Preparation of a Paeonol-Containing Temperature-Sensitive In Situ Gel and Its Preliminary Efficacy on Allergic Rhinitis
Abstract
:Abbreviations
| P407 | poloxamer 407 |
| P188 | poloxamer 188 |
| AR | allergic rhinitis |
| TCM | Traditional Chinese Medicine |
| β-CD | β-cyclodextrin |
| DSC | differential scanning calorimetry |
| LTE4 | leukotriene E4 |
| IgE | immunoglobulin E |
1. Introduction
2. Results and Discussion
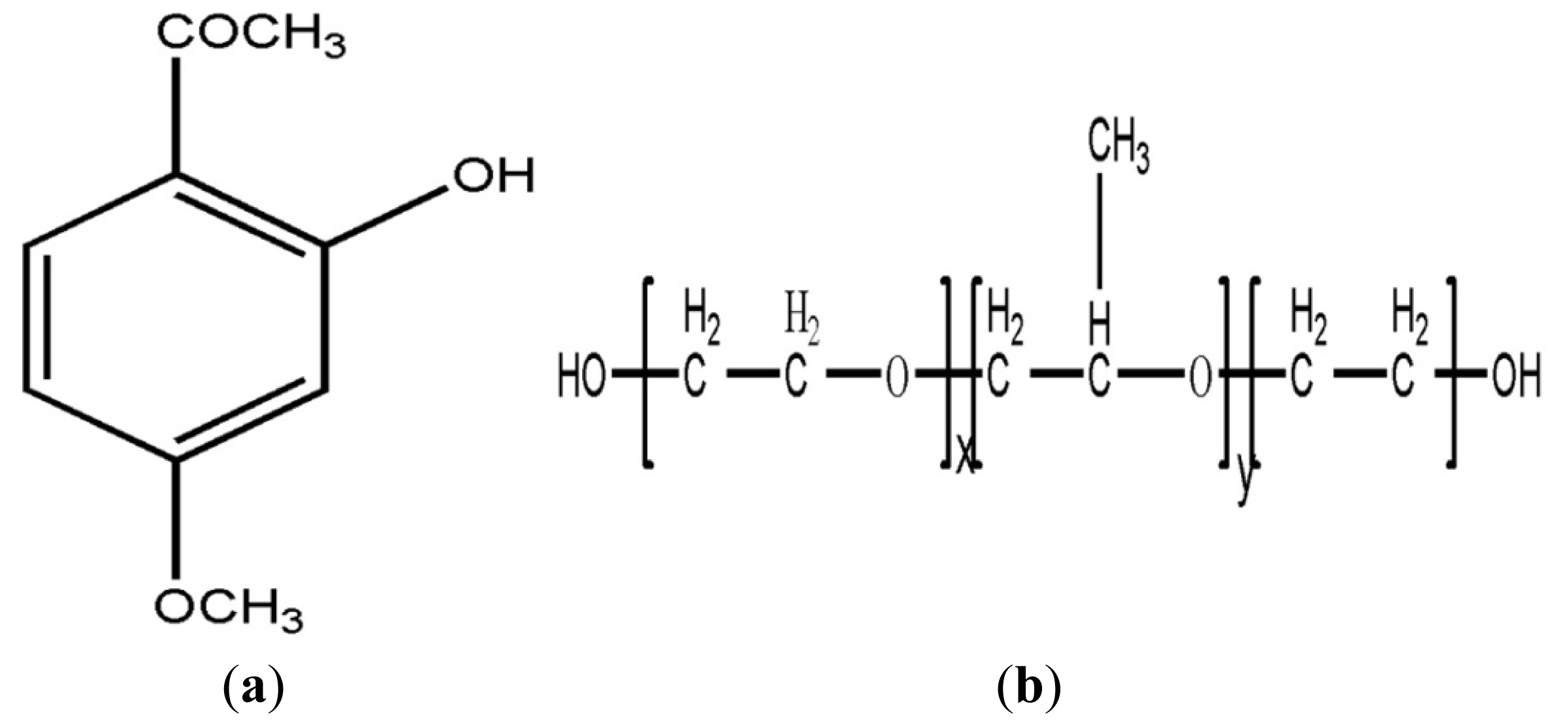
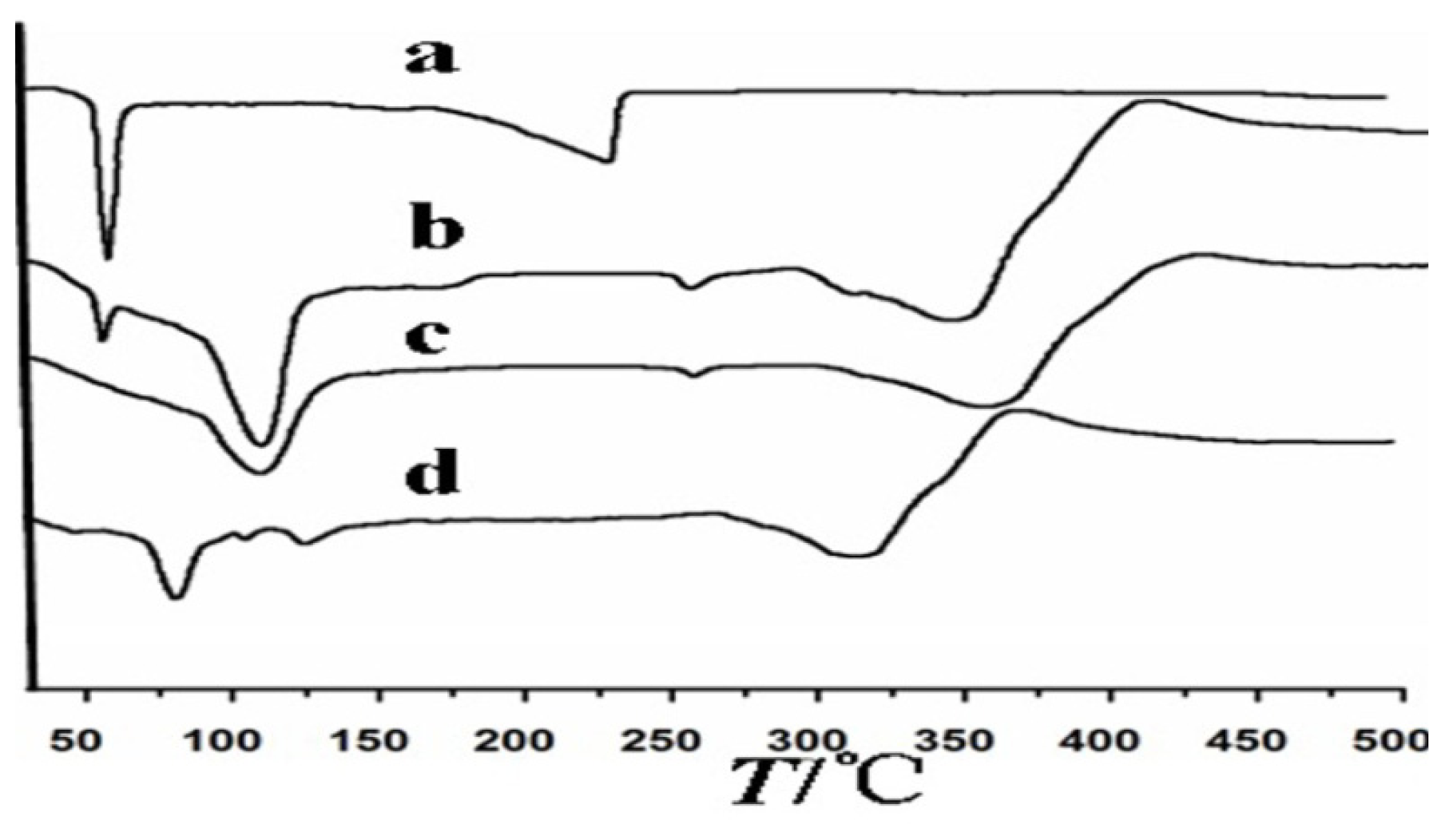
2.1. The Inclusion Complex of Paeonol and β-CD
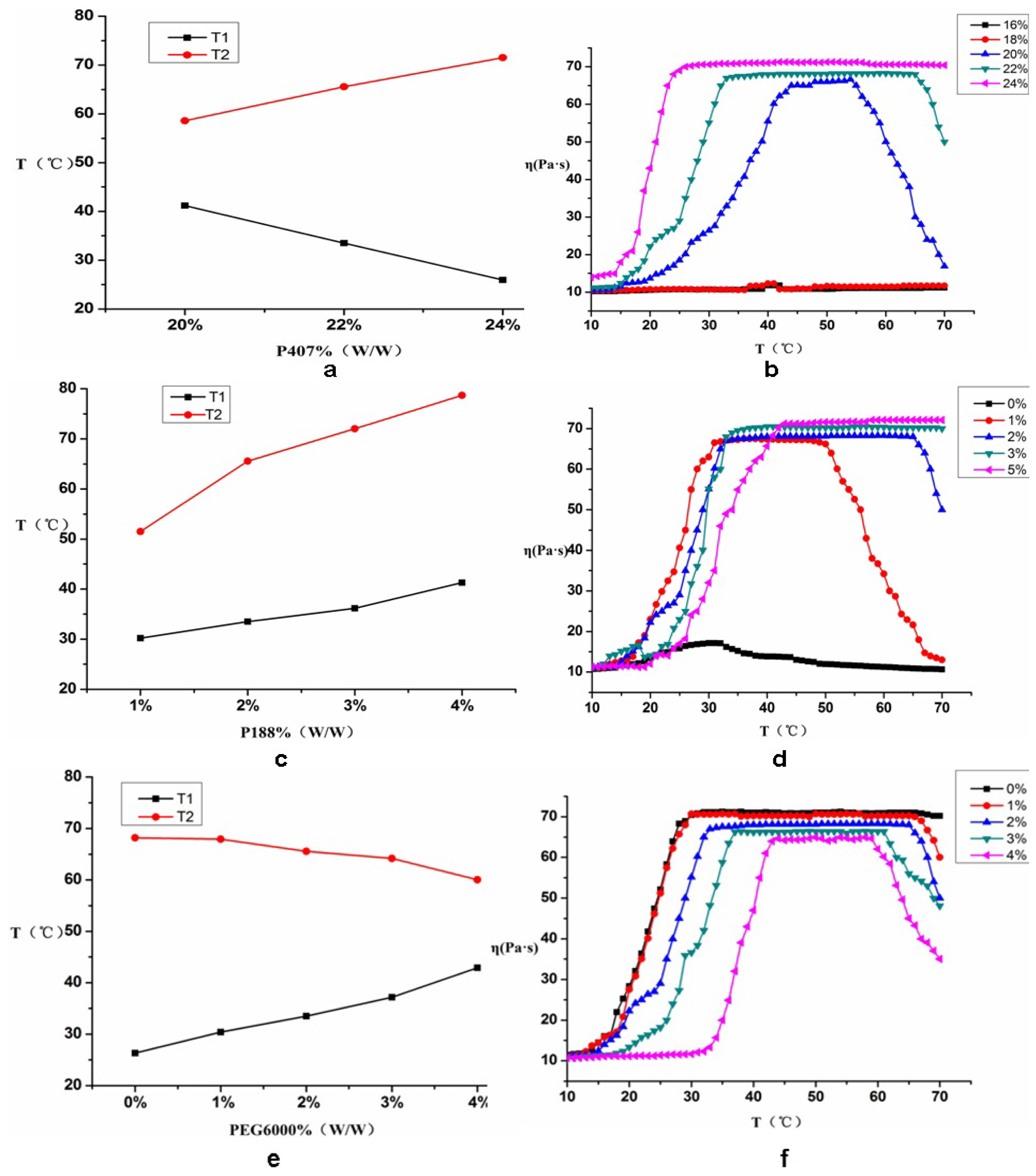
2.2. Composition Screening of the Paeonol Temperature-Sensitive In Situ Gel
2.2.1. Influence of P407 on T1, T2 and η
2.2.2. The Effect of P188 on T1, T2 and η
2.2.3. The Effect of PEG6000 on T1, T2 and η
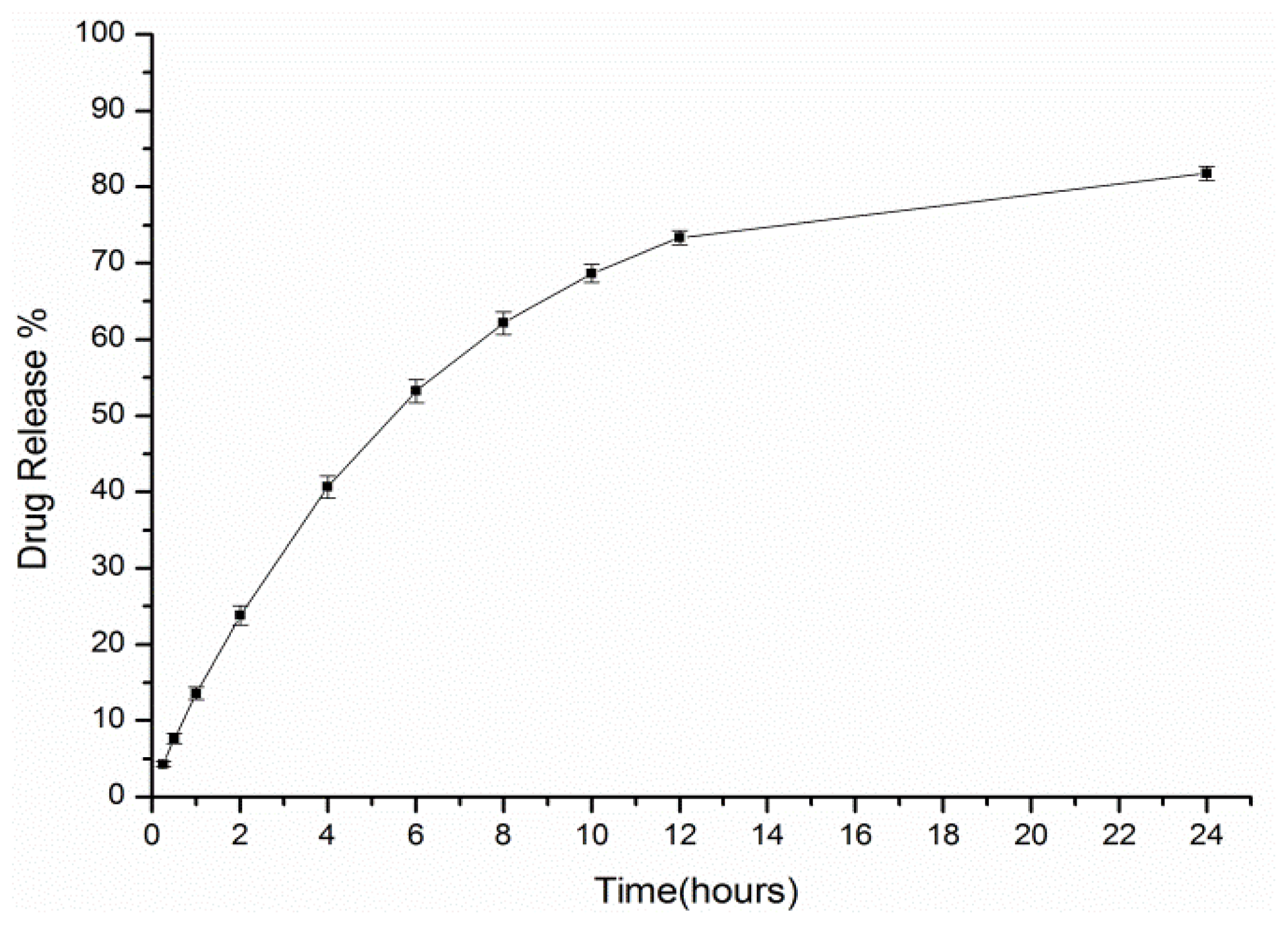
2.3. In Vitro Release Study
2.4. Nasal Ciliary Toxicity Study of the Paeonol Temperature-Sensitive In Situ Gel
2.5. Studies of the Paeonol Temperature-Sensitive In Situ Gel for the Treatment of Allergic Rhinitis
2.5.1. IgE and LTE4 in the Serum
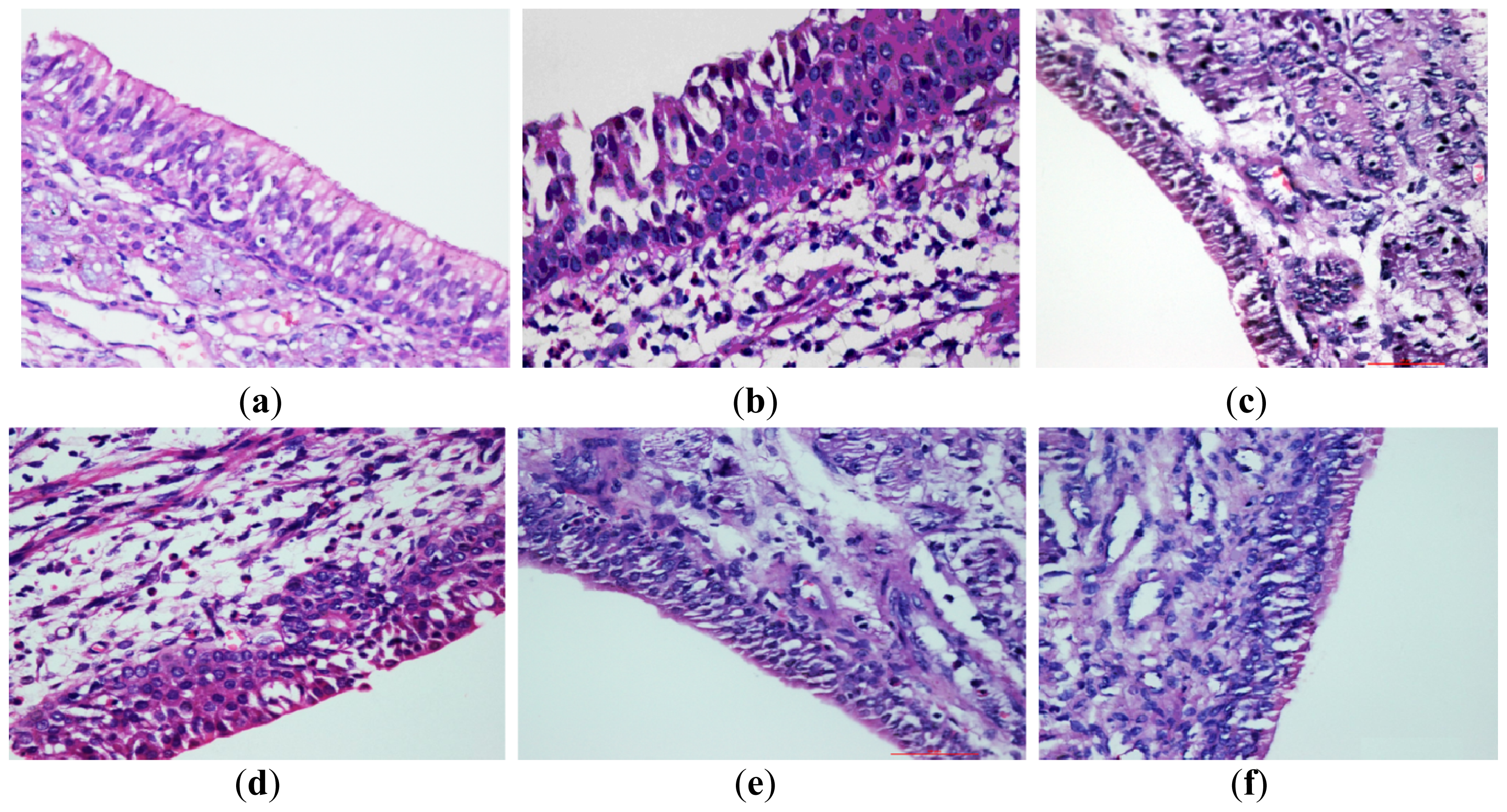
2.5.2. Histological Examination of Nasal Mucosa
3. Experimental Section
3.1. Materials
3.2. Preparation of an Inclusion Complex of Paeonol and β-CD
3.3. The Characterization of the Paeonol-β-CD Complex
3.3.1. Infrared Spectroscopic Analysis
3.3.2. Differential Scanning Calorimetry (DSC) Analysis
3.4. Preparation of the Paeonol Temperature-Sensitive In Situ Gel
3.5. Evaluation of the Paeonol Temperature-Sensitive In Situ Gel
3.5.1. Thermodynamic Study (Gelling Temperature T1 and Gel Melting Temperature T2)
3.5.2. Rheological Properties Study
3.6. In Vitro Release Study
3.7. Nasal Ciliary Toxicity Study
3.8. Preliminary Pharmacodynamic Study
3.8.1. Animals
3.8.2. Grouping and Modeling
3.8.3. Administration and Sample Collection
3.8.4. Determination of IgE and LTE4 in Serum
3.8.5. Histopathological Examination of the Nasal Mucosa
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Schenkel, E.J. Effect of desloratadine on the control of morning symptoms in patients with seasonal and perennial allergic rhinitis. Allergy Asthma Proc 2006, 27, 465–472. [Google Scholar]
- Lu, B. New Techniques and New Dosage Forms of Drugs, 1st ed; People’s Medical Publishing House: Beijing, China, 2002; p. 451. [Google Scholar]
- Gizurason, S. The relevance of nasal physiology to the design of drug absorption studies. Adv. Drug Deliv. Rev 1993, 11, 329–347. [Google Scholar]
- Zhang, C.X.; Zhang, W.T.; Wang, D.K. The research progress of in situ gel, new type of drug delivery system. Chin. J. Hosp. Pharm 2006, 26, 459–461. [Google Scholar]
- Ning, L.L. Characteristics of ophthalmic in situ gel and issues in progress. Chin. J. New Drugs 2007, 16, 988–990. [Google Scholar]
- Schmolka, I.R. Poloxamers in the pharmaceutical industry. In Polymers for Controlled Drug Delivery; Tarcha, P.J., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 189–214. [Google Scholar]
- Chou, T.C. Anti-inflammatory and analgesic effects of paeonol in carrageenan evoked thermal hyperalgesia. Br. J. Pharmacol 2003, 139, 1146–1152. [Google Scholar]
- Ma, Y.L.; Bates, S.S.; Gurney, A.M. The effects of paeonol on the electrophysiological properties of cardiac ventricular myocytes. Eur. J. Pharmacol 2006, 545, 87–92. [Google Scholar]
- Kim, J.; Lee, H.; Lee, Y.; Oh, B.G.; Cho, C.; Kim, Y.; Shin, M.; Hong, M.; Jung, SK.; Bae, H. Inhibition effects of Moutan Cortex Radicis on secretion of eotaxin in A549 human epithelial cells and eosinophil migration. J. Ethnopharmacol. 2007, 114, 186–193. [Google Scholar]
- Kim, S.H.; Kim, S.A.; Park, M.K.; Kim, S.H.; Park, Y.D.; Na, H.J.; Kim, H.M.; Shin, M.K.; Ahn, K.S. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-a. Int. Immunopharmacol 2004, 4, 279–287. [Google Scholar]
- Tang, H.Y.; Yang, S.; Wang, J.B. Verview of preparation process, formulation reform and clinical application of paeonol. Jiangsu J. Trad. Chin. Med 2004, 25, 58–60. [Google Scholar]
- Hirlekar, R.; Kadam, V. Preformulation study of the inclusion complex irbesartan-β-cyelodextrin. AAPS PharmSciTech 2009, 10, 276–281. [Google Scholar]
- Song, T.; Wang, D.K.; Gao, H.; Zhang, C.X.; Ji, B. Thermodynamic and rheological properties of therm osensitive gels for nasal delivery of ribavirin. Chin. J. Pharm 2006, 37, 540–542. [Google Scholar]
- De Santana, D.C.; Pupo, T.T.; Sauaia, M.G.; da Silva, R.S.; Lopez, R.F.V. Nitric oxide photorelease from hydrogels and from skin containing a nitro-ruthenium complex. Int. J. Pharm 2010, 391, 21–28. [Google Scholar]
- Jug, M.; BEeIREvIe-Lacan, M.; Bengez, S. Novel cyclodextrin-based film form ulation intended for buecal delivery of atenolol. Drug Dev. Ind. Pharm 2009, 35, 796–807. [Google Scholar]
- Xu, X.Y. The Identification of principal components of clathrate and complex with infrared spectral subtraction method. Chin. J. Pharm. Anal 1997, 17, 87–89. [Google Scholar]
- Lu, W.Y.; Chen, P.R.; Lin, G.Q. New stereoselective synthesis of thiamphenicol and florfenicol from enantiomerically pure cyanohydrin: A chemo-enzymatic approach. Tetrahedron 2008, 64, 7822–7827. [Google Scholar]
- Schmolka, I.R. Artificial skin I. Preparation and properties and properties of pluronic F-127 gels for the treatment of burns. J. Biomed. Mater. Res 1972, 6, 571–582. [Google Scholar]
- Mao, S.; Shi, Z.; Bi, D. Research of nasal absorption of dipyrone solution. Chin. Pharm. J 1997, 32, 87–90. [Google Scholar]
- Song, C.J.; Wang, Y.; Wang, C.Y. Preparation and evaluation in vivo of scutellaria thermosensitive gel. China J. Chin. Mat. Med 2008, 33, 628–631. [Google Scholar]
- Miyazaki, S.; Nakamura, T.; Takada, M. Thermo-sensitive sol-gel transition of Pluronic F-127. Yakuzaigaku 1991, 51, 36–43. [Google Scholar]
- Azade, T.; Fatemeh, A.; Rassoul, D. Temperature-responsive and biodegradable PVA: PVP k30: poloxamer 407 hydrogel for controlled delivery of human growth hormone (hGH). J. Pediatr. Endocr. Met 2011, 24, 175–179. [Google Scholar]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm 2010, 75, 186–193. [Google Scholar]
- National Pharmacopoeia Committee, Chinese Pharmacopoeia, 1st ed; TCM Science & Technology Publishing House: Beijing, China, 2010; pp. 268–269.
- Jiang, X.G.; Cui, J.B.; Fang, X.L.; Wei, Y.; Xi, N.Z. Toxicity of drugs on nasal mucocilia and the method of its evaluation. Acta Pharm. Sin 1995, 308, 848–853. [Google Scholar]
- Zhang, Q.; Jiang, X.; Xiang, W.; Lu, W.; Su, L.; Shi, Z. Preparation of nimodipineloaded microemulsion for intranasal delivery and evaluation of the targeting efficiency to brain. Int. J. Pharm 2004, 275, 85–96. [Google Scholar]


| Items | Physiological saline | Ephedrine hydrochloride | 1% Sodium deoxycholate | Blank temperaturesensitive in situ gel | Paeonol temperature-sensitive in situ gel |
|---|---|---|---|---|---|
| Continuing swinging time of cilia (min) | 701.60 ± 32.7 | 694. 43 ± 28.21 | 33.4 ± 5.7 * | 676.20 ± 31.3 | 662.60 ± 30.1 |
| Relative percentage (%) | 100.00% | 98.98% | 4.76% * | 96.38% | 94.44% |
| Groups | Number of animals | LTE4 (pg/mL) | IgE (pg/mL) |
|---|---|---|---|
| Blank control group (group A) | 10 | 5.37 ± 2.81 | 0.71 ± 0.45 |
| AR model group (group B) | 10 | 220.88 ± 133.32 ## | 141.38 ± 85.06 ## |
| Rhinocort nasal spray group (group C) | 10 | 32.65 ± 5.60 #* | 18.98 ± 6.93 #* |
| Low-dose of paeonol temperature-sensitive in situ gel group (group D) | 10 | 16.63 ± 4.81 #* | 7.72 ± 2.06 #* |
| Middle-dose of paeonol temperature-sensitive in situ gel group (group E) | 10 | 11.70 ± 2.43 #** | 3.46 ± 1.78 #** |
| High-dose of paeonol temperature-sensitive in situ gel group (group F) | 10 | 10.37 ± 3.03 #** | 3.16 ± 2.54 #** |
| Groups | Number of animals | Number of eosinophil in the nasal mucosa |
|---|---|---|
| Blank control group (group A) | 10 | 4.18 ± 1.66 |
| AR model group (group B) | 10 | 6.46 ± 2.16 ## |
| Rhinocort nasal spray group (group C) | 10 | 3.60 ± 0.81 ##** |
| Low-dose of paeonol temperature-sensitive in situ gel group (group D) | 10 | 4.60 ± 2.28 ##* |
| Middle-dose of paeonol temperature-sensitive in situ gel group (group E) | 10 | 3.36 ± 1.83 ##** |
| High-dose of paeonol temperature-sensitive in situ gel group (group F) | 10 | 3.89 ± 1.32 ##** |
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chu, K.; Chen, L.; Xu, W.; Li, H.; Zhang, Y.; Xie, W.; Zheng, J. Preparation of a Paeonol-Containing Temperature-Sensitive In Situ Gel and Its Preliminary Efficacy on Allergic Rhinitis. Int. J. Mol. Sci. 2013, 14, 6499-6515. https://doi.org/10.3390/ijms14036499
Chu K, Chen L, Xu W, Li H, Zhang Y, Xie W, Zheng J. Preparation of a Paeonol-Containing Temperature-Sensitive In Situ Gel and Its Preliminary Efficacy on Allergic Rhinitis. International Journal of Molecular Sciences. 2013; 14(3):6499-6515. https://doi.org/10.3390/ijms14036499
Chicago/Turabian StyleChu, Kedan, Lidian Chen, Wei Xu, Huang Li, Yuqin Zhang, Weirong Xie, and Jian Zheng. 2013. "Preparation of a Paeonol-Containing Temperature-Sensitive In Situ Gel and Its Preliminary Efficacy on Allergic Rhinitis" International Journal of Molecular Sciences 14, no. 3: 6499-6515. https://doi.org/10.3390/ijms14036499




