Role of EZH2 in the Growth of Prostate Cancer Stem Cells Isolated from LNCaP Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. The LNcap Cell Line Contained More CD44+/CD133+ Cells
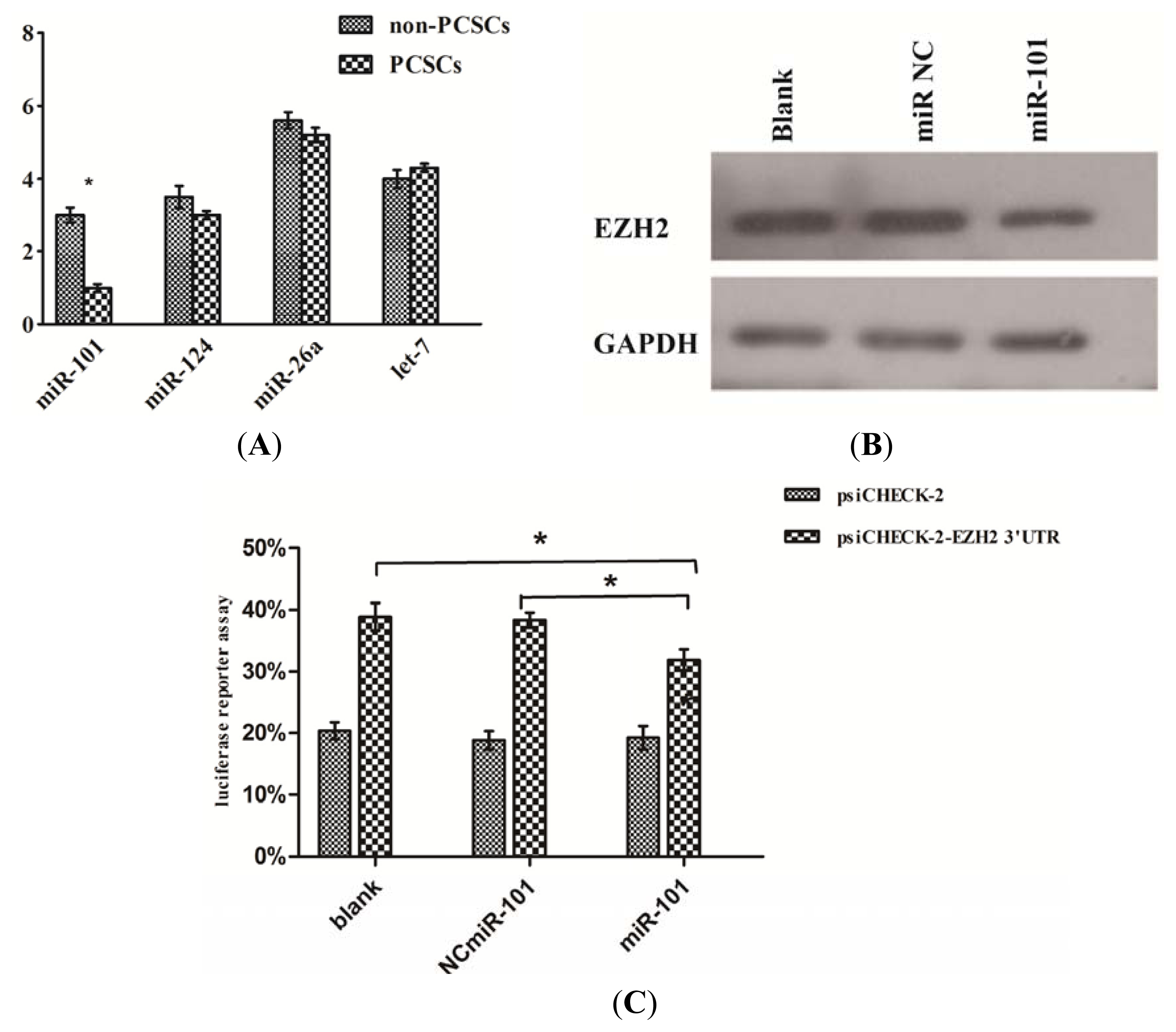
2.2. EZH2 Was Up-Regulated in PCSCs Compared with Non-PCSCs
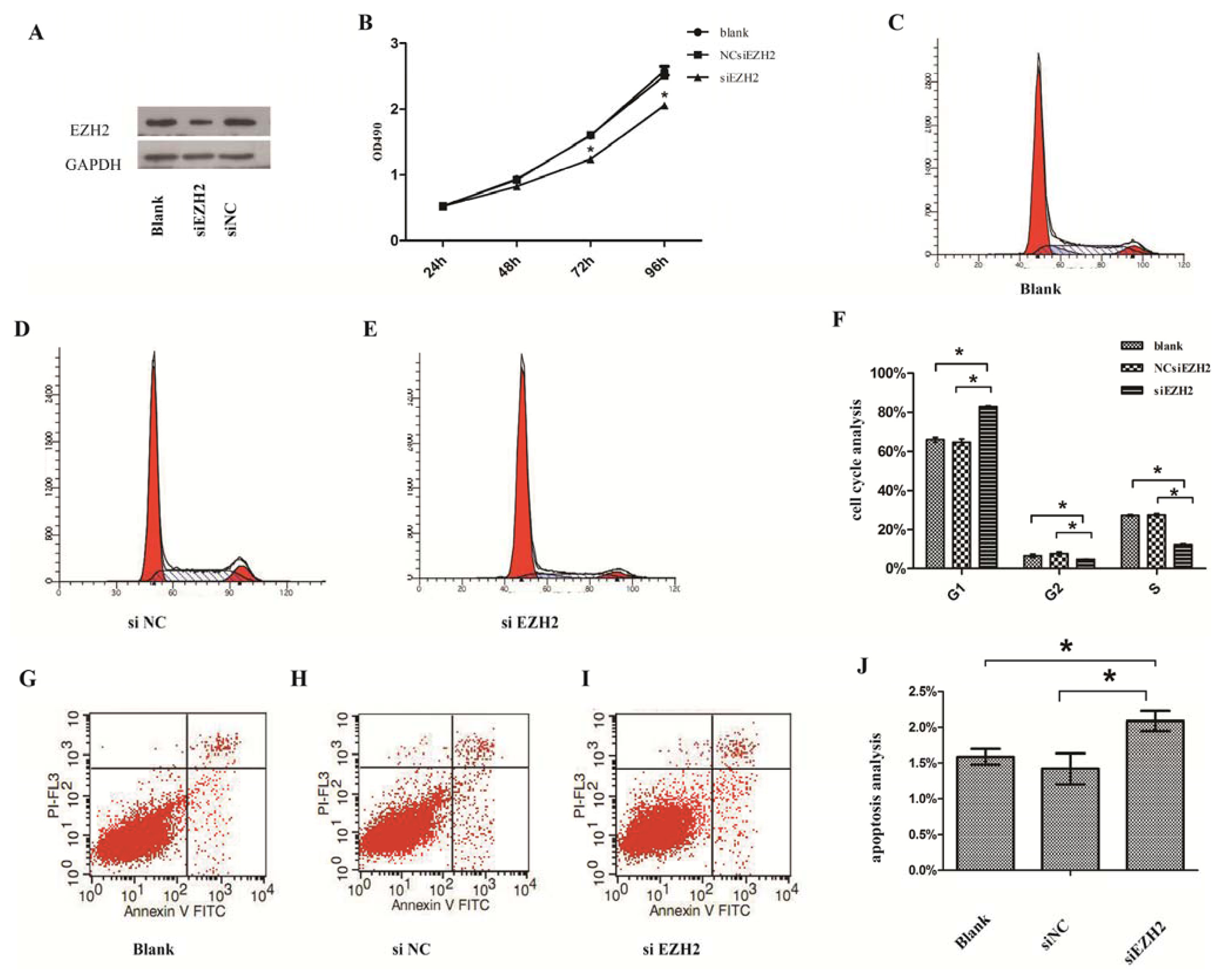
2.3. Silencing of EZH2 Suppressed Proliferation and Inhibits Cell Cycle in PCSCs
2.4. Silencing of EZH2 Promoted the Progression of Apoptosis
2.5. Cyclin E2 as a Cell-Cycle Regulator Was Suppressed by siEZH2
2.6. EZH2 Was a Direct Target of miR-101 in Prostate Cancer Stem Cells
2.7. Discussion
3. Experimental Section
3.1. Cell Culture
3.2. Fluorescence Activated Cell Sorting
3.3. RT-PCR Analysis of mRNAs and miRNA Expression
3.4. Western Blotting
3.5. Cell Proliferation Assay
3.6. Cell Cycle Analysis
3.7. Apoptosis Assays
3.8. Luciferase Reporter Assay
3.9. Transient Transfection
3.10. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-11981-s001.pdfAcknowledgments
Conflict of Interest
References
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar]
- Saraon, P.; Jarvi, K.; Diamandis, E.P. Molecular alterations during progression of prostate cancer to androgen independence. Clin. Chem 2011, 57, 1366–1375. [Google Scholar]
- Al-Hajj, M. Cancer stem cells and oncology therapeutics. Curr. Opin. Oncol 2007, 19, 61–64. [Google Scholar]
- Polyak, K.; Hahn, W.C. Roots and stems: Stem cells in cancer. Nat. Med 2006, 12, 296–300. [Google Scholar]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med 2006, 355, 1253–1261. [Google Scholar]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005, 65, 10946–10951. [Google Scholar]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; de Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar]
- Marian, C.O.; Shay, J.W. Prostate tumor-initiating cells: A new target for telomerase inhibition therapy? Biochim. Biophys. Acta 2009, 1792, 289–296. [Google Scholar]
- Richardson, G.D.; Robson, C.N.; Lang, S.H.; Neal, D.E.; Maitland, N.J.; Collins, A.T. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci 2004, 117, 3539–3545. [Google Scholar]
- Sewalt, R.G.; van der Vlag, J.; Gunster, M.J.; Hamer, K.M.; den Blaauwen, J.L.; Satijn, D.P.; Hendrix, T.; van Driel, R.; Otte, A.P. Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes. Mol. Cell. Biol 1998, 18, 3586–3595. [Google Scholar]
- Raman, J.D.; Mongan, N.P.; Tickoo, S.K.; Boorjian, S.A.; Scherr, D.S.; Gudas, L.J. Increased expression of the polycomb group gene, EZH2, in transitional cell carcinoma of the bladder. Clin. Cancer Res 2005, 11, 8570–8576. [Google Scholar]
- Burdach, S.; Plehm, S.; Unland, R.; Dirksen, U.; Borkhardt, A.; Staege, M.S.; Müller-Tidow, C.; Richter, G.H. Epigenetic maintenance of stemness and malignancy in peripheral neuroectodermal tumors by EZH2. Cell Cycle 2009, 8, 1991–1996. [Google Scholar]
- O’Carroll, D.; Erhardt, S.; Pagani, M.; Barton, S.C.; Surani, M.A.; Jenuwein, T. The polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell. Biol 2001, 21, 4330–4336. [Google Scholar]
- Aoki, R.; Chiba, T.; Miyagi, S.; Negishi, M.; Konuma, T.; Taniguchi, H.; Ogawa, M.; Yokosuka, O.; Iwama, A. The polycomb group gene product Ezh2 regulates proliferation and differentiation of murine hepatic stem/progenitor cells. J. Hepatol 2010, 52, 854–863. [Google Scholar]
- Sher, F.; Rossler, R.; Brouwer, N.; Balasubramaniyan, V.; Boddeke, E.; Copray, S. Differentiation of neural stem cells into oligodendrocytes: Involvement of the polycomb group protein Ezh2. Stem Cells 2008, 26, 2875–2883. [Google Scholar]
- Ezhkova, E.; Pasolli, H.A.; Parker, J.S.; Stokes, N.; Su, I.H.; Hannon, G.; Tarakhovsky, A.; Fuchs, E. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 2009, 136, 1122–1135. [Google Scholar]
- Varambally, S.; Cao, Q.; Mani, R.S.; Shankar, S.; Wang, X.; Ateeq, B.; Laxman, B.; Cao, X.; Jing, X.; Ramnarayanan, K.; et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 2008, 322, 1695–1699. [Google Scholar]
- Wang, H.J.; Ruan, H.J.; He, X.J.; Ma, Y.Y.; Jiang, X.T.; Xia, Y.J.; Ye, Z.Y.; Tao, H.Q. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur. J. Cancer 2010, 46, 2295–2303. [Google Scholar]
- Friedman, J.M.; Liang, G.; Liu, C.C.; Wolff, E.M.; Tsai, Y.C.; Ye, W.; Zhou, X.; Jones, P.A. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res 2009, 69, 2623–2629. [Google Scholar]
- Li, H.; Tang, D.G. Prostate cancer stem cells and their potential roles in metastasis. J. Surg. Oncol 2011, 103, 558–562. [Google Scholar]
- Zheng, F.; Liao, Y.J.; Cai, M.Y.; Liu, Y.H.; Liu, T.H.; Chen, S.P.; Bian, X.W.; Guan, X.Y.; Lin, M.C.; Zeng, Y.X.; et al. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut 2012, 61, 278–289. [Google Scholar]
- Kong, D.; Heath, E.; Chen, W.; Cher, M.L.; Powell, I.; Heilbrun, L.; Li, Y.; Ali, S.; Sethi, S.; Hassan, O.; et al. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One 2012. [Google Scholar] [CrossRef]
- Cai, K.; Wan, Y.; Sun, G.; Shi, L.; Bao, X.; Wang, Z. Let-7a inhibits proliferation and induces apoptosis by targeting EZH2 in nasopharyngeal carcinoma cells. Oncol. Rep 2012, 28, 2101–2106. [Google Scholar]
- Tzatsos, A.; Paskaleva, P.; Lymperi, S.; Contino, G.; Stoykova, S.; Chen, Z.; Wong, K.K.; Bardeesy, N. Lysine-specific demethylase 2B (KDM2B)-let-7-enhancer of zester homolog 2 (EZH2) pathway regulates cell cycle progression and senescence in primary cells. J. Biol. Chem 2011, 286, 33061–33069. [Google Scholar]
- Lu, J.; He, M.L.; Wang, L.; Chen, Y.; Liu, X.; Dong, Q.; Chen, Y.C.; Peng, Y.; Yao, K.T.; Kung, H.F.; et al. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res 2011, 71, 225–233. [Google Scholar]
- Jansen, M.P.; Reijm, E.A.; Sieuwerts, A.M.; Ruigrok-Ritstier, K.; Look, M.P.; Rodríguez-González, F.G.; Heine, A.A.; Martens, J.W.; Sleijfer, S.; Foekens, J.A.; et al. High miR-26a and low CDC2 levels associate with decreased EZH2 expression and with favorable outcome on tamoxifen in metastatic breast cancer. Breast Cancer Res. Treat 2012, 133, 937–947. [Google Scholar]
- Wang, Z.A.; Shen, M.M. Revisiting the concept of cancer stem cells in prostate cancer. Oncogene 2011, 30, 1261–1271. [Google Scholar]
- Boyer, L.A.; Plath, K.; Zeitlinger, J.; Brambrink, T.; Medeiros, L.A.; Lee, T.I.; Levine, S.S.; Wernig, M.; Tajonar, A.; Ray, M.K.; et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 2006, 441, 349–353. [Google Scholar]
- Suva, M.L.; Riggi, N.; Janiszewska, M.; Radovanovic, I.; Provero, P.; Stehle, J.-C.; Baumer, K.; Bitoux, M.-A.L.; Marino, D.; Cironi, L.; et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res 2009, 69, 9211–9218. [Google Scholar]
- Asangani, I.A.; Ateeq, B.; Cao, Q.; Dodson, L.; Pandhi, M.; Kunju, L.P.; Mehra, R.; Lonigro, R.J.; Siddiqui, J.; Palanisamy, N.; et al. Characterization of the EZH2-MMSET histone methyltransferase regulatory axis in cancer. Mol. Cell 2013, 49, 80–93. [Google Scholar]
- West, N.R.; Murray, J.I.; Watson, P.H. Oncostatin-M promotes phenotypic changes associated with mesenchymal and stem cell-like differentiation in breast cancer. Oncogene 2013. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Yang, L.; Dong, L.; Lin, C.; Zhang, J.; Lin, P.; Wang, X. Morusin inhibits human cervical cancer stem cell growth and migration through attenuation of NF-kappaB activity and apoptosis induction. Mol. Cell Biochem 2013, 379, 7–18. [Google Scholar]
- Chang, C.J.; Yang, J.Y.; Xia, W.; Chen, C.T.; Xie, X.; Chao, C.H.; Woodward, W.A.; Hsu, J.M.; Hortobagyi, G.N.; Hung, M.C. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell 2011, 19, 86–100. [Google Scholar]
- Muller-Tidow, C.; Metzger, R.; Kugler, K.; Diederichs, S.; Idos, G.; Thomas, M.; Dockhorn-Dworniczak, B.; Schneider, P.M.; Koeffler, H.P.; Berdel, W.E.; et al. Cyclin E is the only cyclin-dependent kinase 2-associated cyclin that predicts metastasis and survival in early stage non-small cell lung cancer. Cancer Res 2001, 61, 647–653. [Google Scholar]
- Bracken, A.P.; Pasini, D.; Capra, M.; Prosperini, E.; Colli, E.; Helin, K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. Embo. J 2003, 22, 5323–5335. [Google Scholar]
- Fan, X.; Liu, S.; Su, F.; Pan, Q.; Lin, T. Effective enrichment of prostate cancer stem cells from spheres in a suspension culture system. Urol. Oncol 2012, 30, 314–318. [Google Scholar]
- SPSS, version 18.0; software for analyzing data; SPSS Inc: Chicago, IL, USA, 2010.


© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, K.; Liu, C.; Zhou, B.; Bi, L.; Huang, H.; Lin, T.; Xu, K. Role of EZH2 in the Growth of Prostate Cancer Stem Cells Isolated from LNCaP Cells. Int. J. Mol. Sci. 2013, 14, 11981-11993. https://doi.org/10.3390/ijms140611981
Li K, Liu C, Zhou B, Bi L, Huang H, Lin T, Xu K. Role of EZH2 in the Growth of Prostate Cancer Stem Cells Isolated from LNCaP Cells. International Journal of Molecular Sciences. 2013; 14(6):11981-11993. https://doi.org/10.3390/ijms140611981
Chicago/Turabian StyleLi, Kuiqing, Cheng Liu, Bangfen Zhou, Liangkuan Bi, Hai Huang, Tianxin Lin, and Kewei Xu. 2013. "Role of EZH2 in the Growth of Prostate Cancer Stem Cells Isolated from LNCaP Cells" International Journal of Molecular Sciences 14, no. 6: 11981-11993. https://doi.org/10.3390/ijms140611981



