A Cancer-Indicative microRNA Pattern in Normal Prostate Tissue
Abstract
:1. Introduction
2. Results and Discussion
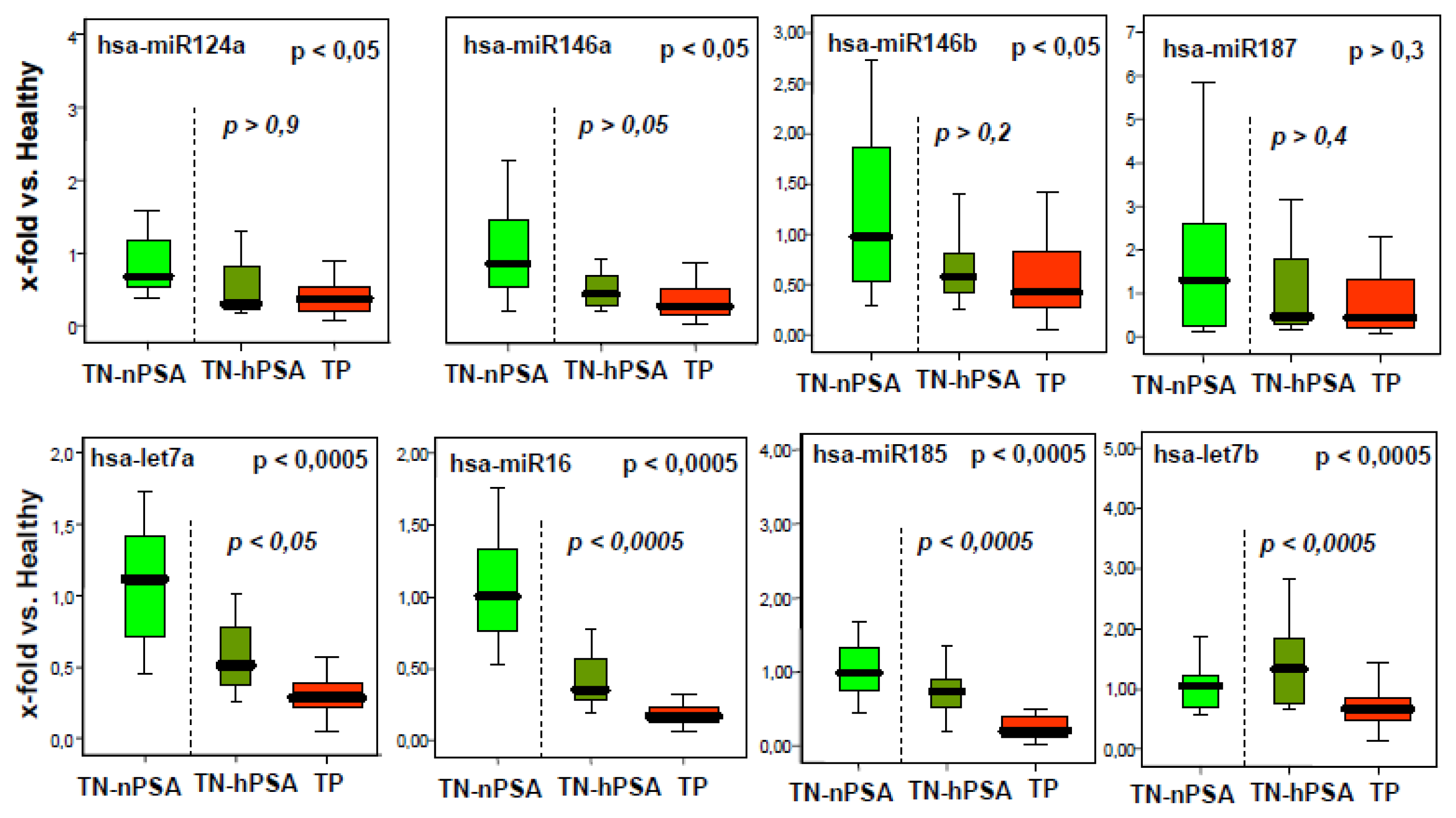
2.1. A Number of microRNAs Discriminate Tumor-Positive from Tumor-Negative Groups
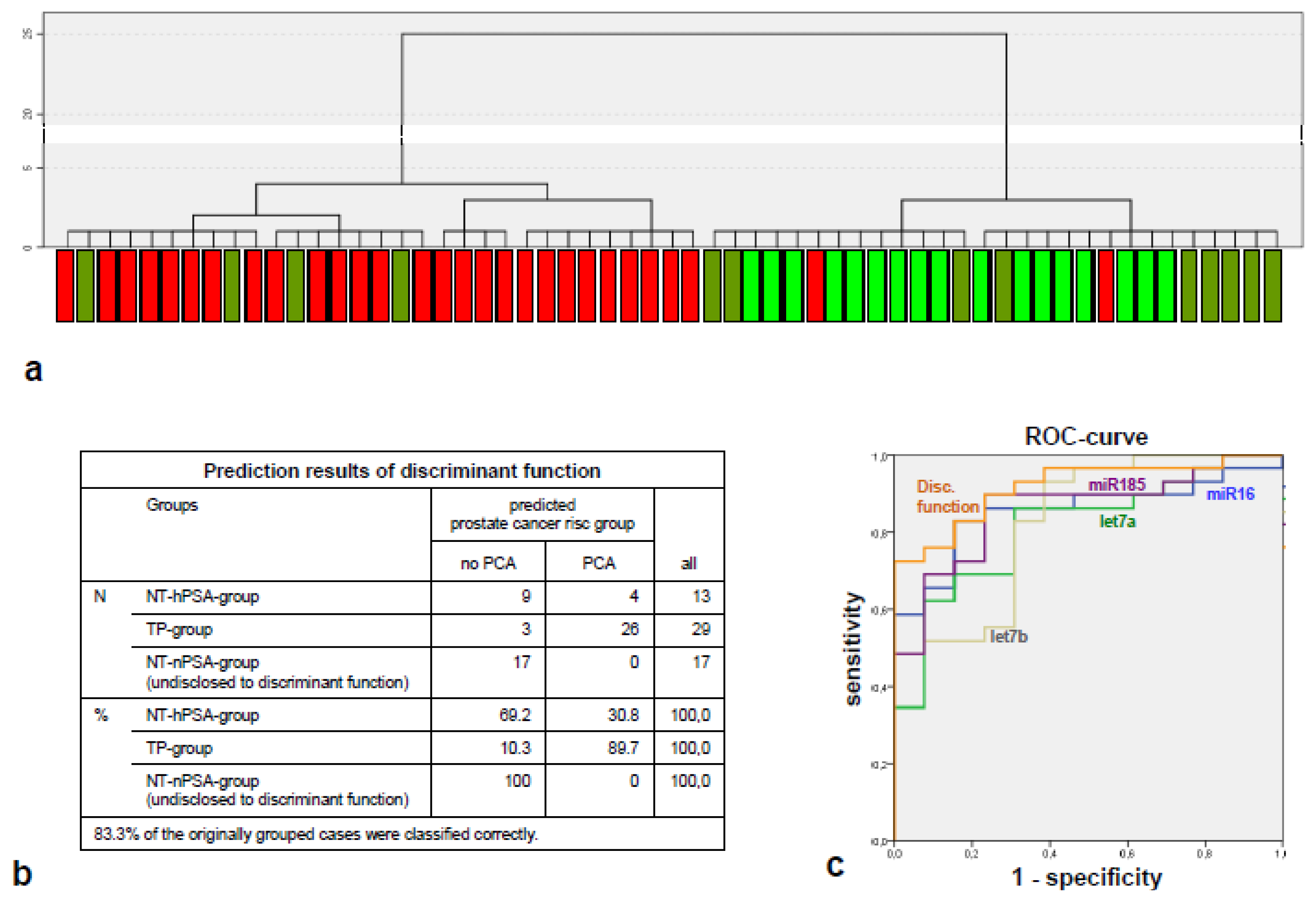
2.2. The miRNA Expression Signature Discriminates the Tumor-Positive from Tumor-Negative Groups Better than Single microRNA Levels
2.3. The Components of the Tumor-Indicative microRNA Signature Have Tumor-Suppressive Properties in Other Tumors
3. Experimental Section
3.1. Pre-Selection of microRNAs
- Are the microRNAs associated with prostate cancer (according to MiRó data)? If yes, then:
- Are (prostate-) cancer-related target transcripts of the microRNAs experimentally validated or else consistently predicted by three independent prediction algorithms (TargetScan, miRanda and PicTar)? If yes, then:
- Are there reports on a role in human early cancer formation, malignant transformation or field cancerization in scientific literature (PubMed search results up to the year 2008)?
3.2. Collectives Analyzed in the Main Experiment
- the tumor-positive group (TP group) composed of patients with proven PCa,
- the tumor negative/high PSA group (TN-hPSA group), composed of patients with an elevated PSA level, but a high likelihood of being cancer-free (as confirmed by multiple repeated negative biopsies [24]),
- the tumor negative/normal PSA group (TN-nPSA group), comprising healthy men with normal serum PSA (<1 ng/mL).
3.3. Tissue Sample Acquisition and RNA Isolation
3.4. Quantitative RT-PCR
3.5. Statistical Evaluation
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Albini, A.; Sporn, M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar]
- Dakubo, G.D.; Jakupciak, J.P.; Birch-Machin, M.A.; Parr, R.L. Clinical implications and utility of field cancerization. Cancer Cell. Int 2007, 7, 2. [Google Scholar]
- Schlomm, T.; Hellwinkel, O.J.; Buness, A.; Ruschhaupt, M.; Lubke, A.M.; Chun, F.K.; Simon, R.; Budaus, L.; Erbersdobler, A.; Graefen, M.; et al. Molecular cancer phenotype in normal prostate tissue. Eur. Urol 2009, 55, 885–890. [Google Scholar]
- Alvarez-Garcia, I.; Miska, E.A. MicroRNA functions in animal development and human disease. Development 2005, 132, 4653–4662. [Google Scholar]
- Yue, J.; Tigyi, G. MicroRNA trafficking and human cancer. Cancer Biol. Ther 2006, 5, 573–578. [Google Scholar]
- Pillai, R.S. MicroRNA function: Multiple mechanisms for a tiny RNA? RNA 2005, 11, 1753–1761. [Google Scholar]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar]
- Chang, T.C.; Yu, D.; Lee, Y.S.; Wentzel, E.A.; Arking, D.E.; West, K.M.; Dang, C.V.; Thomas-Tikhonenko, A.; Mendell, J.T. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet 2008, 40, 43–50. [Google Scholar]
- Sampson, V.B.; Rong, N.H.; Han, J.; Yang, Q.; Aris, V.; Soteropoulos, P.; Petrelli, N.J.; Dunn, S.P.; Krueger, L.J. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res 2007, 67, 9762–9770. [Google Scholar]
- Long, X.B.; Sun, G.B.; Hu, S.; Liang, G.T.; Wang, N.; Zhang, X.H.; Cao, P.P.; Zhen, H.T.; Cui, Y.H.; Liu, Z. Let-7a microRNA functions as a potential tumor suppressor in human laryngeal cancer. Oncol Rep 2009, 22, 1189–1195. [Google Scholar]
- Zhang, X.; Lee, C.; Ng, P.Y.; Rubin, M.; Shabsigh, A.; Buttyan, R. Prostatic neoplasia in transgenic mice with prostate-directed overexpression of the c-myc oncoprotein. Prostate 2000, 43, 278–285. [Google Scholar]
- Ellwood-Yen, K.; Graeber, T.G.; Wongvipat, J.; Iruela-Arispe, M.L.; Zhang, J.; Matusik, R.; Thomas, G.V.; Sawyers, C.L. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 2003, 4, 223–238. [Google Scholar]
- Dong, Q.; Meng, P.; Wang, T.; Qin, W.; Wang, F.; Yuan, J.; Chen, Z.; Yang, A.; Wang, H. MicroRNA let-7a inhibits proliferation of human prostate cancer cells in vitro and in vivo by targeting E2F2 and CCND2. PLoS One 2010, 5, e10147. [Google Scholar]
- Schultz, J.; Lorenz, P.; Gross, G.; Ibrahim, S.; Kunz, M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell. Res 2008, 18, 549–557. [Google Scholar]
- Schaefer, A.; Jung, M.; Mollenkopf, H.J.; Wagner, I.; Stephan, C.; Jentzmik, F.; Miller, K.; Lein, M.; Kristiansen, G.; Jung, K. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J. Cancer 2010, 126, 1166–1176. [Google Scholar]
- Bonci, D.; Coppola, V.; Musumeci, M.; Addario, A.; Giuffrida, R.; Memeo, L.; D’Urso, L.; Pagliuca, A.; Biffoni, M.; Labbaye, C.; et al. The miR-15a-miR-16–1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med 2008, 14, 1271–1277. [Google Scholar]
- Bandi, N.; Zbinden, S.; Gugger, M.; Arnold, M.; Kocher, V.; Hasan, L.; Kappeler, A.; Brunner, T.; Vassella, E. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res 2009, 69, 5553–5559. [Google Scholar]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar]
- Takahashi, Y.; Forrest, A.R.; Maeno, E.; Hashimoto, T.; Daub, C.O.; Yasuda, J. MiR-107 and MiR-185 can induce cell cycle arrest in human non small cell lung cancer cell lines. PLoS One 2009, 4, e6677. [Google Scholar]
- Braakhuis, B.J.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res 2003, 63, 1727–1730. [Google Scholar]
- Kakizoe, T. Development and progression of urothelial carcinoma. Cancer Sci 2006, 97, 821–828. [Google Scholar]
- Roesch-Ely, M.; Nees, M.; Karsai, S.; Ruess, A.; Bogumil, R.; Warnken, U.; Schnolzer, M.; Dietz, A.; Plinkert, P.K.; Hofele, C.; et al. Proteomic analysis reveals successive aberrations in protein expression from healthy mucosa to invasive head and neck cancer. Oncogene 2007, 26, 54–64. [Google Scholar]
- Hockel, M.; Dornhofer, N. The hydra phenomenon of cancer: Why tumors recur locally after microscopically complete resection. Cancer Res 2005, 65, 2997–3002. [Google Scholar]
- Walz, J.; Graefen, M.; Chun, F.K.; Erbersdobler, A.; Haese, A.; Steuber, T.; Schlomm, T.; Huland, H.; Karakiewicz, P.I. High incidence of prostate cancer detected by saturation biopsy after previous negative biopsy series. Eur. Urol 2006, 50, 498–505. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001, 29, e45. [Google Scholar]
- Zubakov, D.; Boersma, A.W.; Choi, Y.; van Kuijk, P.F.; Wiemer, E.A.; Kayser, M. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int. J. Legal Med 2010, 124, 217–226. [Google Scholar]
- Sevli, S.; Uzumcu, A.; Solak, M.; Ittmann, M.; Ozen, M. The function of microRNAs, small but potent molecules, in human prostate cancer. Prostate Cancer Prostatic Dis 2010, 13, 208–217. [Google Scholar]
- Coppola, V.; De Maria, R.; Bonci, D. MicroRNAs and prostate cancer. Endocr. Relat. Cancer 2010, 17, F1–F17. [Google Scholar]
- Gandellini, P.; Folini, M.; Zaffaroni, N. Emerging role of microRNAs in prostate cancer: Implications for personalized medicine. Discov. Med 2010, 9, 212–218. [Google Scholar]
- Gandellini, P.; Folini, M.; Longoni, N.; Pennati, M.; Binda, M.; Colecchia, M.; Salvioni, R.; Supino, R.; Moretti, R.; Limonta, P.; et al. miR-205 Exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res 2009, 69, 2287–2295. [Google Scholar]
| Prostate biopsies with normal, tumor free tissue (30% to 50% epithelia) from | |||
|---|---|---|---|
| Groups | Healthy men (tumor negative) | Healthy men (with initial suspicion for prostate cancer (PCa), but found to be tumor negative) | PCa patients (with proven malignant prostate tumor) |
| Number of individuals | n = 17 | n = 14 | n = 31 |
| Years of age (10%- & 90%-quantiles) | 51 to 64 | 54 to 75 | 54 to 66 |
| Diagnostics | negative palpation, negative diagnostic biopsy series | negative or unclear palpation, multiple negative diagnostic biopsy series | unclear or positive palpation, ≥one tumor positive diagnostic biopsy |
| low prostate specific antigen (PSA) level (mean 0.78 ng/mL [±0.26 SD]) | elevated PSA level (mean 6.44 ng/mL [±4.82 SD]) | elevated PSA level (mean 7.97 ng/mL [±5.21 SD]) | |
| Designations | Tumor-negative/normal PSA group (TN-nPSA group) | Tumor-negative/high PSA group (TN-hPSA group) | Tumor-positive group (TP group) |
| Note | groups representing men with elevated PSA levels submitted to diagnostic biopsy-taking in clinical routine | ||
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hellwinkel, O.J.C.; Sellier, C.; Sylvester, Y.-M.J.; Brase, J.C.; Isbarn, H.; Erbersdobler, A.; Steuber, T.; Sültmann, H.; Schlomm, T.; Wagner, C. A Cancer-Indicative microRNA Pattern in Normal Prostate Tissue. Int. J. Mol. Sci. 2013, 14, 5239-5249. https://doi.org/10.3390/ijms14035239
Hellwinkel OJC, Sellier C, Sylvester Y-MJ, Brase JC, Isbarn H, Erbersdobler A, Steuber T, Sültmann H, Schlomm T, Wagner C. A Cancer-Indicative microRNA Pattern in Normal Prostate Tissue. International Journal of Molecular Sciences. 2013; 14(3):5239-5249. https://doi.org/10.3390/ijms14035239
Chicago/Turabian StyleHellwinkel, Olaf J. C., Christina Sellier, Yu-Mi Jessica Sylvester, Jan C. Brase, Hendrik Isbarn, Andreas Erbersdobler, Thomas Steuber, Holger Sültmann, Thorsten Schlomm, and Christina Wagner. 2013. "A Cancer-Indicative microRNA Pattern in Normal Prostate Tissue" International Journal of Molecular Sciences 14, no. 3: 5239-5249. https://doi.org/10.3390/ijms14035239





