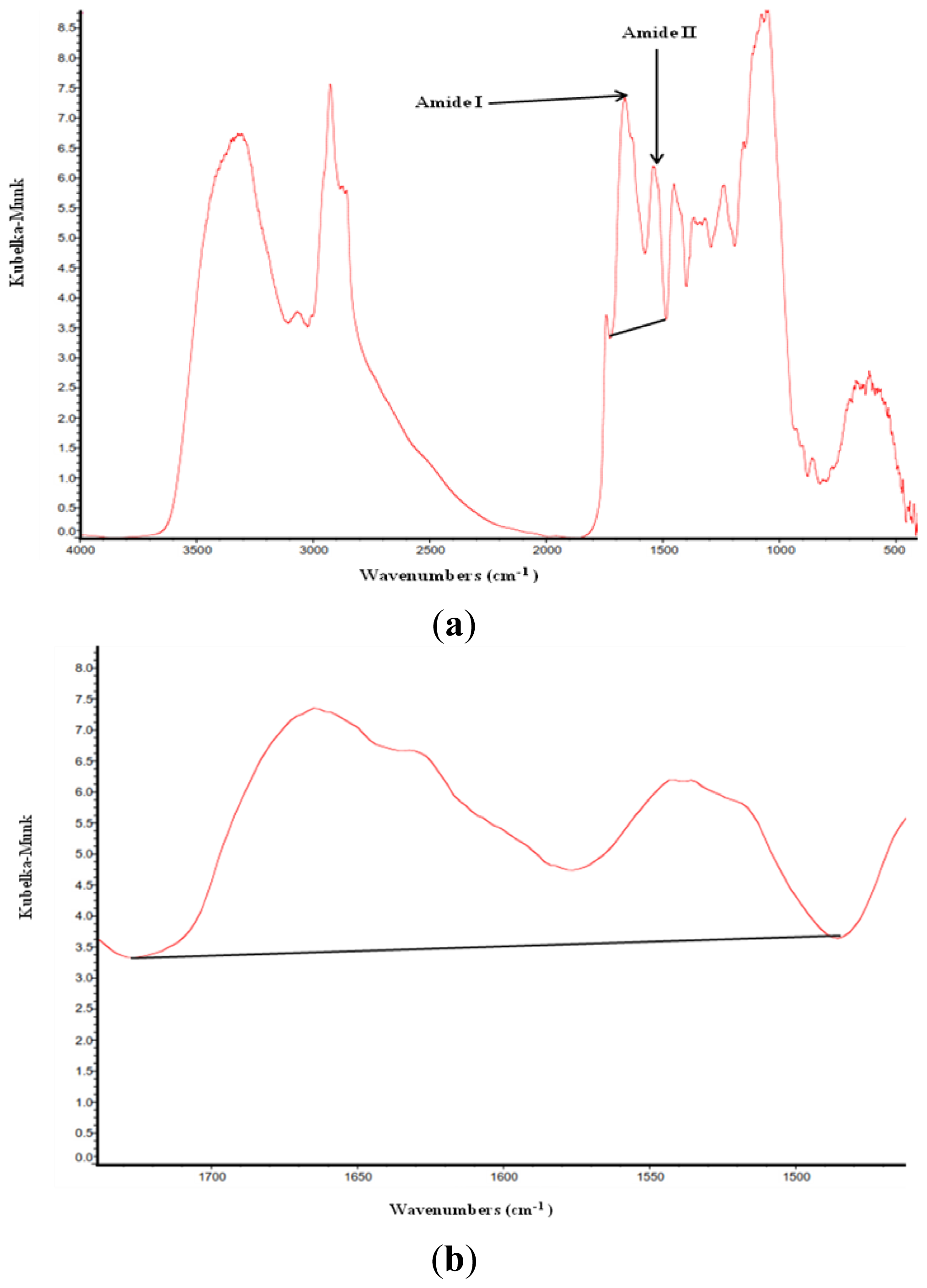

| Items | Number of repetitions for DRIFT analysis | Amide I | Amide II | Ratio of amide I to amide II |
|---|---|---|---|---|
| Amides IR peak center position | ~1655 cm−1 | ~1550 cm−1 | ~1665/~1550 cm−1 | |
| Amides IR peak region | ~1719–1576 cm−1 | ~1576–1450 cm−1 | ||
| Amides IR peak are base line | ~1719–1485 cm−1 | ~1719–1485 cm−1 | ~1719–1485 cm−1 | |
| Based on the protein amide I and II peak area | ||||
| WDDGS 1 | 30 | 220.13 a | 99.40 a | 2.18 a |
| BDDGS1 2 | 20 | 106.66 b | 57.62 b | 1.90 b |
| BDDGS2 3 | 20 | 83.29 b | 44.92 b | 1.83 b |
| SEM 4 | 27.380 | 11.364 | 0.624 | |
| Items | Number of repetitions for DRIFT analysis | Amide I | Amide II | Ratio of amide I to amide II |
|---|---|---|---|---|
| Amides IR peak center position | ~1655 cm−1 | ~1550 cm−1 | ~1665/~1550 cm−1 | |
| Amides IR peak region | ~1719–1576 cm−1 | ~1576–1450 cm−1 | ||
| Amides IR peak are base line | ~1719–1485 cm−1 | ~1719–1485 cm−1 | ~1719–1485 cm−1 | |
| WDDGS | Based on the amide I and II peak area | |||
| Batch1 | 10 | 312.76 | 136.05 | 2.29 a |
| Batch2 | 10 | 200.20 | 92.59 | 2.15 a,b |
| Batch3 | 10 | 147.42 | 65.56 | 2.11b |
| SEM | 39.237 | 15.526 | 0.033 | |
| BDDGS1 | ||||
| Batch1 | 10 | 117.14 | 58.79 | 1.73a |
| Batch2 | 10 | 102.17 | 56.44 | 2.07b |
| SEM | 10 | 8.884 | 4.513 | 0.022 |
| BDDGS2 | ||||
| Batch1 | 10 | 66.32 | 37.68 | 1.74 |
| Batch2 | 10 | 100.27 | 52.30 | 1.91 |
| SEM | 10.145 | 4.608 | 0.039 | |
| Infrared absorption | Protein secondary structures | ||||||
|---|---|---|---|---|---|---|---|
| α-helix | β-sheet | Ratio of α-helix to β-sheet | |||||
| Items | Number of repetition for DRIFT analysis | α-helix peak center (cm−1) | β-sheet peak center (cm−1) | peak base line (cm−1) | ~1663 (cm−1) | ~1632 (cm−1) | ~1663/1632 (cm−1) |
| Based on the protein α-helix and β-sheet peak height | |||||||
| WDDGS | 30 | 1665 | 1632 | ~1718–1485 | 2.54 a | 2.03 a | 1.26 |
| BDDGS1 | 20 | 1663 | 1632 | ~1718–1485 | 1.36 b | 1.11 b | 1.23 |
| BDDGS2 | 20 | 1663 | 1632 | ~1718–1485 | 1.10 b | 0.85 b | 1.29 |
| SEM | 0.325 | 0.260 | 0.022 | ||||
| Infrared absorption | Protein secondary structures | ||||||
|---|---|---|---|---|---|---|---|
| α-helix | β-sheet | Ratio of α-helix to β-sheet | |||||
| Items | Number of repetition for DRIFT analysis | α-helix peak center (cm−1) | β-sheet peak center (cm−1) | peak base line (cm−1) | ~1663 (cm−1) | ~1632 (cm−1) | ~1663/1632 (cm−1) |
| WDDGS | Based on the protein α-helix and β-sheet peak height | ||||||
| Batch1 | 10 | 1664 | 1632 | ~1718–1485 | 3.60 | 2.89 | 1.25 b |
| Batch2 | 10 | 1666 | 1632 | ~1718–1485 | 2.34 | 1.82 | 1.29 a |
| Batch3 | 10 | 1665 | 1632 | ~1718–1485 | 1.71 | 1.38 | 1.23 b |
| SEM | 0.465 | 0.371 | 0.006 | ||||
| BDDGS1 | |||||||
| Batch1 | 10 | 1664 | 1632 | ~1718–1485 | 1.36 | 1.06 | 1.28 |
| Batch2 | 10 | 1661 | 1632 | ~1718–1485 | 1.36 | 1.16 | 1.17 |
| SEM | 0.124 | 0.078 | 0.030 | ||||
| BDDGS2 | |||||||
| Batch1 | 10 | 1662 | 1632 | ~1718–1485 | 0.96 | 0.74 | 1.31 |
| Batch2 | 10 | 1665 | 1632 | ~1718–1485 | 1.23 | 0.97 | 1.28 |
| SEM | 0.143 | 0.095 | 0.027 | ||||
| Items | WDDGS | BDDGS1 | BDDGS2 | SEM |
|---|---|---|---|---|
| Rumen undegraded protein (RUP, g/kg CP) | 376.2 b | 592.7 a | 593.9 a | 20.69 |
| Rumen degraded protein balance (OEB, g/kg DM) | 159.1 a | 82.0 b | 65.8 b | 8.25 |
| In vitro intestinal digestibility 1 of RUP (g/kg RUP) | 806.9 | 781.5 | 788.0 | 15.03 |
| True protein digested and absorbed in the small intestine (DVE, g/kg DM) | 177.7 | 184.8 | 170.4 | 5.00 |
| Items | Correlation with protein α-helix to β-sheet ratios | |
|---|---|---|
| Correlation coefficient, r | p-value | |
| Protein fraction1(g/kg DM) | ||
| PA | −0.21 | 0.280 |
| PB1 | −0.06 | 0.765 |
| PB2 | −0.40 | 0.035 |
| PB3 | 0.24 | 0.219 |
| PC | 0.59 | 0.001 |
| Ruminal and intestinal availability of protein | ||
| Rumen undegraded protein (RUP, g/kg CP) | 0.41 | 0.031 |
| Rumen degraded protein balance (OEB, g/kg DM) | −0.34 | 0.080 |
| In vitro intestinal digestibility of RUP (g/kg RUP) | 0.10 | 0.615 |
| True protein digested and absorbed in the small intestine (DVE, g/kg DM) | 0.15 | 0.448 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Azarfar, A.; Jonker, A.; Yu, P. Protein Structures among Bio-Ethanol Co-Products and Its Relationships with Ruminal and Intestinal Availability of Protein in Dairy Cattle. Int. J. Mol. Sci. 2013, 14, 16802-16816. https://doi.org/10.3390/ijms140816802
Azarfar A, Jonker A, Yu P. Protein Structures among Bio-Ethanol Co-Products and Its Relationships with Ruminal and Intestinal Availability of Protein in Dairy Cattle. International Journal of Molecular Sciences. 2013; 14(8):16802-16816. https://doi.org/10.3390/ijms140816802
Chicago/Turabian StyleAzarfar, Arash, Arjan Jonker, and Peiqiang Yu. 2013. "Protein Structures among Bio-Ethanol Co-Products and Its Relationships with Ruminal and Intestinal Availability of Protein in Dairy Cattle" International Journal of Molecular Sciences 14, no. 8: 16802-16816. https://doi.org/10.3390/ijms140816802




