Versatile Redox Chemistry Complicates Antioxidant Capacity Assessment: Flavonoids as Milieu-Dependent Anti- and Pro-Oxidants
Abstract
:1. Introduction
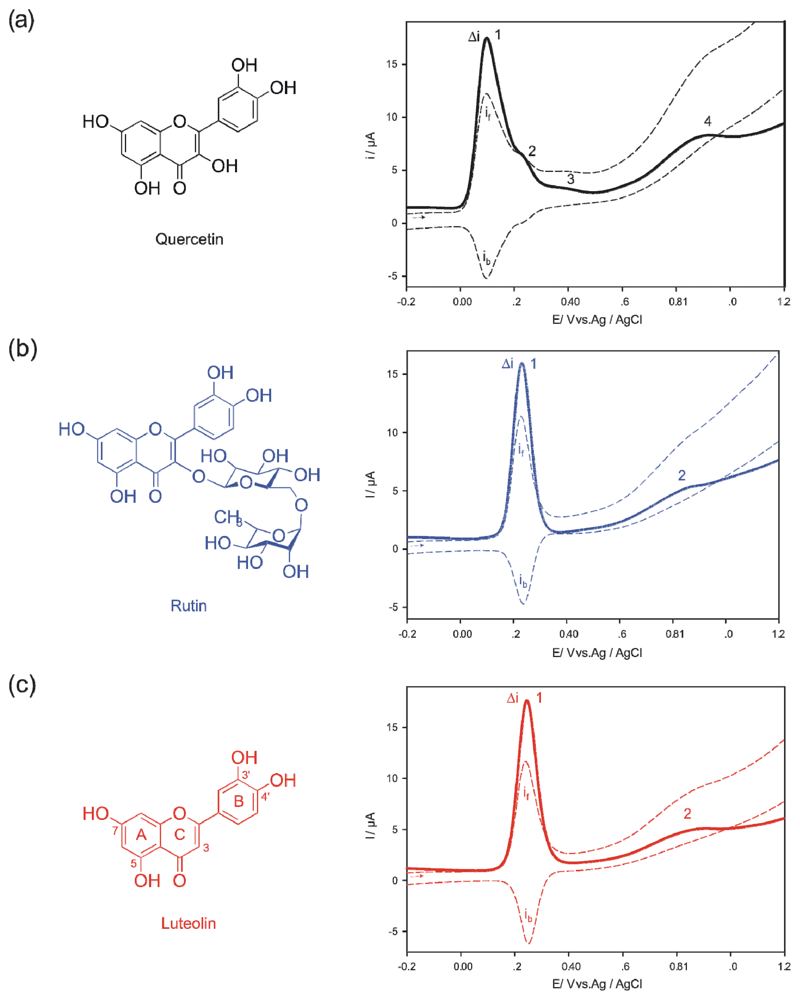
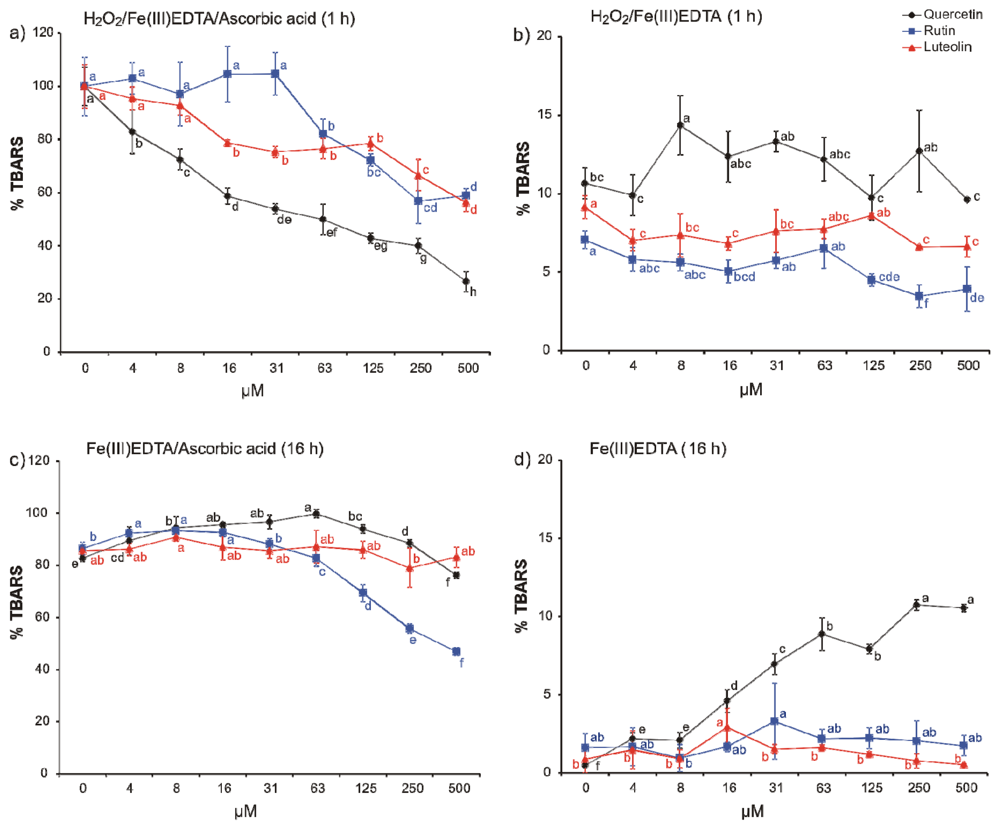
2. Results and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Square-Wave Voltammetry
3.3. Deoxyribose Degradation Assay Variants
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev 2002, 82, 47–95. [Google Scholar]
- Kell, D.B. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch. Toxicol 2010, 84, 825–889. [Google Scholar]
- Halliwell, B. Free radicals and antioxidants—quo vadis? Trends Pharmacol. Sci 2011, 32, 125–130. [Google Scholar]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Meth 2009, 2, 41–60. [Google Scholar]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric 2006, 86, 2046–2056. [Google Scholar]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem 2005, 53, 1841–1856. [Google Scholar]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar]
- Aruoma, O.I. Deoxyribose Assay for Detecting Hydroxyl Radicals. In Oxygen Radicals in Biological Systems; Sies, H., Abelson, J., Melvin, S., Eds.; Academic Press Inc: San Diego, CA, USA, 1994; Volume 233, pp. 57–66. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method—A simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem 1987, 165, 215–219. [Google Scholar]
- Moore, J.; Yin, J.J.; Yu, L.L. Novel fluorometric assay for hydroxyl radical scavenging capacity (HOSC) estimation. J. Agric. Food Chem 2006, 54, 617–626. [Google Scholar]
- Saran, M.; Summer, K.H. Assaying for hydroxyl radicals: Hydroxylated terephthalate is a superior fluorescence marker than hydroxylated benzoate. Free Radic. Res 1999, 31, 429–436. [Google Scholar]
- Stoyanova, S.; Geuns, J.; Hideg, E.; van den Ende, W. The food additives inulin and stevioside counteract oxidative stress. Int. J. Food Sci. Nutr 2011, 62, 207–214. [Google Scholar]
- Bonini, M.G.; Rota, C.; Tomasi, A.; Mason, R.P. The oxidation of 2′,7′-dichlorofluorescein to reactive oxygen species: A self-fulfilling prophesy? Free Radic. Biol. Med 2006, 40, 968–975. [Google Scholar]
- Rota, C.; Chignell, C.F.; Mason, R.P. Evidence for free radical formation during the oxidation of 2′,7′-dichlorofluorescein to the fluorescent dye 2′,7′-dichlorofluorescein by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Radic. Biol. Med 1999, 27, 873–881. [Google Scholar]
- Sies, H.; Degroot, H. Role of reactive oxygen species in cell toxicity. Toxicol. Lett. 1992, 64–65, 547–551. [Google Scholar]
- Yin, J.J.; Fu, P.P.; Lutterodt, H.; Zhou, Y.T.; Antholine, W.E.; Wamer, W. Dual role of selected antioxidants found in dietary supplements: Crossover between anti- and pro-oxidant activities in the presence of copper. J. Agric. Food Chem 2012, 60, 2554–2561. [Google Scholar]
- Chobot, V. Simultaneous detection of pro- and antioxidative effects in the variants of the deoxyribose degradation assay. J. Agric. Food Chem. 2010, 58, 2088–2094. Correction. J. Agric. Food Chem 2012, 60, 8772–8772. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med 1996, 20, 933–956. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 2005, 7, 581–591. [Google Scholar]
- Hadacek, F.; Bachmann, G.; Egelmeier, D.; Chobot, V. Hormesis and a chemical raison d’être for secondary plant metabolites. Dose-Response 2011, 9, 79–116. [Google Scholar]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem 1996, 7, 66–76. [Google Scholar]
- Silva, M.M.; Santos, M.R.; Caroco, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res 2002, 36, 1219–1227. [Google Scholar]
- Liu, P.Y.; Li, K.; Zhang, J.; Zhang, D.W.; Lin, H.H.; Yu, X.Q. Who is the king? The α-hydroxy-β-oxo-α,β-enone moiety or the catechol B ring: Relationship between the structure of quercetin derivatives and their pro-oxidative abilities. Chem. Biodivers 2010, 7, 236–244. [Google Scholar]
- Cao, G.H.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med 1997, 22, 749–760. [Google Scholar]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys 2008, 476, 107–112. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 3rd ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2006; p. 250. [Google Scholar]
- Brett, C.M.A.; Oliveira-Brett, A.M. Electrochemistry: Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993; p. 427. [Google Scholar]
- Novak, I.; Šeruga, M.; Komorsky-Lovrić, Š. Square-wave and cyclic voltammetry of epicatechin gallate on glassy carbon electrode. J. Electroanal. Chem 2009, 631, 71–75. [Google Scholar]
- Oliveira-Brett, A.M.; Ghica, M.E. Electrochemical oxidation of quercetin. Electroanalysis 2003, 15, 1745–1750. [Google Scholar]
- Ghica, M.E.; Oliveira-Brett, A.M. Electrochemical oxidation of rutin. Electroanalysis 2005, 17, 313–318. [Google Scholar]
- Liu, A.L.; Zhang, S.B.; Huang, L.Y.; Cao, Y.Y.; Yao, H.; Chen, W.; Lin, X.H. Electrochemical oxidation of luteolin at a glassy carbon electrode and its application in pharmaceutical analysis. Chem. Pharm. Bull 2008, 56, 745–748. [Google Scholar]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta Gen. Subj 2005, 1721, 174–184. [Google Scholar]
- Medvidovic-Kosanovic, M.; Seruga, M.; Jakobek, L.; Novak, I. Electrochemical and Antioxidant Properties of (+)-Catechin, Quercetin and Rutin. Croat. Chem. Acta 2010, 83, 197–207. [Google Scholar]
- Horvathova, K.; Novotny, L.; Vachalkova, A. The free radical scavenging activity of four flavonoids determined by the comet assay. Neoplasma 2003, 50, 291–295. [Google Scholar]
- Brown, J.E.; Khodr, H.; Hider, R.C.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J 1998, 330, 1173–1178. [Google Scholar]
- Tsimogiannis, D.I.; Oreopoulou, V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′,4′-hydroxy substituted members. Innov. Food Sci. Emerg. Technol 2006, 7, 140–146. [Google Scholar]
- Shaw, L.J.; Morris, P.; Hooker, J.E. Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ. Microbiol 2006, 8, 1867–1880. [Google Scholar]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol 2008, 147, 1251–1263. [Google Scholar]
- Orthen, B.; Popp, M.; Smirnoff, N. Hydroxyl radical scavenging properties of cyclitols. Proc. R. Soc. Edinb. Sec. B 1994, 102, 269–272. [Google Scholar]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, E.; van den Ende, W. Towards understanding vacuolar antioxidant mechanisms: A role for fructans? J. Exp. Bot 2013, 64, 1025–1038. [Google Scholar]
- Schubert, M.; Melnikova, A.N.; Mesecke, N.; Zubkova, E.K.; Fortte, R.; Batashev, D.R.; Barth, I.; Sauer, N.; Gamalei, Y.V.; Mamushina, N.S.; et al. Two novel disaccharides, rutinose and methylrutinose, are involved in carbon metabolism in Datisca glomerata. Planta 2010, 231, 507–521. [Google Scholar]
- Martearena, M.R.; Daz, M.; Ellenrieder, G. Synthesis of rutinosides and rutinose by reverse hydrolysis catalyzed by fungal α-l-rhamnosidases. Biocatal. Biotransform 2008, 26, 177–185. [Google Scholar]
- Halliwell, B.; Aeschbach, R.; Loliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol 1995, 33, 601–617. [Google Scholar]
- Genaro-Mattos, T.C.; Dalvi, L.T.; Oliveira, R.G.; Ginani, J.S.; Hermes-Lima, M. Reevaluation of the 2-deoxyribose assay for determination of free radical formation. Biochim. Biophys. Acta Gen. Subj 2009, 1790, 1636–1642. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chobot, V.; Kubicova, L.; Bachmann, G.; Hadacek, F. Versatile Redox Chemistry Complicates Antioxidant Capacity Assessment: Flavonoids as Milieu-Dependent Anti- and Pro-Oxidants. Int. J. Mol. Sci. 2013, 14, 11830-11841. https://doi.org/10.3390/ijms140611830
Chobot V, Kubicova L, Bachmann G, Hadacek F. Versatile Redox Chemistry Complicates Antioxidant Capacity Assessment: Flavonoids as Milieu-Dependent Anti- and Pro-Oxidants. International Journal of Molecular Sciences. 2013; 14(6):11830-11841. https://doi.org/10.3390/ijms140611830
Chicago/Turabian StyleChobot, Vladimir, Lenka Kubicova, Gert Bachmann, and Franz Hadacek. 2013. "Versatile Redox Chemistry Complicates Antioxidant Capacity Assessment: Flavonoids as Milieu-Dependent Anti- and Pro-Oxidants" International Journal of Molecular Sciences 14, no. 6: 11830-11841. https://doi.org/10.3390/ijms140611830





