Understanding Resolvin Signaling Pathways to Improve Oral Health
Abstract
:1. Introduction
2. Inflammation and Polyunsaturated Fatty Acids
3. Signaling Pathways
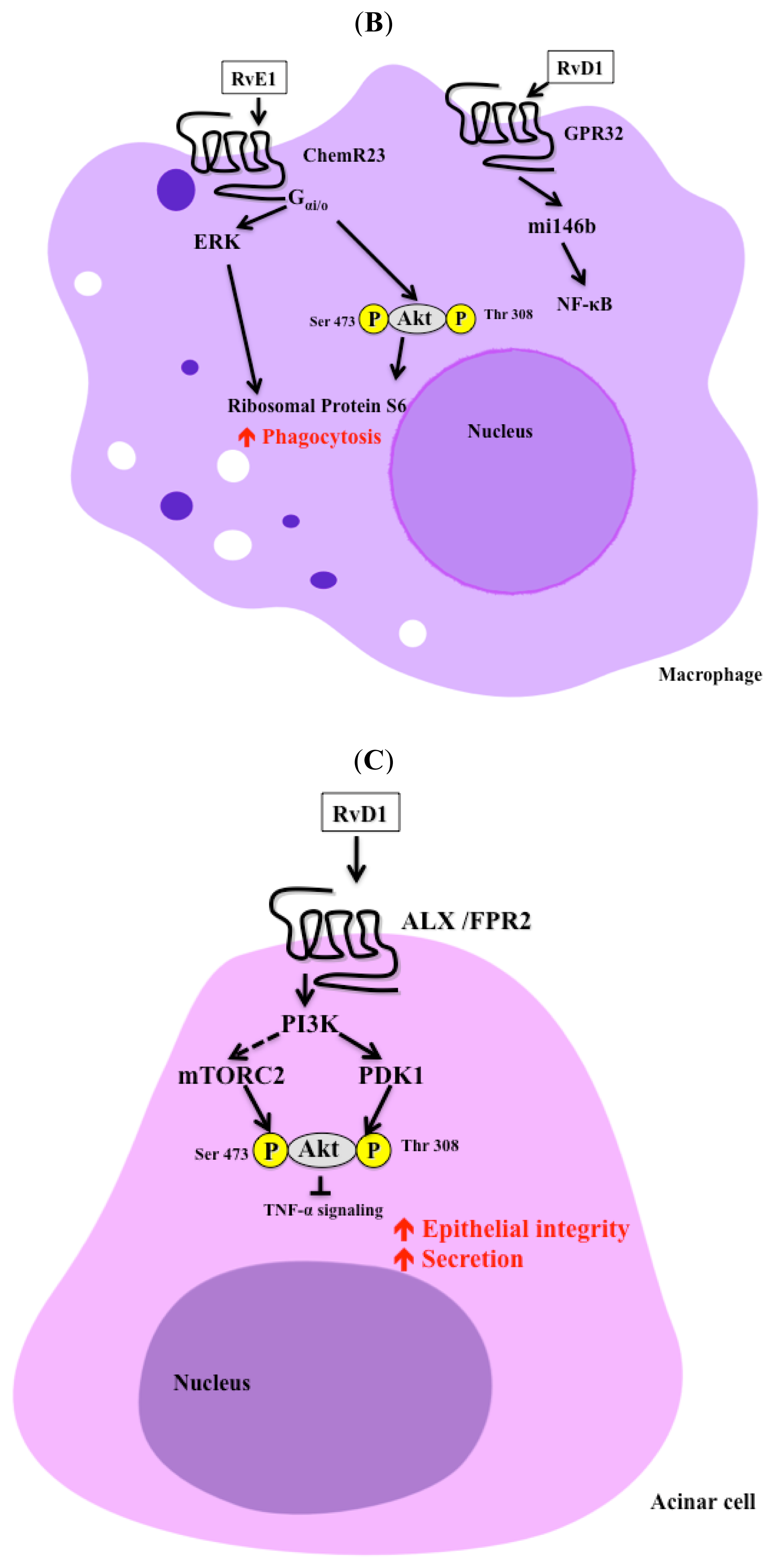
4. Resolvins and Immune System
5. Resolvins and Pain
6. Resolvins and Coagulation
7. Resolvins and Periodontitis
8. Resolvins and Salivary Gland Function
9. Conclusions
Acknowledgements
Conflict of Interest
References
- Fan, Y.Y.; Monk, J.M.; Hou, T.Y.; Callway, E.; Vincent, L.; Weeks, B.; Yang, P.; Chapkin, R.S. Characterization of an arachidonic acid-deficient (Fads1 knockout) mouse model. J. Lipid Res 2012, 53, 1287–1295. [Google Scholar]
- Basu, S.; Nachat-Kappes, R.; Caldefie-Chezet, F.; Vasson, M.P. Eicosanoids and adipokines in breast cancer: From molecular mechanisms to clinical considerations. Antioxid. Redox Signal 2013, 18, 323–360. [Google Scholar]
- Cathcart, M.C.; O’Byrne, K.J.; Reynolds, J.V.; O’Sullivan, J.; Pidgeon, G.P. COX-derived prostanoid pathways in gastrointestinal cancer development and progression: Novel targets for prevention and intervention. Biochim. Biophys. Acta 2012, 1825, 49–63. [Google Scholar]
- Stanger, M.J.; Thompson, L.A.; Young, A.J.; Lieberman, H.R. Anticoagulant activity of select dietary supplements. Nutr. Rev 2012, 70, 107–117. [Google Scholar]
- Pezato, R.; Swierczynska-Krepa, M.; Nizankowska-Mogilnicka, E.; Derycke, L.; Bachert, C.; Perez-Novo, C.A. Role of imbalance of eicosanoid pathways and staphylococcal superantigens in chronic rhinosinusitis. Allergy 2012, 67, 1347–1356. [Google Scholar] [Green Version]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr 2012, 107, S171–S184. [Google Scholar]
- Rogerio, A.P.; Anibal, F.F. Role of leukotrienes on protozoan and helminth infections. Mediators Inflamm 2012, 2012, 595694. [Google Scholar]
- Navab, M.; Reddy, S.T.; van Lenten, B.J.; Buga, G.M.; Hough, G.; Wagner, A.C.; Fogelman, A.M. High-density lipoprotein and 4F peptide reduce systemic inflammation by modulating intestinal oxidized lipid metabolism: Novel hypotheses and review of literature. Arterioscler. Thromb. Vasc. Biol 2012, 32, 2553–2560. [Google Scholar]
- Rius, B.; Lopez-Vicario, C.; Gonzalez-Periz, A.; Moran-Salvador, E.; Garcia-Alonso, V.; Claria, J.; Titos, E. Resolution of inflammation in obesity-induced liver disease. Front. Immunol 2012, 3, 257. [Google Scholar]
- Johnson, G.H.; Fritsche, K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: A systematic review of randomized controlled trials. J. Acad. Nutr. Diet. 2012, 112. [Google Scholar] [CrossRef]
- Calabrese, C.; Triggiani, M.; Marone, G.; Mazzarella, G. Arachidonic acid metabolism in inflammatory cells of patients with bronchial asthma. Allergy 2000, 55, 27–30. [Google Scholar]
- Campbell, W.B. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol. Sci 2000, 21, 125–127. [Google Scholar]
- Morteau, O. Prostaglandins and inflammation: The cyclooxygenase controversy. Arch. Immunol. Ther. Exp 2000, 48, 473–480. [Google Scholar]
- Norling, L.V.; Serhan, C.N. Profiling in resolving inflammatory exudates identifies novel anti-inflammatory and pro-resolving mediators and signals for termination. J. Intern. Med 2010, 268, 15–24. [Google Scholar]
- Gonzalez-Periz, A.; Claria, J. Resolution of adipose tissue inflammation. ScientificWorldJournal 2010, 10, 832–856. [Google Scholar]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat 2012, 97, 73–82. [Google Scholar]
- Zhang, M.J.; Spite, M. Resolvins: Anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu. Rev. Nutr 2012, 32, 203–227. [Google Scholar]
- Samuelsson, B.; Dahlen, S.E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and lipoxins: Structures, biosynthesis, and biological effects. Science 1987, 237, 1171–1176. [Google Scholar]
- Zeldin, D.C. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem 2001, 276, 36059–36062. [Google Scholar]
- Kristensen, S.D.; de Caterina, R.; Schmidt, E.B.; Endres, S. Fish oil and ischaemic heart disease. Br. Heart J 1993, 70, 212–214. [Google Scholar]
- Khair-el-Din, T.A.; Sicher, S.C.; Vazquez, M.A.; Wright, W.J.; Lu, C.Y. Docosahexaenoic acid, a major constituent of fetal serum and fish oil diets, inhibits IFN gamma-induced Ia-expression by murine macrophages in vitro. J. Immunol 1995, 154, 1296–1306. [Google Scholar]
- McLennan, P.; Howe, P.; Abeywardena, M.; Muggli, R.; Raederstorff, D.; Mano, M.; Rayner, T.; Head, R. The cardiovascular protective role of docosahexaenoic acid. Eur. J. Pharmacol 1996, 300, 83–89. [Google Scholar]
- Rapp, J.H.; Connor, W.E.; Lin, D.S.; Porter, J.M. Dietary eicosapentaenoic acid and docosahexaenoic acid from fish oil. Their incorporation into advanced human atherosclerotic plaques. Arterioscler. Thromb 1991, 11, 903–911. [Google Scholar]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med 2002, 196, 1025–1037. [Google Scholar]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.L.; Serhan, C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem 2003, 278, 14677–14687. [Google Scholar]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med 2005, 201, 713–722. [Google Scholar]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med 2000, 192, 1197–1204. [Google Scholar]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Gronert, K.; Chiang, N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: A novel mechanism for NSAID and N-3 PUFA therapeutic actions. J. Physiol. Pharmacol 2000, 51, 643–654. [Google Scholar]
- Halpern, G.M. Anti-inflammatory effects of a stabilized lipid extract of Perna canaliculus (Lyprinol). Allerg. Immunol 2000, 32, 272–278. [Google Scholar]
- Pompeia, C.; Lopes, L.R.; Miyasaka, C.K.; Procopio, J.; Sannomiya, P.; Curi, R. Effect of fatty acids on leukocyte function. Braz. J. Med. Biol. Res 2000, 33, 1255–1268. [Google Scholar]
- Serhan, C.N.; Chiang, N.; van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol 2008, 8, 349–361. [Google Scholar]
- Serhan, C.N. Controlling the resolution of acute inflammation: A new genus of dual anti-inflammatory and proresolving mediators. J. Periodontol 2008, 79, 1520–1526. [Google Scholar]
- Serhan, C.N.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol 2008, 153, S200–S215. [Google Scholar]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; Inoue, M.; Arai, H. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem 2012, 287, 10525–10534. [Google Scholar]
- Bannenberg, G.L.; Chiang, N.; Ariel, A.; Arita, M.; Tjonahen, E.; Gotlinger, K.H.; Hong, S.; Serhan, C.N. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol 2005, 174, 4345–4355. [Google Scholar]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Arita, M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: An overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat 2004, 73, 155–172. [Google Scholar]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem 2007, 282, 9323–9334. [Google Scholar]
- Arita, M.; Yoshida, M.; Hong, S.; Tjonahen, E.; Glickman, J.N.; Petasis, N.A.; Blumberg, R.S.; Serhan, C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2005, 102, 7671–7676. [Google Scholar]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol 2005, 6, 1191–1197. [Google Scholar]
- Elston, C.; Geddes, D. Inflammation in cystic fibrosis—When and why? Friend or foe? Semin. Respir. Crit. Care Med 2007, 28, 286–294. [Google Scholar]
- Wyllie, D.H.; Sogaard, K.C.; Holland, K.; Yaobo, X.; Bregu, M.; Hill, A.V.; Kiss-Toth, E. Identification of 34 novel proinflammatory proteins in a genome-wide macrophage functional screen. PLoS One 2012, 7, e42388. [Google Scholar]
- Torchinsky, M.B.; Garaude, J.; Blander, J.M. Infection and apoptosis as a combined inflammatory trigger. Curr. Opin. Immunol 2010, 22, 55–62. [Google Scholar]
- Stone, O.J. A mechanism of peripheral spread or localization of inflammatory reactions—Role of the localized ground substance adaptive phenomenon. Med. Hypotheses 1989, 29, 167–169. [Google Scholar]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol 1995, 146, 3–15. [Google Scholar]
- Doukas, J.; Majno, G.; Mordes, J.P. Anti-endothelial cell autoantibodies in BB rats with spontaneous and induced IDDM. Diabetes 1996, 45, 1209–1216. [Google Scholar]
- Gilroy, D.W.; Lawrence, T.; Perretti, M.; Rossi, A.G. Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov 2004, 3, 401–416. [Google Scholar]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med 2008, 233, 674–688. [Google Scholar]
- Simopoulos, A.P.; Norman, H.A.; Gillaspy, J.E. Purslane in human nutrition and its potential for world agriculture. World Rev. Nutr. Diet 1995, 77, 47–74. [Google Scholar]
- Sijben, J.W.; Calder, P.C. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc. Nutr. Soc 2007, 66, 237–259. [Google Scholar]
- Eaton, S.B.; Konner, M. Paleolithic nutrition. A consideration of its nature and current implications. N. Engl. J. Med 1985, 312, 283–289. [Google Scholar]
- Kasuga, K.; Yang, R.; Porter, T.F.; Agrawal, N.; Petasis, N.A.; Irimia, D.; Toner, M.; Serhan, C.N. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. J. Immunol 2008, 181, 8677–8687. [Google Scholar]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol 2007, 25, 101–137. [Google Scholar]
- Duffield, J.S.; Hong, S.; Vaidya, V.S.; Lu, Y.; Fredman, G.; Serhan, C.N.; Bonventre, J.V. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol 2006, 177, 5902–5911. [Google Scholar]
- Tjonahen, E.; Oh, S.F.; Siegelman, J.; Elangovan, S.; Percarpio, K.B.; Hong, S.; Arita, M.; Serhan, C.N. Resolvin E2: Identification and anti-inflammatory actions: Pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem. Biol 2006, 13, 1193–1202. [Google Scholar]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr 2006, 83, 1505S–1519S. [Google Scholar]
- Trebble, T.; Arden, N.K.; Stroud, M.A.; Wootton, S.A.; Burdge, G.C.; Miles, E.A.; Ballinger, A.B.; Thompson, R.L.; Calder, P.C. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br. J. Nutr 2003, 90, 405–412. [Google Scholar]
- Caughey, G.E.; Mantzioris, E.; Gibson, R.A.; Cleland, L.G.; James, M.J. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr 1996, 63, 116–122. [Google Scholar]
- Nielsen, A.A.; Jorgensen, L.G.; Nielsen, J.N.; Eivindson, M.; Gronbaek, H.; Vind, I.; Hougaard, D.M.; Skogstrand, K.; Jensen, S.; Munkholm, P.; et al. Omega-3 fatty acids inhibit an increase of proinflammatory cytokines in patients with active Crohn’s disease compared with omega-6 fatty acids. Aliment. Pharmacol. Ther 2005, 22, 1121–1128. [Google Scholar]
- Ghosh, S.; Novak, E.M.; Innis, S.M. Cardiac proinflammatory pathways are altered with different dietary n-6 linoleic to n-3 alpha-linolenic acid ratios in normal, fat-fed pigs. Am. J. Physiol. Heart Circ. Physiol 2007, 293, H2919–H2927. [Google Scholar]
- McDaniel, J.C.; Ahijevych, K.; Belury, M. Effect of n-3 oral supplements on the n-6/n-3 ratio in young adults. West. J. Nurs. Res 2010, 32, 64–80. [Google Scholar]
- Hu, F.B.; Manson, J.E.; Willett, W.C. Types of dietary fat and risk of coronary heart disease: A critical review. J. Am. Coll. Nutr 2001, 20, 5–19. [Google Scholar]
- Kris-Etherton, P.M.; Taylor, D.S.; Yu-Poth, S.; Huth, P.; Moriarty, K.; Fishell, V.; Hargrove, R.L.; Zhao, G.; Etherton, T.D. Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr 2000, 71, 179S–88S. [Google Scholar]
- Liou, Y.A.; King, D.J.; Zibrik, D.; Innis, S.M. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J. Nutr 2007, 137, 945–952. [Google Scholar]
- Ferreira, S.H. Inflammation, prostaglandins and aspirin-like drugs. Trans. Med. Soc. Lond 1973, 89, 20–31. [Google Scholar]
- Moncada, S.; Ferreira, S.H.; Vane, J.R. Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature 1973, 246, 217–219. [Google Scholar]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Invest 2001, 108, 15–23. [Google Scholar]
- Zurier, R.B. Role of prostaglandins E in inflammation and immune responses. Adv. Prostaglandin Thromboxane Leukot. Res 1991, 21B, 947–953. [Google Scholar]
- Yokomizo, T.; Izumi, T.; Chang, K.; Takuwa, Y.; Shimizu, T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 1997, 387, 620–624. [Google Scholar]
- Tager, A.M.; Dufour, J.H.; Goodarzi, K.; Bercury, S.D.; von Andrian, U.H.; Luster, A.D. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J. Exp. Med 2000, 192, 439–446. [Google Scholar]
- Woo, C.H.; You, H.J.; Cho, S.H.; Eom, Y.W.; Chun, J.S.; Yoo, Y.J.; Kim, J.H. Leukotriene B(4) stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J. Biol. Chem 2002, 277, 8572–8578. [Google Scholar]
- Peters-Golden, M.; Canetti, C.; Mancuso, P.; Coffey, M.J. Leukotrienes: Underappreciated mediators of innate immune responses. J. Immunol 2005, 174, 589–594. [Google Scholar]
- Weylandt, K.H.; Kang, J.X.; Wiedenmann, B.; Baumgart, D.C. Lipoxins and resolvins in inflammatory bowel disease. Inflamm. Bowel. Dis 2007, 13, 797–799. [Google Scholar]
- Arita, M.; Oh, S.F.; Chonan, T.; Hong, S.; Elangovan, S.; Sun, Y.P.; Uddin, J.; Petasis, N.A.; Serhan, C.N. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem 2006, 281, 22847–22854. [Google Scholar]
- Hasturk, H.; Kantarci, A.; Ohira, T.; Arita, M.; Ebrahimi, N.; Chiang, N.; Petasis, N.A.; Levy, B.D.; Serhan, C.N.; van Dyke, T.E. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J 2006, 20, 401–403. [Google Scholar]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of inflammation: State of the art, definitions and terms. FASEB J 2007, 21, 325–332. [Google Scholar]
- Shikano, M.; Masuzawa, Y.; Yazawa, K. Effect of docosahexaenoic acid on the generation of platelet-activating factor by eosinophilic leukemia cells, Eol-1. J. Immunol 1993, 150, 3525–3533. [Google Scholar]
- Shikano, M.; Masuzawa, Y.; Yazawa, K.; Takayama, K.; Kudo, I.; Inoue, K. Complete discrimination of docosahexaenoate from arachidonate by 85 kDa cytosolic phospholipase A2 during the hydrolysis of diacyl- and alkenylacylglycerophosphoethanolamine. Biochim. Biophys. Acta 1994, 1212, 211–216. [Google Scholar]
- Ogawa, S.; Urabe, D.; Yokokura, Y.; Arai, H.; Arita, M.; Inoue, M. Total synthesis and bioactivity of resolvin E2. Org. Lett 2009, 11, 3602–3605. [Google Scholar]
- Seki, H.; Tani, Y.; Arita, M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat 2009, 89, 126–130. [Google Scholar]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar]
- Arita, M.; Ohira, T.; Sun, Y.P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol 2007, 178, 3912–3917. [Google Scholar]
- Samson, M.; Edinger, A.L.; Stordeur, P.; Rucker, J.; Verhasselt, V.; Sharron, M.; Govaerts, C.; Mollereau, C.; Vassart, G.; Doms, R.W.; Parmentier, M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur. J. Immunol 1998, 28, 1689–1700. [Google Scholar]
- Kaur, J.; Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem. Biophys. Res. Commun 2010, 391, 1762–1768. [Google Scholar]
- De Palma, G.; Castellano, G.; del Prete, A.; Sozzani, S.; Fiore, N.; Loverre, A.; Parmentier, M.; Gesualdo, L.; Grandaliano, G.; Schena, F.P. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int 2011, 79, 1228–1235. [Google Scholar] [Green Version]
- Seki, H.; Fukunaga, K.; Arita, M.; Arai, H.; Nakanishi, H.; Taguchi, R.; Miyasho, T.; Takamiya, R.; Asano, K.; Ishizaka, A.; et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol 2010, 184, 836–843. [Google Scholar]
- Baker, O. Identification of RvE1 receptors in Salivary Glands 2013, unpublished work.
- Keyes, K.T.; Ye, Y.; Lin, Y.; Zhang, C.; Perez-Polo, J.R.; Gjorstrup, P.; Birnbaum, Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol 2010, 299, H153–H164. [Google Scholar]
- Zhang, F.; Yang, H.; Pan, Z.; Wang, Z.; Wolosin, J.M.; Gjorstrup, P.; Reinach, P.S. Dependence of resolvin-induced increases in corneal epithelial cell migration on EGF receptor transactivation. Invest. Ophthalmol. Vis. Sci 2010, 51, 5601–5609. [Google Scholar]
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 formation and impact in inflammation resolution. J. Immunol 2012, 188, 4527–4534. [Google Scholar]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Fredman, G.; Serhan, C.N. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol 2012, 180, 2018–2027. [Google Scholar]
- Odusanwo, O.; Chinthamani, S.; McCall, A.; Duffey, M.E.; Baker, O.J. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am. J. Physiol. Cell Physiol 2012, 302, C1331–C1345. [Google Scholar]
- Dartt, D.A.; Hodges, R.R.; Li, D.; Shatos, M.A.; Lashkari, K.; Serhan, C.N. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J. Immunol 2011, 186, 4455–4466. [Google Scholar]
- Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C.N. MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J 2011, 25, 544–560. [Google Scholar]
- Gianotti, L.; Braga, M.; Fortis, C.; Soldini, L.; Vignali, A.; Colombo, S.; Radaelli, G.; di Carlo, V. A prospective, randomized clinical trial on perioperative feeding with an arginine-, omega-3 fatty acid-, and RNA-enriched enteral diet: Effect on host response and nutritional status. J. Parenter. Enteral Nutr 1999, 23, 314–320. [Google Scholar]
- Hasturk, H.; Kantarci, A.; Goguet-Surmenian, E.; Blackwood, A.; Andry, C.; Serhan, C.N.; van Dyke, T.E. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol 2007, 179, 7021–7029. [Google Scholar]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar]
- Kim, T.H.; Kim, G.D.; Jin, Y.H.; Park, Y.S.; Park, C.S. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol 2012, 14, 384–391. [Google Scholar]
- Haworth, O.; Cernadas, M.; Levy, B.D. NK cells are effectors for resolvin E1 in the timely resolution of allergic airway inflammation. J. Immunol 2011, 186, 6129–6135. [Google Scholar]
- Lund, T.; Mangsbo, S.M.; Scholz, H.; Gjorstrup, P.; Totterman, T.H.; Korsgren, O.; Foss, A. Resolvin E1 reduces proinflammatory markers in human pancreatic islets in vitro. Exp. Clin. Endocrinol. Diabetes 2010, 118, 237–244. [Google Scholar]
- Leirisalo-Repo, M. The present knowledge of the inflammatory process and the inflammatory mediators. Pharmacol. Toxicol 1994, 75, 1–3. [Google Scholar]
- Poch, B.; Gansauge, F.; Rau, B.; Wittel, U.; Gansauge, S.; Nussler, A.K.; Schoenberg, M.; Beger, H.G. The role of polymorphonuclear leukocytes and oxygen-derived free radicals in experimental acute pancreatitis: Mediators of local destruction and activators of inflammation. FEBS Lett 1999, 461, 268–272. [Google Scholar]
- Rau, B.; Poch, B.; Gansauge, F.; Bauer, A.; Nussler, A.K.; Nevalainen, T.; Schoenberg, M.H.; Beger, H.G. Pathophysiologic role of oxygen free radicals in acute pancreatitis: Initiating event or mediator of tissue damage? Ann. Surg 2000, 231, 352–360. [Google Scholar]
- Henson, P.M.; Bratton, D.L.; Fadok, V.A. Apoptotic cell removal. Curr. Biol 2001, 11, R795–R805. [Google Scholar]
- Fadok, V.A.; Bratton, D.L.; Henson, P.M. Phagocyte receptors for apoptotic cells: Recognition, uptake, and consequences. J. Clin. Invest 2001, 108, 957–962. [Google Scholar]
- Fadok, V.A.; Bratton, D.L.; Guthrie, L.; Henson, P.M. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: Role of proteases. J. Immunol 2001, 166, 6847–6854. [Google Scholar]
- Huynh, M.L.; Fadok, V.A.; Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest 2002, 109, 41–50. [Google Scholar]
- Palmer, C.D.; Mancuso, C.J.; Weiss, J.P.; Serhan, C.N.; Guinan, E.C.; Levy, O. 17(R)-Resolvin D1 differentially regulates TLR4-mediated responses of primary human macrophages to purified LPS and live E. coli. J. Leukoc. Biol 2011, 90, 459–470. [Google Scholar]
- Campbell, E.L.; Louis, N.A.; Tomassetti, S.E.; Canny, G.O.; Arita, M.; Serhan, C.N.; Colgan, S.P. Resolvin E1 promotes mucosal surface clearance of neutrophils: A new paradigm for inflammatory resolution. FASEB J 2007, 21, 3162–3170. [Google Scholar]
- Aoki, H.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Kawata, T.; Shimizu, Y.; Okajima, F.; Dobashi, K.; Mori, M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun 2008, 367, 509–515. [Google Scholar]
- Chiang, N.; Fredman, G.; Backhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar]
- Lima-Garcia, J.F.; Dutra, R.C.; da Silva, K.; Motta, E.M.; Campos, M.M.; Calixto, J.B. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br. J. Pharmacol 2011, 164, 278–293. [Google Scholar]
- Ji, R.R.; Xu, Z.Z.; Strichartz, G.; Serhan, C.N. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci 2011, 34, 599–609. [Google Scholar]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med 2010, 16, 592–597. [Google Scholar]
- Xu, Z.Z.; Berta, T.; Ji, R.R. Resolvin E1 Inhibits Neuropathic Pain and Spinal Cord Microglial Activation Following Peripheral Nerve Injury. J. Neuroimmune Pharmacol 2012, 8, 37–41. [Google Scholar]
- Ramasamy, I. Inherited bleeding disorders: Disorders of platelet adhesion and aggregation. Crit. Rev. Oncol. Hematol 2004, 49, 1–35. [Google Scholar]
- Andre, P.; LaRocca, T.; Delaney, S.M.; Lin, P.H.; Vincent, D.; Sinha, U.; Conley, P.B.; Phillips, D.R. Anticoagulants (thrombin inhibitors) and aspirin synergize with P2Y12 receptor antagonism in thrombosis. Circulation 2003, 108, 2697–2703. [Google Scholar]
- Fuse, I.; Higuchi, W.; Mito, M.; Aizawa, Y. DDAVP normalized the bleeding time in patients with congenital platelet TxA2 receptor abnormality. Transfusion 2003, 43, 563–567. [Google Scholar]
- Lee, Y.Y.; Lee, S.; Jin, J.L.; Yun-Choi, H.S. Platelet anti-aggregatory effects of coumarins from the roots of Angelica genuflexa and A. gigas. Arch. Pharm. Res 2003, 26, 723–726. [Google Scholar]
- Dona, M.; Fredman, G.; Schwab, J.M.; Chiang, N.; Arita, M.; Goodarzi, A.; Cheng, G.; von Andrian, U.H.; Serhan, C.N. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 2008, 112, 848–855. [Google Scholar]
- Fredman, G.; van Dyke, T.E.; Serhan, C.N. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler. Thromb. Vasc. Biol 2010, 30, 2005–2013. [Google Scholar]
- Van Dyke, T.E.; Serhan, C.N. Resolution of inflammation: A new paradigm for the pathogenesis of periodontal diseases. J. Dent. Res 2003, 82, 82–90. [Google Scholar]
- Blair, H.C.; Robinson, L.J.; Zaidi, M. Osteoclast signalling pathways. Biochem. Biophys. Res. Commun 2005, 328, 728–738. [Google Scholar]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.; Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989, 245, 855–857. [Google Scholar]
- Sarrazin, P.; Hackett, J.A.; Fortier, I.; Gallant, M.A.; de Brum-Fernandes, A. Role of EP3 and EP4 prostaglandin receptors in reorganization of the cytoskeleton in mature human osteoclasts. J. Rheumatol 2004, 31, 1598–1606. [Google Scholar]
- Suzawa, T.; Miyaura, C.; Inada, M.; Maruyama, T.; Sugimoto, Y.; Ushikubi, F.; Ichikawa, A.; Narumiya, S.; Suda, T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology 2000, 141, 1554–1559. [Google Scholar]
- Herrera, B.S.; Ohira, T.; Gao, L.; Omori, K.; Yang, R.; Zhu, M.; Muscara, M.N.; Serhan, C.N.; Van Dyke, T.E.; Gyurko, R. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br. J. Pharmacol 2008, 155, 1214–1223. [Google Scholar]
- Fox, P.C.; Bowman, S.J.; Segal, B.; Vivino, F.B.; Murukutla, N.; Choueiri, K.; Ogale, S.; McLean, L. Oral involvement in primary Sjogren syndrome. J. Am. Dent. Assoc 2008, 139, 1592–1601. [Google Scholar]
- Daniels, T.E.; Fox, P.C. Salivary and oral components of Sjogren’s syndrome. Rheum. Dis. Clin. North Am 1992, 18, 571–589. [Google Scholar]
- Horrobin, D.F.; Campbell, A. Sjogren’s syndrome and the sicca syndrome: The role of prostaglandin E1 deficiency. Treatment with essential fatty acids and vitamin C. Med. Hypotheses 1980, 6, 225–232. [Google Scholar]
- Jonsson, R.; Moen, K.; Vestrheim, D.; Szodoray, P. Current issues in Sjogren’s syndrome. Oral Dis 2002, 8, 130–140. [Google Scholar]


© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Keinan, D.; Leigh, N.J.; Nelson, J.W.; De Oleo, L.; Baker, O.J. Understanding Resolvin Signaling Pathways to Improve Oral Health. Int. J. Mol. Sci. 2013, 14, 5501-5518. https://doi.org/10.3390/ijms14035501
Keinan D, Leigh NJ, Nelson JW, De Oleo L, Baker OJ. Understanding Resolvin Signaling Pathways to Improve Oral Health. International Journal of Molecular Sciences. 2013; 14(3):5501-5518. https://doi.org/10.3390/ijms14035501
Chicago/Turabian StyleKeinan, David, Noel J. Leigh, Joel W. Nelson, Laura De Oleo, and Olga J. Baker. 2013. "Understanding Resolvin Signaling Pathways to Improve Oral Health" International Journal of Molecular Sciences 14, no. 3: 5501-5518. https://doi.org/10.3390/ijms14035501



