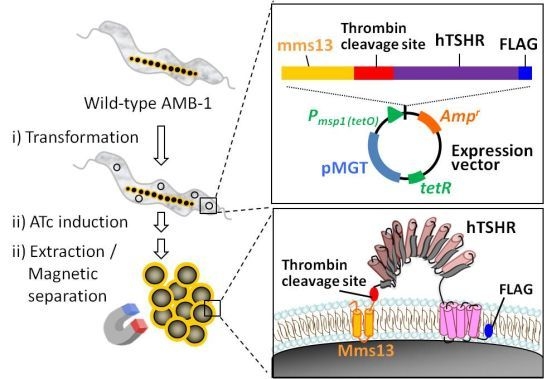
Functional Expression of Thyroid-Stimulating Hormone Receptor on Nano-Sized Bacterial Magnetic Particles in Magnetospirillum magneticum AMB-1
Abstract
:1. Introduction
2. Results
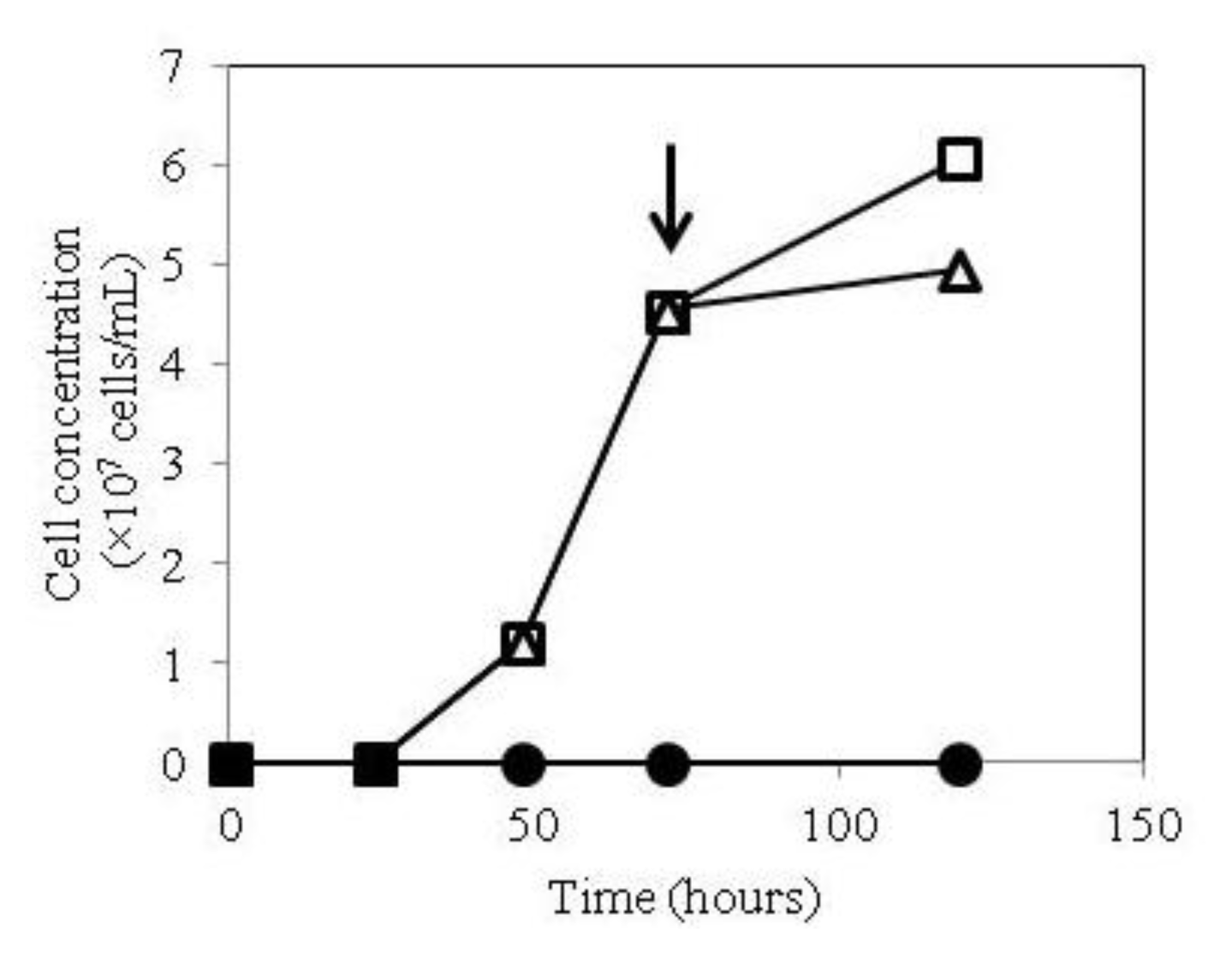
2.1. Growth of hTSHR Transformants in a Tetracycline-Inducible Expression System
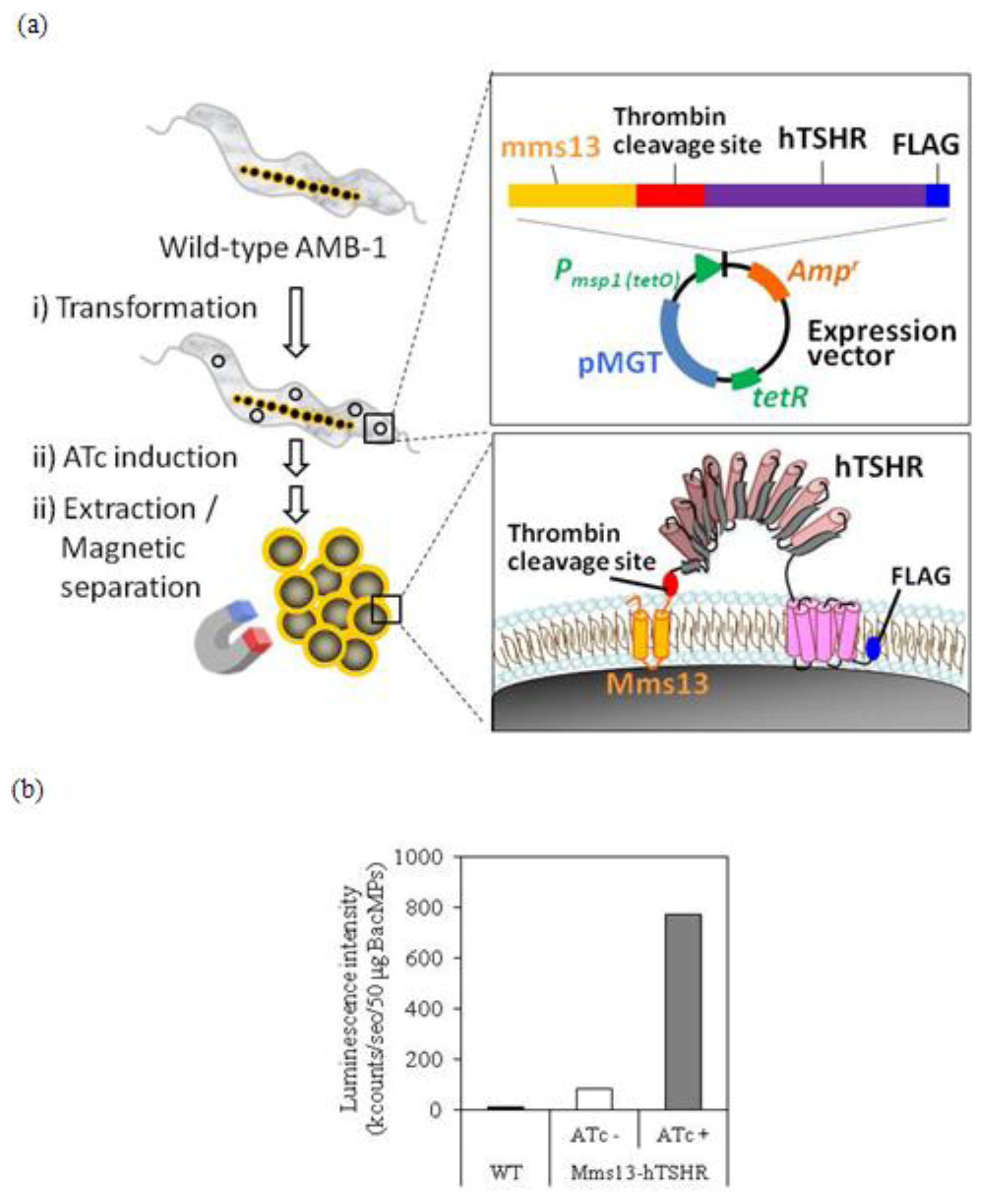
2.2. Isolation of hTSHR-Displaying BacMPs
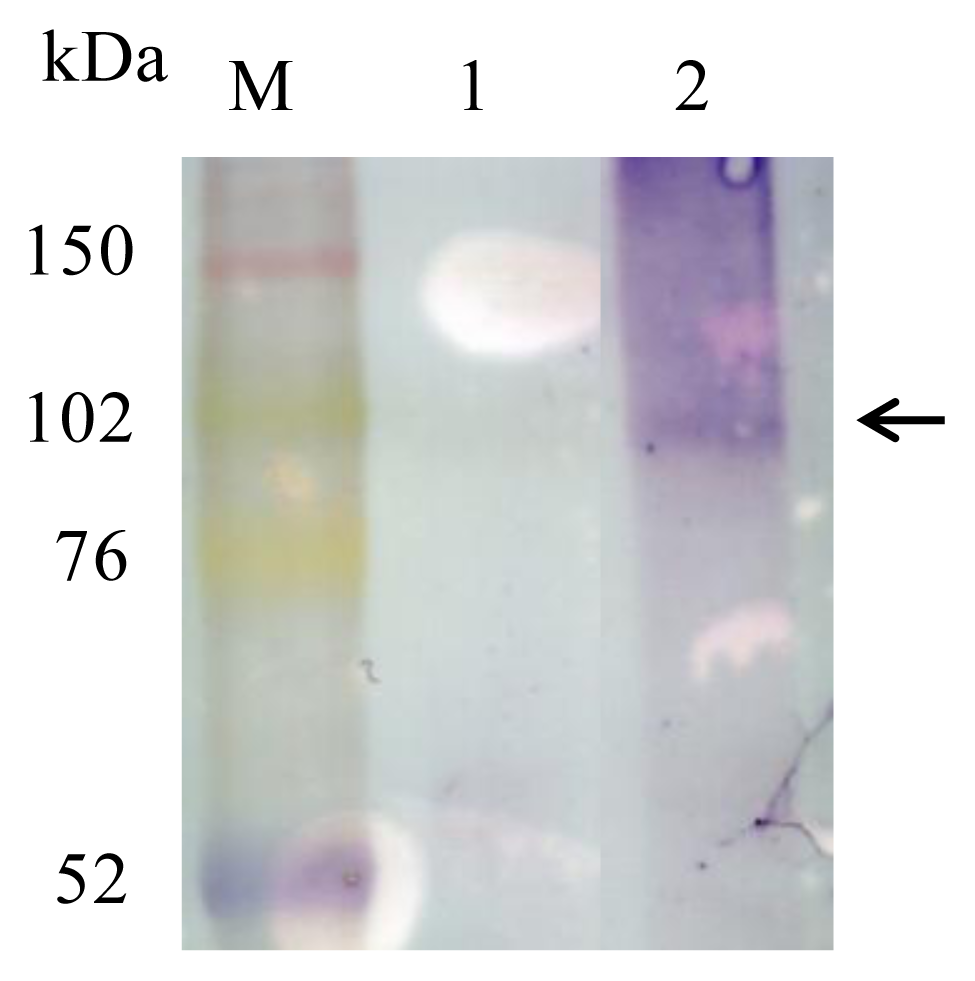
2.3. Detection of Mms13-hTSHR Fusion Protein by Immunoblotting
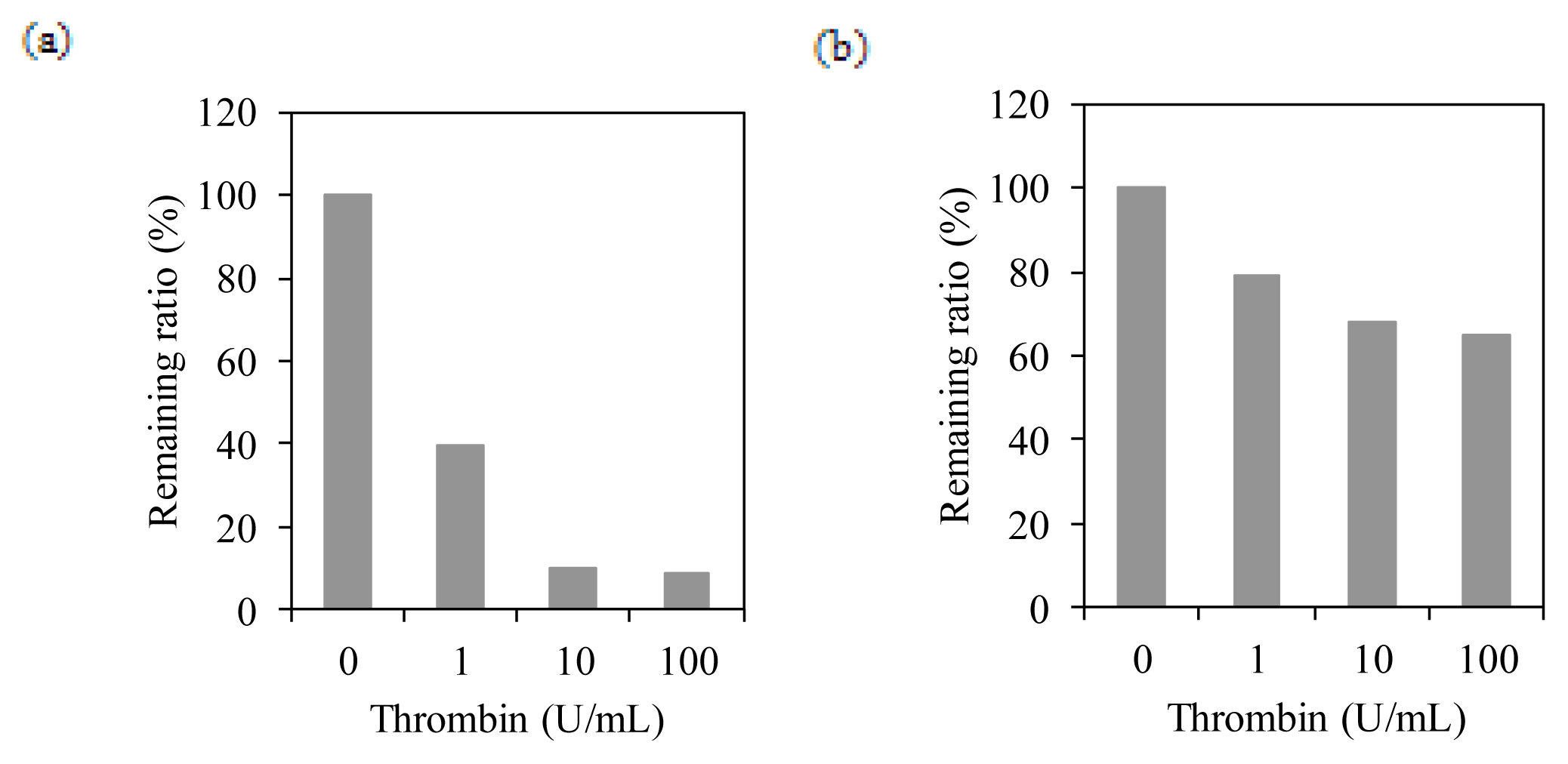
2.4. Membrane Integration Analysis
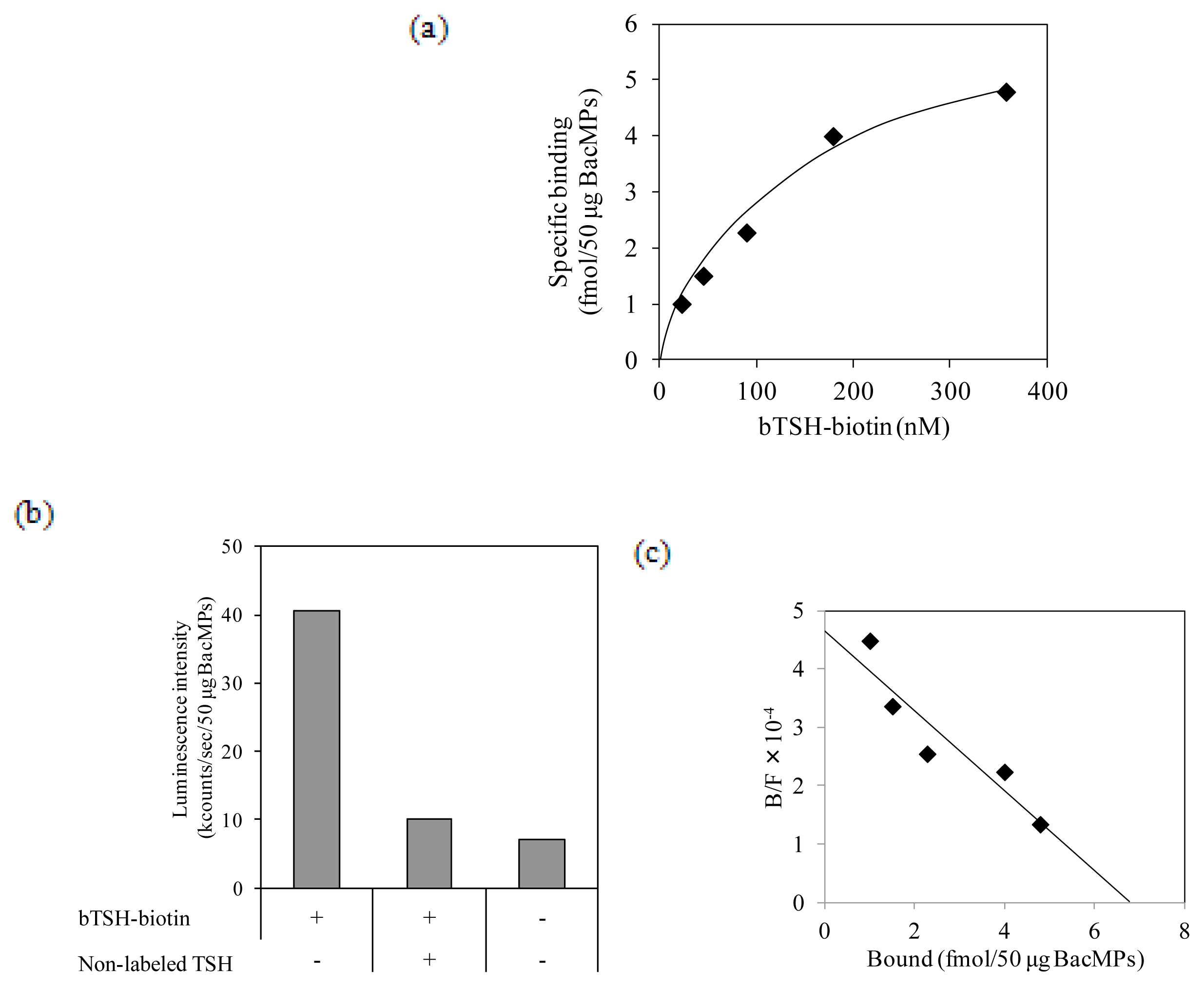
2.5. Binding of TSH to hTSHR on BacMPs
2.6. Binding of Autoantibody to hTSHR on BacMPs
3. Discussion
4. Experimental Section
4.1. Bacterial Cell Strains
4.2. Vector Construction
4.3. Preparation of BacMPs
4.4. Expression Analysis of hTSHR on BacMPs
4.4.1. ELISA
4.4.2. Western Blot Analysis
4.5. Thrombin Cleavage Assay on BacMPs
4.6. Ligand and Autoantibody Binding Assay on BacMPs
4.7. Determination of Kd Value for the Interaction of TSH and hTSHR
5. Conclusions
Conflict of Interest
References
- Farid, N.R.; Szkudlinski, M.W. Minireview: Structural and functional evolution of the thyrotropin receptor. Endocrinology 2004, 145, 4048–4057. [Google Scholar]
- Kamath, C.; Adlan, M.A.; Premawardhana, L.D. The role of thyrotrophin receptor antibody assays in Graves’ disease. J. Thyroid Res 2012, 2012, 525936. [Google Scholar]
- Zöphel, K.; Roggenbuck, D.; Schott, M. Clinical review about TRAb assay’s history. Autoimm. Rev 2010, 9, 695–700. [Google Scholar]
- Bolton, J.; Sanders, J.; Oda, Y. Measurement of thyroid-stimulating hormone receptor autoantibodies by ELISA. Clin. Chem 1999, 45, 2285–2287. [Google Scholar]
- Costagliola, S.; Morgenthaler, N.G.; Hoermann, R.; Badenhoop, K.; Struck, J.; Freitag, D.; Poertl, S.; Dumont, J.E.; Bergmann, A.; Mann, K.; et al. Second generation assay for thyrotropin receptor graves’ disease. J. Clin. Endocrinol. Metab 1999, 84, 90–97. [Google Scholar]
- Smith, B.R.; Bolton, J.; Young, S.; Collyer, A.; Weeden, A.; Bradbury, J.; Weightman, D.; Perros, P.; Sanders, J.; Furmaniak, J. A new assay for thyrotropin receptor autoantibodies. Thyroid 2004, 14, 830–835. [Google Scholar]
- Rapoport, B.; Chazenbalk, G.D.; Jaume, J.C.; McLachlan, S.M. The thyrotropin (TSH) receptor: Interaction with TSH and autoantibodies. Endocr. Rev 1998, 19, 673–716. [Google Scholar]
- Arakaki, A.; Webb, J.; Matsunaga, T. A novel protein tightly bound to bacterial magnetic particles in Magnetospirillum magneticum strain AMB-1. J. Biol. Chem 2003, 278, 8745–8750. [Google Scholar]
- Tanaka, T.; Matsunaga, T. Fully automated chemiluminescence immunoassay of insulin using antibody-protein A-bacterial magnetic particle complexes. Anal. Chem 2000, 72, 3518–3522. [Google Scholar]
- Yoshino, T.; Takahashi, M.; Takeyama, H.; Okamura, Y.; Kato, F.; Matsunaga, T. Assembly of G protein-coupled receptors onto nanosized bacterial magnetic particles using Mms16 as an anchor molecule. Society 2004, 70, 2880–2885. [Google Scholar]
- Yoshino, T.; Matsunaga, T. Efficient and stable display of functional proteins on bacterial magnetic particles using Mms13 as a novel anchor molecule. Appl. Environ. Microbiol 2006, 72, 465–471. [Google Scholar]
- Yoshino, T.; Hirabe, H.; Takahashi, M.; Kuhara, M.; Takeyama, H.; Matsunaga, T. Magnetic cell separation using nano-sized bacterial magnetic particles with reconstructed magnetosome membrane. Biotechnol. Bioeng 2008, 101, 470–477. [Google Scholar]
- Yoshino, T.; Shimojo, A.; Maeda, Y.; Matsunaga, T. Inducible expression of transmembrane proteins on bacterial magnetic particles in Magnetospirillum magneticum AMB-1. Appl. Environ. Microbiol 2010, 76, 1152–1157. [Google Scholar]
- Ban, T.; Kosugi, S.; Kohn, L.D. Specific antibody to the thyrotropin receptor identifies multiple receptor forms in membranes of cells transfected with wild-type receptor complementary deoxyribonucleic acid: Characterization of their relevance to receptor synthesis, processing, structur. Endocrinology 1992, 131, 815–829. [Google Scholar]
- Bobovnikova, Y.; Graves, P.N.; Vlase, H.; Davies, T.F. Characterization of soluble, disulfide bond-stabilized, prokaryotically expressed human thyrotropin receptor ectodomain. Endocrinology 1997, 138, 588–593. [Google Scholar]
- Graves, P.N.; Vlase, H.; Davies, T.F. Folding of the recombinant human thyrotropin (TSH) receptor extracellular domain: Identification of folded monomeric and tetrameric complexes that bind TSH receptor autoantibodies. Endocrinology 1995, 136, 521–527. [Google Scholar]
- Harfst, E.; Johnstone, A.P.; Nussey, S.S. Characterization of the extracellular region of the human thyrotrophin receptor expressed as a recombinant protein. J. Mol. Endocrinol 1992, 9, 227–236. [Google Scholar]
- Huang, G.C.; Collison, K.S.; McGregor, A.M.; Banga, J.P. Expression of a human thyrotrophin receptor fragment in Escherichia coli and its interaction with the hormone and autoantibodies from patients with Graves’ disease. J. Mol. Endocrinol 1992, 8, 137–144. [Google Scholar]
- Costagliola, S.; Alcalde, L.; Ruf, J.; Vassart, G.; Ludgate, M. Overexpression of the extracellular domain of the thyrotrophin receptor in bacteria; production of thyrotrophin-binding inhibiting immunoglobulins. J. Mol. Endocrinol 1994, 13, 11–21. [Google Scholar]
- Busuttil, B.E.; Turney, K.L.; Frauman, A.G. The expression of soluble, full-length, recombinant human TSH receptor in a prokaryotic system. Protein Express. Purific 2001, 23, 369–373. [Google Scholar]
- Seetharamaiah, G.S.; Kurosky, A.; Desai, R.K.; Dallas, J.S.; Prabhakar, B.S. A recombinant extracellular domain of the thyrotropin (TSH) receptor binds TSH in the absence of membranes. Endocrinology 1994, 134, 549–554. [Google Scholar]
- Shi, Y.; Zou, M.; Parhar, R.S.; Farid, N.R. High-affinity binding of thyrotropin to the extracellular domain of its receptor transfected in Chinese hamster ovary cells. Thyroid 1993, 3, 129–133. [Google Scholar]
- Nagayama, Y.; Namba, H.; Yokoyama, N.; Yamashita, S.; Niwa, M. Role of asparagine-linked oligosaccharides in protein folding, membrane targeting, and thyrotropin and autoantibody binding of the human thyrotropin receptor. J. Biol. Chem 1998, 273, 33423–33428. [Google Scholar]
- Costagliola, S.; Panneels, V.; Bonomi, M. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J 2002, 21, 504–513. [Google Scholar]
- Misrahi, M.; Ghinea, N.; Sar, S.; Saunier, B.; Jolivet, A.; Loosfelt, H.; Cerutti, M.; Devauchelle, G.; Milgrom, E. Processing of the precursors of the human thyroid-stimulating hormone receptor in various eukaryotic cells (human thyrocytes, transfected L cells and baculovirus-infected insect cells). Eur. J. Biochem 1994, 222, 711–719. [Google Scholar]
- Rapoport, B.; McLachlan, S.M.; Kakinuma, A.; Chazenbalk, G.D. Critical relationship between autoantibody recognition and thyrotropin receptor maturation as reflected in the acquisition of complex carbohydrate. J. Clin. Endocrinol. Metab 1996, 81, 2525–2533. [Google Scholar]
- Yoshino, T.; Matsunaga, T. Development of efficient expression system for protein display on bacterial magnetic particles. Biochem. Biophys. Res. Commun 2005, 338, 1678–1681. [Google Scholar]
- Kanetsuki, Y.; Tanaka, M.; Tanaka, T.; Matsunaga, T.; Yoshino, T. Effective expression of human proteins on bacterial magnetic particles in an anchor gene deletion mutant of Magnetospirillum magneticum AMB-1. Biochem. Biophys. Res. Commun 2012, 426, 7–11. [Google Scholar]
- Kanetsuki, Y.; Tanaka, T.; Matsunaga, T.; Yoshino, T. Enhanced heterologous protein display on bacterial magnetic particles using a lon protease gene deletion mutant in Magnetospirillum magneticum AMB-1. J. Biosci. Bioeng 2013, 116, 65–70. [Google Scholar]
- De Marco, A.; Deuerling, E.; Mogk, A.; Tomoyasu, T.; Bukau, B. Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnol 2007, 7, 32. [Google Scholar]
- De Marco, A. Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb. Cell Factor 2009, 8, 26. [Google Scholar]
- Gassner, D.; Stock, W.; Golla, R.; Roth, H.-J. First automated assay for thyrotropin receptor autoantibodies. Clin. Chem. Lab. Med 2009, 47, 1091–1095. [Google Scholar]
- Tozzoli, R.; Kodermaz, G.; Villalta, D.; Bagnasco, M.; Pesce, G.; Bizzaro, N. Accuracy of receptor-based methods for detection of thyrotropin-receptor autoantibodies: A new automated third-generation immunoassay shows higher analytical and clinical sensitivity for the differential diagnosis of hyperthyroidism. Autoimm. Highlights 2010, 1, 95–100. [Google Scholar]
- Tozzoli, R.; Bagnasco, M.; Giavarina, D.; Bizzaro, N. TSH receptor autoantibody immunoassay in patients with Graves’ disease: Improvement of diagnostic accuracy over different generations of methods. Systematic review and meta-analysis. Autoimm. Rev 2012, 12, 107–113. [Google Scholar]
- Matsunaga, T.; Sakaguchi, T.; Tadokoro, F. Magnetite formation by a magnetic bacterium capable of growing aerobically. Appl. Microbiol. Biotechnol 1991, 35, 651–655. [Google Scholar]
- Okamura, Y.; Takeyama, H.; Matsunaga, T. Two-dimensional analysis of proteins specific to the bacterial magnetic particle membrane from magnetospirillum sp. AMB-1. Appl. Microbiol. Biotechnol 2000, 84, 441–446. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sugamata, Y.; Uchiyama, R.; Honda, T.; Tanaka, T.; Matsunaga, T.; Yoshino, T. Functional Expression of Thyroid-Stimulating Hormone Receptor on Nano-Sized Bacterial Magnetic Particles in Magnetospirillum magneticum AMB-1. Int. J. Mol. Sci. 2013, 14, 14426-14438. https://doi.org/10.3390/ijms140714426
Sugamata Y, Uchiyama R, Honda T, Tanaka T, Matsunaga T, Yoshino T. Functional Expression of Thyroid-Stimulating Hormone Receptor on Nano-Sized Bacterial Magnetic Particles in Magnetospirillum magneticum AMB-1. International Journal of Molecular Sciences. 2013; 14(7):14426-14438. https://doi.org/10.3390/ijms140714426
Chicago/Turabian StyleSugamata, Yasuhiro, Ryo Uchiyama, Toru Honda, Tsuyoshi Tanaka, Tadashi Matsunaga, and Tomoko Yoshino. 2013. "Functional Expression of Thyroid-Stimulating Hormone Receptor on Nano-Sized Bacterial Magnetic Particles in Magnetospirillum magneticum AMB-1" International Journal of Molecular Sciences 14, no. 7: 14426-14438. https://doi.org/10.3390/ijms140714426