Digoxin Downregulates NDRG1 and VEGF through the Inhibition of HIF-1α under Hypoxic Conditions in Human Lung Adenocarcinoma A549 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Digoxin Inhibits the Viability of A549 Cells
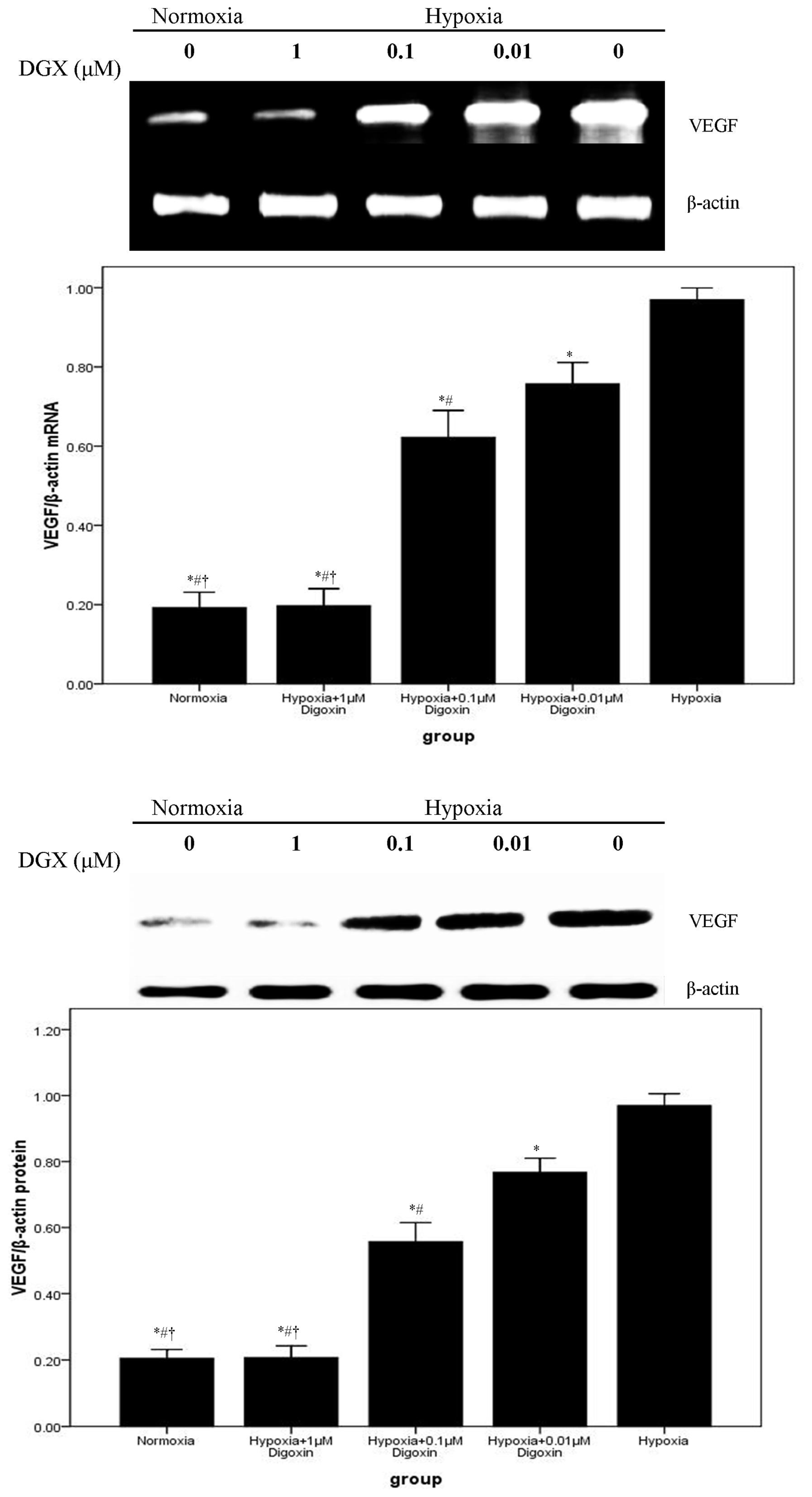
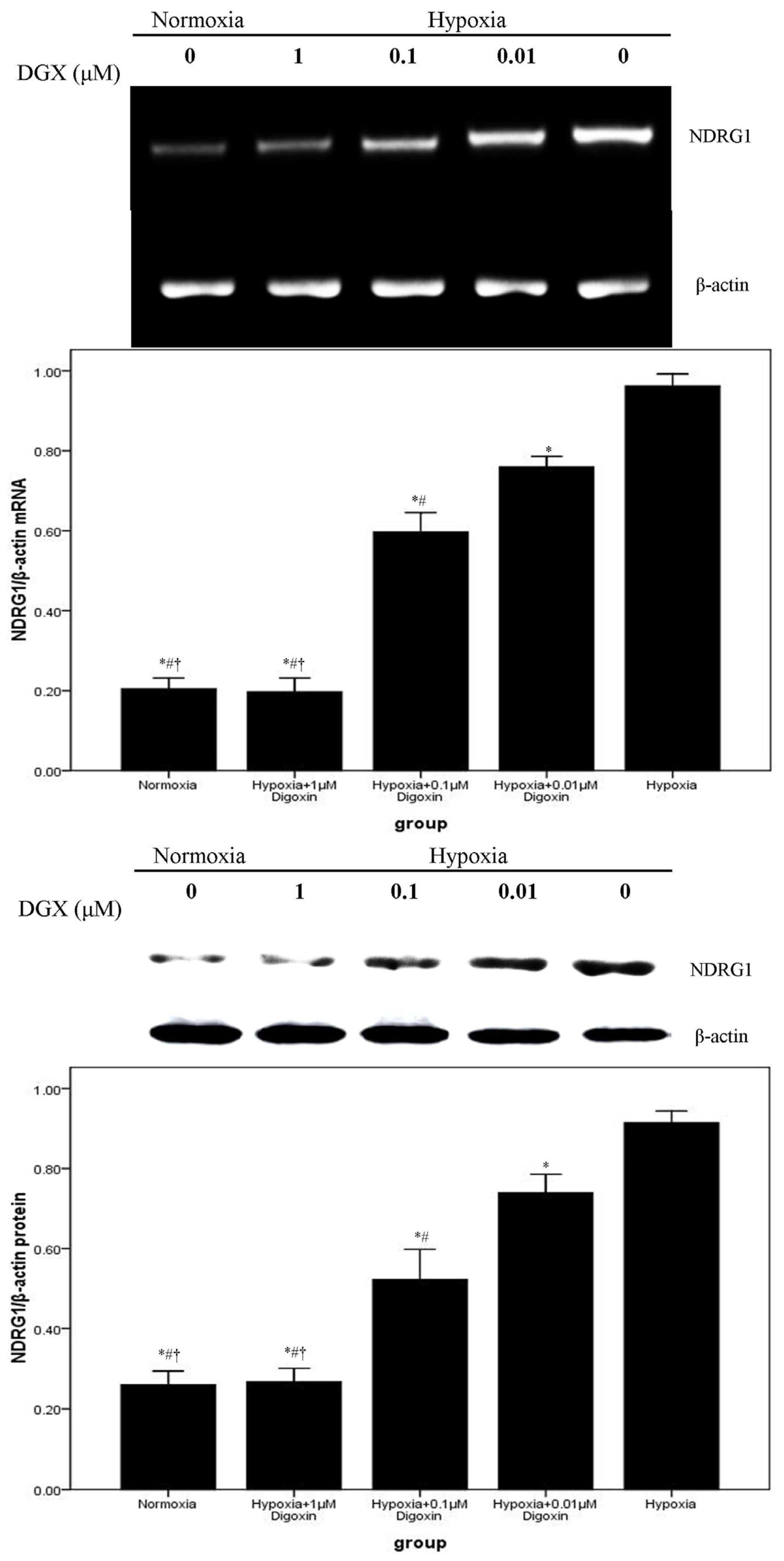
2.2. Digoxin Attenuates mRNA and Protein Expression of VEGF and NDRG1 in A549 Cells
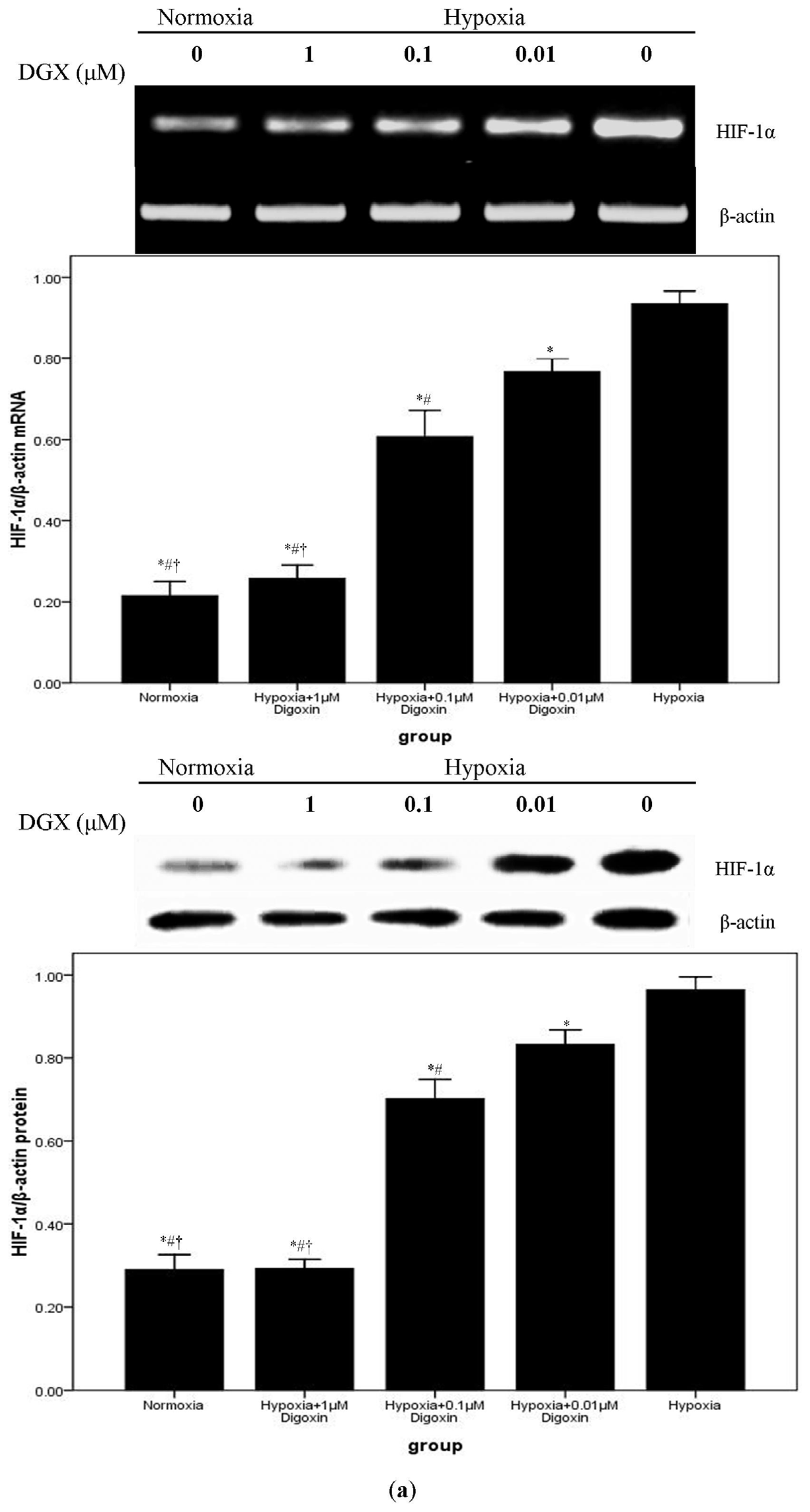
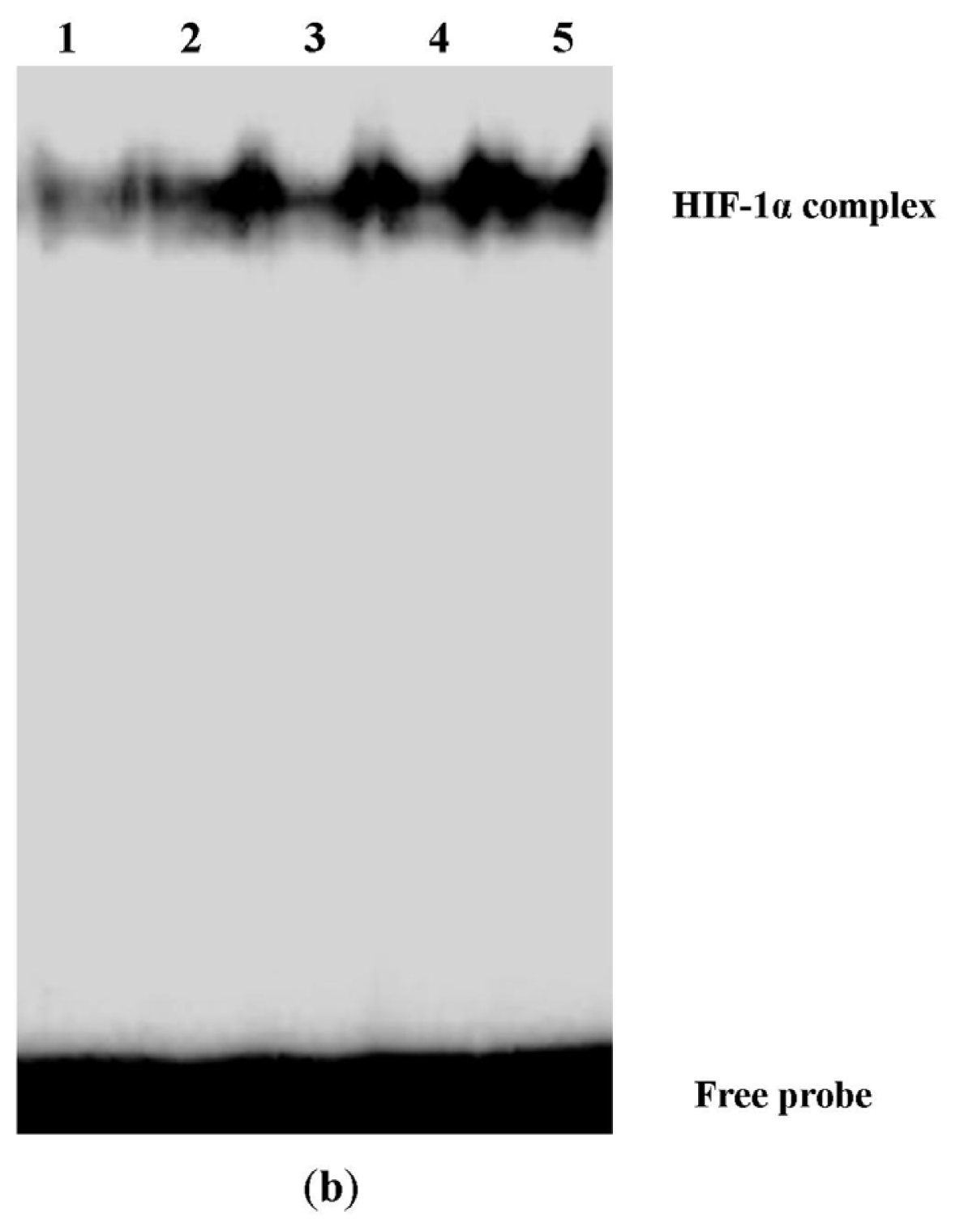
2.3. Digoxin Suppresses mRNA and Protein Expression of HIF-1α and Reduces the Level of HIF-1/DNA Complex in A549 Cells
3. Experiment Section
3.1. Reagents
3.2. Cell Culture and MTT Assay
3.3. RT-PCR
3.4. Western Blotting
3.5. Electrophoretic Mobility Shift Assay (EMSA)
3.6. Statistical Analysis
4. Conclusions
Conflict of Interest
References
- Aisner, D.L.; Marshall, C.B. Molecular pathology of non-small cell lung cancer: A practical guide. Am. J. Clin. Pathol 2012, 138, 332–346. [Google Scholar]
- Cagle, P.T.; Chirieac, L.R. Advances in treatment of lung cancer with targeted therapy. Arch. Pathol. Lab. Med 2012, 136, 504–509. [Google Scholar]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar]
- Hirota, K.; Semenza, G.L. Regulation of angiogenesis by hypoxia inducible factor 1. Crit. Rev. Oncol. Hematol 2006, 59, 15–26. [Google Scholar]
- Brown, J.M.; Giaccia, A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res 1998, 58, 1408–1416. [Google Scholar]
- Harris, A.L. Hypoxia—A key regulatory factor in tumor growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar]
- Kaelin, W.G., Jr; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar]
- Maxwell, P.H. The HIF pathway in cancer. Semin. Cell Dev. Biol 2005, 16, 523–530. [Google Scholar]
- Greijer, A.E.; van der Groep, P.; Kemming, D.; Shvarts, A.; Semenza, G.L.; Meijer, G.A.; van de Wiel, M.A.; Belien, J.A.; van Diest, P.J.; van der Wall, E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J. Pathol 2005, 206, 291–304. [Google Scholar]
- Kaelin, W.G. Proline hydroxylation and gene expression. Annu. Rev. Biochem 2005, 74, 115–128. [Google Scholar]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev 2004, 25, 581–611. [Google Scholar]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar]
- Heroult, M.; Schaffner, F.; Augustin, H.G. Eph receptor and ephrin ligandmediated interactions during angiogenesis and tumor progression. Exp. Cell Res 2006, 312, 642–650. [Google Scholar]
- Chen, C.J.; Hsu, M.H.; Huang, L.J.; Yamori, T.; Chung, J.G.; Lee, F.Y.; Teng, C.M.; Kuo, S.C. Anticancer mechanisms of YC-1 in human lung cancer cell line, NCI-H226. Biochem. Pharmacol 2008, 75, 360–368. [Google Scholar]
- Mojsilovic-Petrovic, J.; Callaghan, D.; Cui, H.; Dean, C.; Stanimirovic, D.B.; Zhang, W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J. Neuroinflamm 2007, 4, 12. [Google Scholar]
- Zhang, L.; Ge, W.; Hu, K.; Zhang, Y.; Li, C.; Xu, X.; He, D.; Zhao, Z.; Zhang, J.; Jie, F.; et al. Endostar down-regulates HIF-1 and VEGF expression and enhances the radioresponse to human lung adenocarcinoma cancer cells. Mol. Biol. Rep 2012, 39, 89–95. [Google Scholar]
- Said, H.M.; Polat, B.; Staab, A.; Hagemann, C.; Stein, S.; Flentje, M.; Theobald, M.; Katzer, A.; Vordermark, D. Rapid detection of the hypoxia-regulated CA-IX and NDRG1 gene expression in different glioblastoma cells in vitro. Oncol. Rep 2008, 20, 413–419. [Google Scholar]
- Said, H.M.; Stein, S.; Hagemann, C.; Polat, B.; Staab, A.; Anacker, J.; Schoemig, B.; Theobald, M.; Flentje, M.; Vordermark, D. Oxygen-dependent regulation of NDRG1 in human glioblastoma cells in vitro and in vivo. Oncol. Rep 2009, 21, 237–246. [Google Scholar]
- Zhang, H.; Qian, D.Z.; Tan, Y.S.; Lee, K.; Gao, P.; Ren, Y.R.; Rey, S.; Hammers, H.; Chang, D.; Pili, R.; et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19579–19586. [Google Scholar]
- Bos, R.; van der Groep, P.; Greijer, A.E.; Shvarts, A.; Meijer, S.; Pinedo, H.M.; Semenza, G.L.; van Diest, P.J.; van der Wall, E. Levels of hypoxia-Inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 2003, 97, 1573–1581. [Google Scholar]
- Oike, T.; Suzuki, Y.; Al-Jahdari, W.; Mobaraki, A.; Saitoh, J.I.; Torikai, K.; Shirai, K.; Nakano, T. Suppression of HIF-1α expression and radiation resistance in acute hypoxic conditions. Exp. Ther. Med 2012, 3, 141–145. [Google Scholar]
- Abud, E.M.; Maylor, J.; Undem, C.; Punjabi, A.; Zaiman, A.L.; Myers, A.C.; Sylvester, J.T.; Semenza, G.L.; Shimoda, L.A. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 1239–1244. [Google Scholar]
- Elbaz, H.A.; Stueckle, T.A.; Tse, W.; Rojanasakul, Y.; Dinu, C.Z. Digitoxin and its analogs as novel cancer therapeutics. Exp. Hematol. Oncol 2012, 1, 4, :1–4:10.. [Google Scholar]
- Liu, L.Z.; Fang, J.; Zhou, Q.; Hu, X.; Shi, X.; Jiang, B.H. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: Implication of chemoprevention of lung cancer. Mol. Pharmacol 2005, 68, 635–643. [Google Scholar]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med 1971, 285, 1182–1186. [Google Scholar]
- Berse, B.; Brown, L.F.; van de Water, L.; Dvorak, H.F.; Senger, D.R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell 1992, 3, 211–220. [Google Scholar]
- Salnikow, K.; Blagosklonny, M.V.; Ryan, H.; Johnson, R.; Costa, M. Carcinogenic nickel induces genes involved with hypoxic stress. Cancer Res 2000, 60, 38–41. [Google Scholar]
- Cangul, H.; Salnikow, K.; Yee, H.; Zagzag, D.; Commes, T.; Costa, M. Enhanced overexpression of an HIF-1/hypoxia-related protein in cancer cells. Environ. Health Perspect 2002, 110, 783–788. [Google Scholar]
- Ellen, T.P.; Ke, Q.; Zhang, P.; Costa, M. NDRG1, a growth and cancer related gene: Regulation of gene expression and function in normal and disease states. Carcinogenesis 2008, 29, 2–8. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Pietras, K.; Ostman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res 2010, 316, 1324–1331. [Google Scholar]
- Wang, Y.; Qiu, Q.; Shen, J.J.; Li, D.D.; Jiang, X.J.; Si, S.Y.; Shao, R.G.; Wang, Z. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int. J. Biochem. Cell Biol 2012, 44, 1813–1824. [Google Scholar]
- Prassas, I.; Karagiannis, G.S.; Batruch, I.; Dimitromanolakis, A.; Datti, A.; Diamandis, E.P. Digitoxin-induced cytotoxicity in cancer cells is mediated through distinct kinase and interferon signaling networks. Mol. Cancer Ther 2011, 10, 2083–2093. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wei, D.; Peng, J.-J.; Gao, H.; Li, H.; Li, D.; Tan, Y.; Zhang, T. Digoxin Downregulates NDRG1 and VEGF through the Inhibition of HIF-1α under Hypoxic Conditions in Human Lung Adenocarcinoma A549 Cells. Int. J. Mol. Sci. 2013, 14, 7273-7285. https://doi.org/10.3390/ijms14047273
Wei D, Peng J-J, Gao H, Li H, Li D, Tan Y, Zhang T. Digoxin Downregulates NDRG1 and VEGF through the Inhibition of HIF-1α under Hypoxic Conditions in Human Lung Adenocarcinoma A549 Cells. International Journal of Molecular Sciences. 2013; 14(4):7273-7285. https://doi.org/10.3390/ijms14047273
Chicago/Turabian StyleWei, Dong, Jing-Jing Peng, Hui Gao, Hua Li, Dong Li, Yong Tan, and Tao Zhang. 2013. "Digoxin Downregulates NDRG1 and VEGF through the Inhibition of HIF-1α under Hypoxic Conditions in Human Lung Adenocarcinoma A549 Cells" International Journal of Molecular Sciences 14, no. 4: 7273-7285. https://doi.org/10.3390/ijms14047273



