Sesquiterpenoids Lactones: Benefits to Plants and People
Abstract
:1. Introduction
2. Nutritional Factors
3. Structure
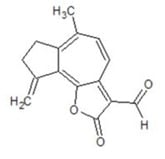
3.1. Function of the α-Methylene-γ-Lactone Group
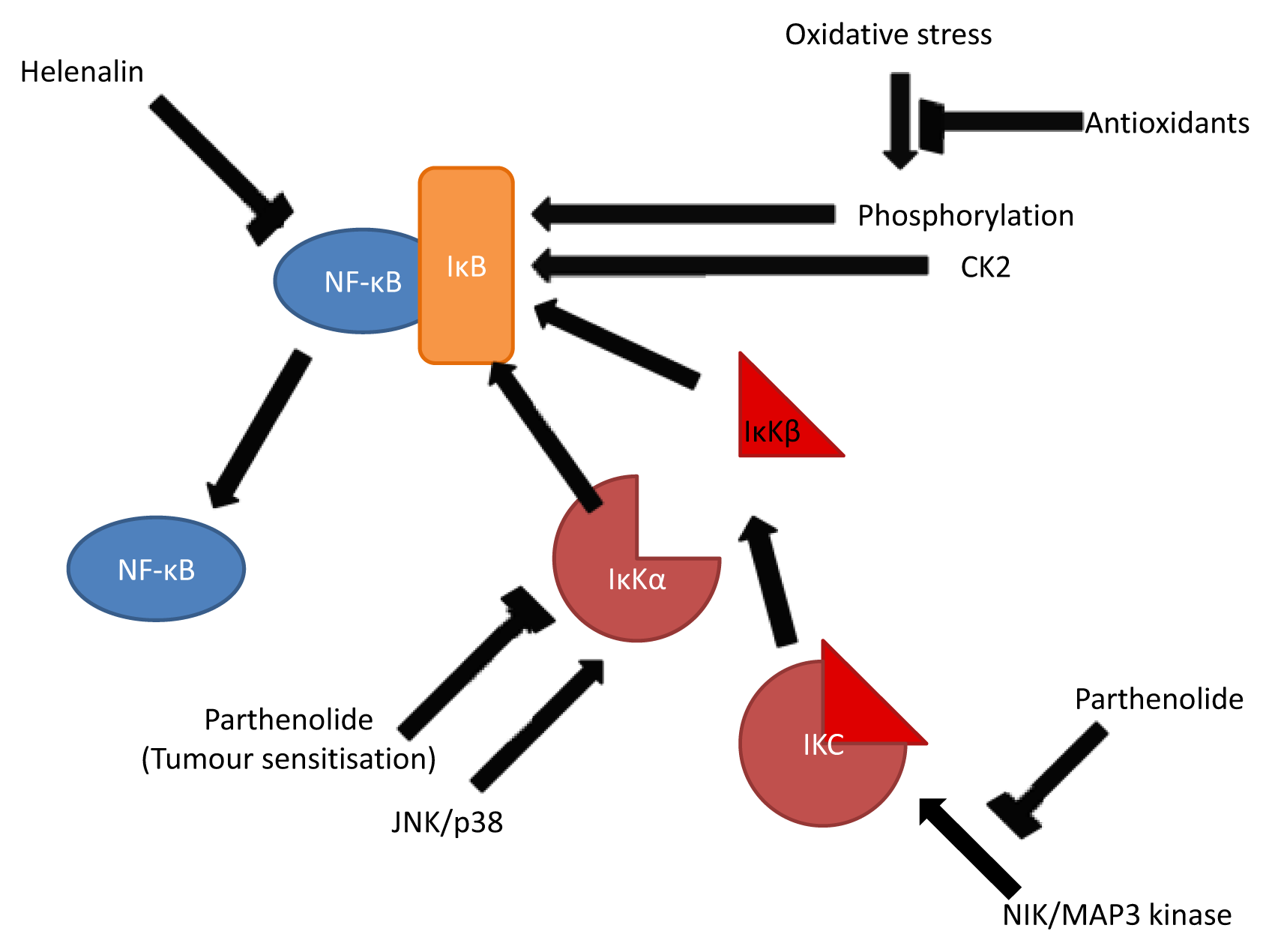
3.2. Inhibition of NF-κB
4. Function in People
4.1. Tumor Inhibition by Sesquiterpene Lactones
4.2. Alternative Mechanisms of Anti-inflammatory Effects
4.3. The Role of Parthenolide in Anti-Cancer Treatments
4.4. Sesquiterpenoids in Traditional Medicine
4.5. Extraction of Artemisinin
4.6. Antioxidant Function of Sesquiterpene Lactones
5. Bitterness
6. Function in Plants
6.1. Anti-Herbivory Effects of Sesquiterpene Lactones
6.2. Antimicrobial Function
6.3. Using Sesquiterpene Lactones to Defend against Ozone Damage
6.4. Allelopathic Function
6.5. Environmental Function of Allelochemicals
7. Implications for Crop Production
8. Conclusions
Acknowledgments
Conflict of Interest
References
- Heinrich, M.; Robles, M.; West, J.E.; Ortiz de Montellano, B.R.; Rodriguez, E. Ethnopharmacology of mexican Asteraceae (Compositae). Annu. Rev. Pharmacol. Toxicol 1998, 38, 539–565. [Google Scholar]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Tod 2010, 15, 668–678. [Google Scholar]
- Zhang, S.; Won, Y.-K.; Ong, C.-N.; Shen, H.-M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents 2005, 5, 239–249. [Google Scholar]
- Merfort, I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targ 2011, 12, 1560–1573. [Google Scholar]
- Wedge, D.E.; Galindo, J.C.G.; Macías, F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar]
- Canales, M.; Hernández, T.; Caballero, J.; de Vivar, A.R.; Avila, G.; Duran, A.; Lira, R. Informant consensus factor and antibacterial activity of the medicinal plants used by the people of San Rafael Coxcatlán, Puebla, México. J. Ethnopharmacol 2005, 97, 429–439. [Google Scholar]
- Rodriguez, E.; Towers, G.N.H.; Mitchell, J.C. Biological activities of sesquiterpene lactones. Phytochemistry 1976, 15, 1573–1580. [Google Scholar]
- Ivie, G.W.; Witzel, D.A.; Rushing, D.D. Toxicity and milk bittering properties of tenulin, the major sesquiterpene lactone constituent of Helenium amarum (bitter sneezeweed). J. Agric. Food Chem 1975, 23, 845–849. [Google Scholar]
- De Luque, A.P.; Galindo, J.C.G.; Macías, F.A.; Jorrín, J. Sunflower sesquiterpene lactone models induce Orobanche cumana seed germination. Phytochemistry 2000, 53, 45–50. [Google Scholar]
- Macías, F.A.; Torres, A.; Molinllo, J.G.; Varela, R.M.; Castellano, D. Potential allelopathic sesquiterpene lactones from sunflower leaves. Phytochemistry 1996, 43, 1205–1215. [Google Scholar]
- Food and agriculture organisation FAOStat lettuce and chicory production. Available online: http://faostat.fao.org/ (access on 15 April 2013).
- Thompson, B.; Demark-Wahnefried, W.; Taylor, G.; McClelland, J.W.; Stables, G.; Havas, S.; Feng, Z.; Topor, M.; Heimendinger, J.; Reynolds, K.D.; et al. Baseline fruit and vegetable intake among adults in seven 5 a day study centers located in diverse geographic areas. J. Am. Diet. Assoc 1999, 99, 1241–1248. [Google Scholar]
- Sorensen, G.; Stoddard, A.; Peterson, K.; Cohen, N.; Hunt, M.K.; Stein, E.; Palombo, R.; Lederman, R. Increasing fruit and vegetable consumption through worksites and families in the Treatwell 5-a-day study. Am. J. Public Health 1999, 89, 54–60. [Google Scholar]
- Casagrande, S.S.; Wang, Y.; Anderson, C.; Gary, T.L. Have Americans increased their fruit and vegetable intake? The trends between 1988 and 2002. Am. J. Prevent. Med 2007, 32, 257–263. [Google Scholar]
- Rogers, S.; Pryer, J.A. Who consumed 5 or more portions of fruit and vegetables per day in 1986–1987 and in 2000–2001? Pub. Health Nutr 2012, 15, 1240–1247. [Google Scholar]
- Bork, P.M.; Schmitz, M.L.; Kuhnt, M.; Escher, C.; Heinrich, M. Sesquiterpene lactone containing mexican indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett 1997, 402, 85–90. [Google Scholar]
- Lyß, G.; Knorre, A.; Schmidt, T.J.; Pahl, H.L.; Merfort, I. The Anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-κB by directly targeting p65. J. Biol. Chem 1998, 273, 33508–33516. [Google Scholar]
- Yu, F.; Utsumi, R. Diversity, regulation, and genetic manipulation of plant mono- and sesquiterpenoid biosynthesis. Cell. Mol. Life Sci 2009, 66, 3043–3052. [Google Scholar]
- Bennett, M.H.; Mansfield, J.W.; Lewis, M.J.; Beale, M.H. Cloning and expression of sesquiterpene synthase genes from Lettuce (Lactuca sativa L.). Phytochem 2002, 60, 255–261. [Google Scholar]
- Cheng, A.-X.; Xiang, C.-Y.; Li, J.-X.; Yang, C.-Q.; Hu, W.-L.; Wang, L.-J.; Lou, Y.-G.; Chen, X.-Y. The rice (E)-β-Caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar]
- Picaud, S.; Olsson, M.E.; Brodelius, P.E. Improved conditions for production of recombinant plant sesquiterpene synthases in Escherichia coli. Prot. Expr. Purif 2007, 51, 71–79. [Google Scholar]
- Lange, G.L.; Lee, M. Synthesis of four sesquiterpenoid Lactone skeletons, germacranolide, elemanolide, cadinanolide, and guaianolide, from a single photoadduct. J. Org. Chem 1987, 52, 325–331. [Google Scholar]
- Little, D.B.; Croteau, R.B. Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases δ-selinene synthase and γ-humulene synthase. Arch. Biochem. Biophys 2002, 402, 120–135. [Google Scholar]
- Schnee, C.; Kollner, T.G.; Gershenzon, J.; Degenhardt, J. The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol 2002, 130, 2049–2060. [Google Scholar]
- Zhang, Z.; Gao, Z.H.; Wei, J.H.; Xu, Y.H.; Li, Y.; Yang, Y.; Meng, H.; Sui, C.; Wang, M.X. The mechanical wound transcriptome of three-year-old Aquilaria sinensis. Acta Pharma. Sin 2012, 47, 1106–1110. [Google Scholar]
- Seaman, F.C. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot. Rev 1982, 48, 121–595. [Google Scholar]
- Cordell, G.A. Biosynthesis of sesquiterpenes. Chem. Rev 1976, 76, 425–460. [Google Scholar]
- Ruzicka, L. The isoprene rule and the biogenesis of terpenic compounds. Cell. Mol. Life Sci 1953, 9, 357–367. [Google Scholar]
- Michalska, K.; Stojakowska, A.; Malarz, J.; Dolezalová, I.; Lebeda, A.; Kisiel, W. Systematic implications of sesquiterpene lactones in Lactuca species. Biochem. System. Ecol 2009, 37, 174–179. [Google Scholar]
- Ren, Y.L.; Zhou, Y.W.; Ye, Y.H. Chemical components of Lactuca and their bioactivites. Yao Xue Xue Bao 2004, 39, 954–960. [Google Scholar]
- Kupchan, S.M.; Eakin, M.A.; Thomas, A.M. Tumor Inhibitors. 69. Structure-cytotoxicity relations among the sesquiterpene lactones. J. Med. Chem 1971, 14, 1147–1152. [Google Scholar]
- Mitchell, J.C.; Fritig, B.; Singh, B.; Towers, G.H.N. Allergic contact dermatitis from frullania and compositae. The role of sesquiterpene lactones. J. Investig. Dermatol 1970, 54, 233–239. [Google Scholar]
- Wong, H.R.; Menendez, I.Y. Sesquiterpene lactones inhibit inducible nitric oxide synthase gene expression in cultured rat aortic smooth muscle cells. Biochem. Biophys. Res. Commun 1999, 262, 375–380. [Google Scholar]
- Mazor, R.L.; Menendez, I.Y.; Ryan, M.A.; Fiedler, M.A.; Wong, H.R. Sesquiterpene lactones are potent inhibitors of interleukin 8 gene expression in cultured human respiratory epithelium. Cytokine 2000, 12, 239–245. [Google Scholar]
- Schomburg, C.; Schuehly, W.; da Costa, F.B.; Klempnauer, K.-H.; Schmidt, T.J. Natural sesquiterpene Lactones as inhibitors of myb-dependent gene expression: Structure-activity relationships. Eur. J. Med. Chem 2013, 63, 313–320. [Google Scholar]
- Lee, K.-H.; Huang, E.-S.; Piantadosi, C.; Pagano, J.S.; Geissman, T.A. Cytotoxicity of sesquiterpene lactones. Cancer Res 1971, 31, 1649–1654. [Google Scholar]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic acid derivatives as anticancer agents—A review. Curr. Med. Chem 2011, 18, 1672–1703. [Google Scholar]
- Bennett, M.H. The phytoalexin response of lettuce to challenge by Botrytis cinerea, Bremia lactucae, and Pseudomonas syringae pv. phaseolicola. Physiol. Mol. Plant Pathol 1994, 44, 321–333. [Google Scholar]
- Ding, X.C.; Beck, H.-P.; Raso, G. Plasmodium sensitivity to artemisinins: Magic bullets hit elusive targets. Trends Parasitol 2011, 27, 73–81. [Google Scholar]
- Baud, V.; Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov 2009, 8, 33–40. [Google Scholar] [Green Version]
- Hehner, S.P.; Heinrich, M.; Bork, P.M.; Vogt, M.; Ratter, F.; Lehmann, V.; Schulze-Osthoff, K.; Droge, W.; Schmitz, M.L. Sesquiterpene lactones specifically inhibit activation of NF-κB by preventing the degradation of IκB-α and IκB-β. J. Biol. Chem 1998, 273, 1288–1297. [Google Scholar]
- Hehner, S.P.; Hofmann, T.G.; Droge, W.; Schmitz, M.L. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-κB by targeting the IκB kinase complex. J. Immunol 1999, 163, 5617–5623. [Google Scholar]
- Siedle, B.; Garcia-Pineres, A.J.; Murillo, R.; Schulte-Monting, J.; Castro, V.; Rungeler, P.; Klaas, C.A.; da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-κB. J. Med. Chem 2004, 47, 6042–6054. [Google Scholar]
- Chen, F.E.; Huang, D.-B.; Chen, Y.-Q.; Ghosh, G. Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA. Nature 1998, 391, 410–413. [Google Scholar]
- Garc a-Piñeres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Strunck, E.; Pahl, H.L.; Merfort, I. Cysteine 38 in p65/NF-κB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem 2001, 276, 39713–39720. [Google Scholar]
- Pan, L.; Lantvit, D.D.; Riswan, S.; Kardono, L.B.S.; Chai, H.-B.; de Blanco, E.J.; Farnsworth, N.R.; Soejarto, D.D.; Swanson, S.M.; Kinghorn, A.D. Bioactivity-guided isolation of cytotoxic sesquiterpenes of Rolandra fruticosa. Phytochemistry 2010, 71, 635–640. [Google Scholar]
- Takada, Y.; Murakami, A.; Aggarwal, B.B. Zerumbone abolishes NF-κB and IκBα kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene 2005, 24, 6957–6969. [Google Scholar]
- Rüngeler, P.; Castro, V.; Mora, G.; Gören, N.; Vichnewski, W.; Pahl, H.L.; Merfort, I.; Schmidt, T.J. Inhibition of transcription factor NF-κB by sesquiterpene lactones: A proposed molecular mechanism of action. Bioorg. Med. Chem 1999, 7, 2343–2352. [Google Scholar]
- Youl Cho, J. Sesquiterpene lactones as a potent class of NF-B activation inhibitors. Curr. Enz. Inhib 2006, 2, 329–341. [Google Scholar]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar]
- Ravi, R.; Bedi, A. NF-κB in cancer—A friend turned foe. Drug Resist. Updates 2004, 7, 53–67. [Google Scholar]
- Wesolowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol 2006, 107, 254–258. [Google Scholar]
- Prehn, J.H.M.; Krieglstein, J. Platelet-activating factor antagonists reduce excitotoxic damage in cultured neurons from embryonic chick telencephalon and protect the rat hippocampus and neocortex from ischemic injury in vivo. J. Neurosci. Res 1993, 34, 179–188. [Google Scholar]
- Ahlemeyer, B.; Möwes, A.; Krieglstein, J. Inhibition of serum deprivation- and staurosporine-induced neuronal apoptosis by Ginkgo biloba extract and some of its constituents. Eur. J. Pharmacol 1999, 367, 423–430. [Google Scholar]
- Giordano, O.S.; Guerreiro, E.; Pestchanker, M.J.; Guzman, J.; Pastor, D.; Guardia, T. The gastric cytoprotective effect of several sesquiterpene lactones. J. Nat. Prod 1990, 53, 803–809. [Google Scholar]
- Kressmann, S.; Biber, A.; Wonnemann, M.; Schug, B.; Blume, H.H.; Muller, W.E. Influence of pharmaceutical quality on the bioavailability of active components from Ginkgo biloba preparations. J. Pharm. Pharmacol 2002, 54, 1507–1514. [Google Scholar]
- Tamaki, H.; Robinson, R.W.; Anderson, J.L.; Stoewsand, G.S. Sesquiterpene lactones in virus-resistant lettuce. J. Agric. Food Chem 1995, 43, 6–8. [Google Scholar]
- Price, K.D.; MS; Shepherd, R.; Chan, HW-S.; Fenwick, GR. Relationship between the chemical and sensory properties of exotic salad crops- coloured lettuce (Lactuca sativa) and chicory (Chicorium intybus). J. Sci. Food Agric 1990, 53, 185–192. [Google Scholar]
- Sweeney, C.J.; Mehrotra, S.; Sadaria, M.R.; Kumar, S.; Shortle, N.H.; Roman, Y.; Sheridan, C.; Campbell, R.A.; Murry, D.J.; Badve, S.; et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol. Can. Therap 2005, 4, 1004–1012. [Google Scholar]
- Calera, M.R.; Soto, F.; Sanchez, P.; Bye, R.; Hernandez-Bautista, B.; Anaya, A.L.; Lotina-Hennsen, B.; Mata, R. Biochemically active sesquiterpene lactones from Ratibida mexicana. Phytochemistry 1995, 40, 419–425. [Google Scholar]
- Chaves, J.S.; Leal, P.C.; Pianowisky, L.; Calixto, J.B. Pharmacokinetics and tissue distribution of the sesquiterpene α-humulene in mice. Planta Med 2008, 74, 1678–1683. [Google Scholar]
- Cavin, C.; Delannoy, M.; Malnoe, A.; Debefve, E.; Touché, A.; Courtois, D.; Schilter, B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Comm 2005, 327, 742–749. [Google Scholar]
- Blanco, J.G.; Gil, R.R.; Bocco, J.L.; Meragelman, T.L.; Genti-Raimondi, S.; Flury, A. Aromatase inhibition by an 11,13-dihydroderivative of a sesquiterpene lactone. J. Pharmacol. Exptl. Therap 2001, 297, 1099–1105. [Google Scholar]
- deGraffenried, L.A.; Chandrasekar, B.; Friedrichs, W.E.; Donzis, E.; Silva, J.; Hidalgo, M.; Freeman, J.W.; Weiss, G.R. NF-κB inhibition markedly enhances sensitivity of resistant breast cancer tumor cells to tamoxifen. Annu. Oncol 2004, 15, 885–890. [Google Scholar]
- Nakshatri, H.; Rice, S.E.; Bhat-Nakshatri, P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene 2004, 23, 7330–7344. [Google Scholar]
- Zhang, S.; Lin, Z.-N.; Yang, C.-F.; Shi, X.; Ong, C.-N.; Shen, H.-M. Suppressed NF-κB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-α-induced apoptosis in human cancer cells. Carcinogenesis 2004, 25, 2191–2199. [Google Scholar]
- Lamb, J.A.; Ventura, J.-J.; Hess, P.; Flavell, R.A.; Davis, R.J. JunD mediates survival signaling by the JNK signal transduction pathway. Mol. Cell 2003, 11, 1479–1489. [Google Scholar]
- Wen, J.; You, K.-R.; Lee, S.-Y.; Song, C.-H.; Kim, D.-G. Oxidative stress-mediated apoptosis. J. Biol. Chem 2002, 277, 38954–38964. [Google Scholar]
- Lee, K.; Hall, I.; Mar, E.; Starnes, C.; ElGebaly, S.; Waddell, T.; Hadgraft, R.; Ruffner, C.; Weidner, I. Sesquiterpene antitumor agents: Inhibitors of cellular metabolism. Science 1977, 196, 533–536. [Google Scholar]
- Zhang, S.; Ong, C.-N.; Shen, H.-M. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett 2004, 208, 143–153. [Google Scholar]
- Price, R.N.; Nosten, F.; Luxemburger, C.; ter Kuile, F.O.; Paiphun, L.; Chongsuphajaisiddhi, T.; White, N.J. Effects of artemisinin derivatives on malaria transmissibility. Lancet 1996, 347, 1654–1658. [Google Scholar]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Eng. J. Med 2009, 361, 455–467. [Google Scholar]
- Asawamahasakda, W.; Ittarat, I.; Pu, Y.M.; Ziffer, H.; Meshnick, S.R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother 1994, 38, 1854–1858. [Google Scholar]
- Eckstein-Ludwig, U.; Webb, R.J.; van Goethem, I.D.A.; East, J.M.; Lee, A.G.; Kimura, M.; O’Neill, P.M.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar]
- Jambou, R.; Legrand, E.; Niang, M.; Khim, N.; Lim, P.; Volney, B.; Ekala, M.T.; Bouchier, C.; Esterre, P.; Fandeur, T.; et al. Resistance of Plasmodium falciparum field isolates to in vitro artemether and point mutations of the Serca-type PfATPase 6. Lancet 2005, 366, 1960–1963. [Google Scholar]
- Wang, J.; Huang, L.; Li, J.; Fan, Q.; Long, Y.; Li, Y.; Zhou, B. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS One 2010, 5, e9582. [Google Scholar]
- Tournier, H.; Schinella, G.; Balsa, E.M.; Buschiazzo, H.; Manez, S.; Buschiazzo, P.M. Effect of the chloroform extract of Tanacetum vulgare and one of its active principles, parthenolide, on experimental gastric Ulcer in rats. J. Pharm. Pharmacol 1999, 51, 215–219. [Google Scholar]
- Paulsen, E.; Christensen, L.P.; Andersen, K.E. Possible cross-reactivity between para-phenylenediamine and sesquiterpene lactones. Contact Derm 2008, 58, 120–122. [Google Scholar]
- Mark, K.A.; Brancaccio, R.R.; Soter, N.A.; Cohen, D.E. Allergic contact and photoallergic contact dermatitis to plant and pesticide allergens. Arch. Dermatol 1999, 135, 67–70. [Google Scholar]
- Ferreira, J.F.S.; Janick, J. Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell, Tissue Organ Cult 1996, 44, 211–217. [Google Scholar]
- Mannan, A.; Liu, C.; Arsenault, P.R.; Towler, M.J.; Vail, D.R.; Lorence, A.; Weathers, P.J. DMSO triggers the generation of ROS Leading to an increase in artemisinin and dihydroartemisinic acid in Artemisia annua shoot cultures. Plant Cell Rep 2010, 29, 143–152. [Google Scholar]
- De Jesus-Gonzalez, L.; Weathers, P.J. Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 2003, 21, 809–813. [Google Scholar]
- Delabays, N.; Simonnet, X.; Gaudin, M. The genetics of artemisinin content in Artemisia annua L. and the breeding of high yielding cultivars. Curr. Med. Chem 2001, 8, 1795–1801. [Google Scholar]
- Kindermans, J.-M.; Pilloy, J.; Olliaro, P.; Gomes, M. Ensuring sustained ACT production and reliable artemisinin supply. Malaria J 2007, 6, 125. [Google Scholar]
- Wallaart, T.E.; Pras, N.; Beekman, A.C.; Quax, W.J. Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: Proof for the existence of chemotypes. Planta Med 2000, 66, 57–62. [Google Scholar]
- Kapoor, R.; Chaudhary, V.; Bhatnagar, A. Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 2007, 17, 581–587. [Google Scholar]
- Fehsenfeld, F.; Calvert, J.; Fall, R.; Goldan, P.; Guenther, A.B.; Hewitt, C.N.; Lamb, B.; Liu, S.; Trainer, M.; Westberg, H.; et al. Emissions of volatile organic compounds from vegetation and the implications for atmospheric chemistry. Global Biogeochem. Cycles 1992, 6, 389–430. [Google Scholar]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem 2000, 69, 167–174. [Google Scholar]
- Ungnade, H.E.; Hendley, E.C. The bitter principle of Helenium Tenuifolium. J. Am. Chem. Soc 1948, 70, 3921–3924. [Google Scholar]
- Van Beek, T.A.; Maas, P.; King, B.M.; Leclercq, E.; Voragen, A.G.J.; de Groot, A. Bitter sesquiterpene lactones from chicory roots. J. Agric. Food Chem 1990, 38, 1035–1038. [Google Scholar]
- Peters, A.M.; Haagsma, N.; Gensch, K.-H.; van Amerongen, A. Production and characterization of polyclonal antibodies against the bitter sesquiterpene lactones of chicory (Cichorium intybus L.). J. Agric. Food Chem 1996, 44, 3611–3615. [Google Scholar]
- Brockhoff, A.; Behrens, M.; Massarotti, A.; Appendino, G.; Meyerhof, W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, Strychnine, and denatonium. J. Agric. Food Chem 2007, 55, 6236–6243. [Google Scholar]
- Brockhoff, A.; Behrens, M.; Niv, M.Y.; Meyerhof, W. Structural requirements of bitter taste receptor activation. Proc. Natl. Acad. Sci. USA 2010, 107, 11110–11115. [Google Scholar]
- Kim, U.; Wooding, S.; Ricci, D.; Jorde, L.B.; Drayna, D. Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat 2005, 26, 199–204. [Google Scholar]
- Behrens, M.; Reichling, C.; Batram, C.; Brockhoff, A.; Meyerhof, W. Bitter taste receptors and their cells. Annu. N. Y. Acad. Sci 2009, 1170, 111–115. [Google Scholar]
- Loreto, F.; Ciccioli, P.; Brancaleoni, E.; Cecinato, A.; Frattoni, M.; Sharkey, T.D. Different sources of reduced carbon contribute to form three classes of terpenoid emitted by Quercus ilex L. Leaves. Proc. Natl. Acad. Sci. USA 1996, 93, 9966–9969. [Google Scholar]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci 2004, 9, 529–533. [Google Scholar]
- Knight, A.; Light, D. Attractants from bartlett pear for codling moth, Cydia pomonella (L.), larvae. Naturwissenschaften 2001, 88, 339–342. [Google Scholar]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci 2010, 15, 154–166. [Google Scholar]
- Wijeratne, E.M.K.; Turbyville, T.J.; Zhang, Z.; Bigelow, D.; Pierson, L.S.; VanEtten, H.D.; Whitesell, L.; Canfield, L.M.; Gunatilaka, A.A.L. Cytotoxic constituents of Aspergillus terreus from the rhizosphere of Opuntia versicolor of the sonoran desert. J. Nat. Prod 2003, 66, 1567–1573. [Google Scholar]
- Lin, C.; Owen, S.M.; Peñuelas, J. Volatile organic compounds in the roots and rhizosphere of Pinus spp. Soil Biol. Biochem 2007, 39, 951–960. [Google Scholar]
- Poecke, R.M.P.v.; Dicke, M. Signal transduction downstream of salicylic and jasmonic acid in Herbivory-induced parasitoid attraction by Arabidopsis is independent of JAR1 and NPR1. Plant Cell Environ 2003, 26, 1541–1548. [Google Scholar]
- Kubo, I.; Ganjian, I. Insect antifeedant terpenes, hot tasting to humans. Experientia 1981, 37, 1063–1064. [Google Scholar]
- Caputi, L.; Carlin, S.; Ghiglieno, I.; Stefanini, M.; Valenti, L.; Vrhovsek, U.; Mattivi, F. Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the “peppery” character of wine. J. Agric. Food Chem 2011, 59, 5565–5571. [Google Scholar]
- Ikemoto, Y.; Matsuzawa, Y.; Mizutani, J. The effect of antifeedants against the level of biogenic amines in the central nervous system of the Lepidopteran insect (Spodoptera litura). Pesticide Biochem. Physiol 1995, 52, 60–70. [Google Scholar]
- Koul, O. Phytochemicals and insect control: An antifeedant approach. Crit. Rev. Plant Sci 2008, 27, 1–24. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev 1999, 12, 564–582. [Google Scholar]
- Akiyama, K.; Matsuzaki, K.-i.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar]
- Thaler, J.S.; Farag, M.A.; Paré, P.W.; Dicke, M. Jasmonate-deficient plants have reduced direct and indirect defences against Herbivores. Ecol. Lett 2002, 5, 764–774. [Google Scholar]
- Guenther, A. The contribution of reactive carbon emissions from vegetation to the carbon balance of terrestrial ecosystems. Chemosphere 2002, 49, 837–844. [Google Scholar]
- Ormeño, E.; Bousquet-Mélou, A.; Mévy, J.-P.; Greff, S.; Robles, C.; Bonin, G.; Fernandez, C. Effect of intraspecific competition and substrate type on terpene emissions from some mediterranean plant species. J. Chem. Ecol 2007, 33, 277–286. [Google Scholar]
- Millán, M.; Salvador, R.; Mantilla, E.; Artnano, B. Meteorology and photochemical air pollution in southern europe: Experimental results from EC research projects. Atmos. Environ 1996, 30, 1909–1924. [Google Scholar]
- Karban, R.; Baldwin, I.T.; Baxter, K.J.; Laue, G.; Felton, G.W. Communication between plants: Induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 2000, 125, 66–71. [Google Scholar]
- Ninkovic, V. Volatile communication between barley plants affects biomass allocation. J. Exp. Bot 2003, 54, 1931–1939. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plantsand other organisms. Annu. Rev. Plant Biol 2006, 57, 233–266. [Google Scholar]
- Ormeño, E.; Fernandez, C.; Mévy, J.-P. Plant coexistence alters terpene emission and content of mediterranean species. Phytochemistry 2007, 68, 840–852. [Google Scholar]
- Karban, R.; Shiojiri, K. Self-recognition affects plant communication and defense. Ecol. Lett 2009, 12, 502–506. [Google Scholar]
- Ninkovic, V.; Åhman, I. Aphid acceptance of Hordeum genotypes is affected by plant volatile exposure and is correlated with aphid growth. Euphytica 2009, 169, 177–185. [Google Scholar]
- Karban, R.; Shiojiri, K.; Ishizaki, S.; Wetzel, W.C.; Evans, R.Y. Kin recognition affects plant communication and defence. Proc. R. Soc. B 2013. [Google Scholar] [CrossRef]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite profiling of sesquiterpene lactones from Lactuca species. J. Biol. Chem 2000, 275, 26877–26884. [Google Scholar]
- An, Y.; Shen, Y.-B.; Wu, L.-J.; Zhang, Z.-X. A change of phenolic acids content in poplar leaves induced by methyl salicylate and methyl jasmonate. J. For. Res 2006, 17, 107–110. [Google Scholar]
- Preston, C.A.; Betts, H.; Baldwin, I.T. Methyl jasmonate as an allelopathic agent: Sagebrush inhibits germination of a neighboring tobacco, Nicotiana Attenuata. J. Chem. Ecol 2002, 28, 2343–2369. [Google Scholar]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci 2010, 15, 126–132. [Google Scholar]
- Köllner, T.G.; Schnee, C.; Gershenzon, J.; Degenhardt, J. The sesquiterpene hydrocarbons of maize (Zea mays) form five groups with distinct developmental and organ-specific distributions. Phytochemistry 2004, 65, 1895–1902. [Google Scholar]
- Ma, C.; Wang, H.; Lu, X.; Xu, G.; Liu, B. Metabolic fingerprinting investigation of Artemisia annua L. in different stages of development by gas chromatography and gas chromatography-mass spectrometry. J. Chromatog. A 2008, 1186, 412–419. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780-12805. https://doi.org/10.3390/ijms140612780
Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids Lactones: Benefits to Plants and People. International Journal of Molecular Sciences. 2013; 14(6):12780-12805. https://doi.org/10.3390/ijms140612780
Chicago/Turabian StyleChadwick, Martin, Harriet Trewin, Frances Gawthrop, and Carol Wagstaff. 2013. "Sesquiterpenoids Lactones: Benefits to Plants and People" International Journal of Molecular Sciences 14, no. 6: 12780-12805. https://doi.org/10.3390/ijms140612780