Maternal Methyl Donors Supplementation during Lactation Prevents the Hyperhomocysteinemia Induced by a High-Fat-Sucrose Intake by Dams
Abstract
:1. Introduction
2. Results and Discussion
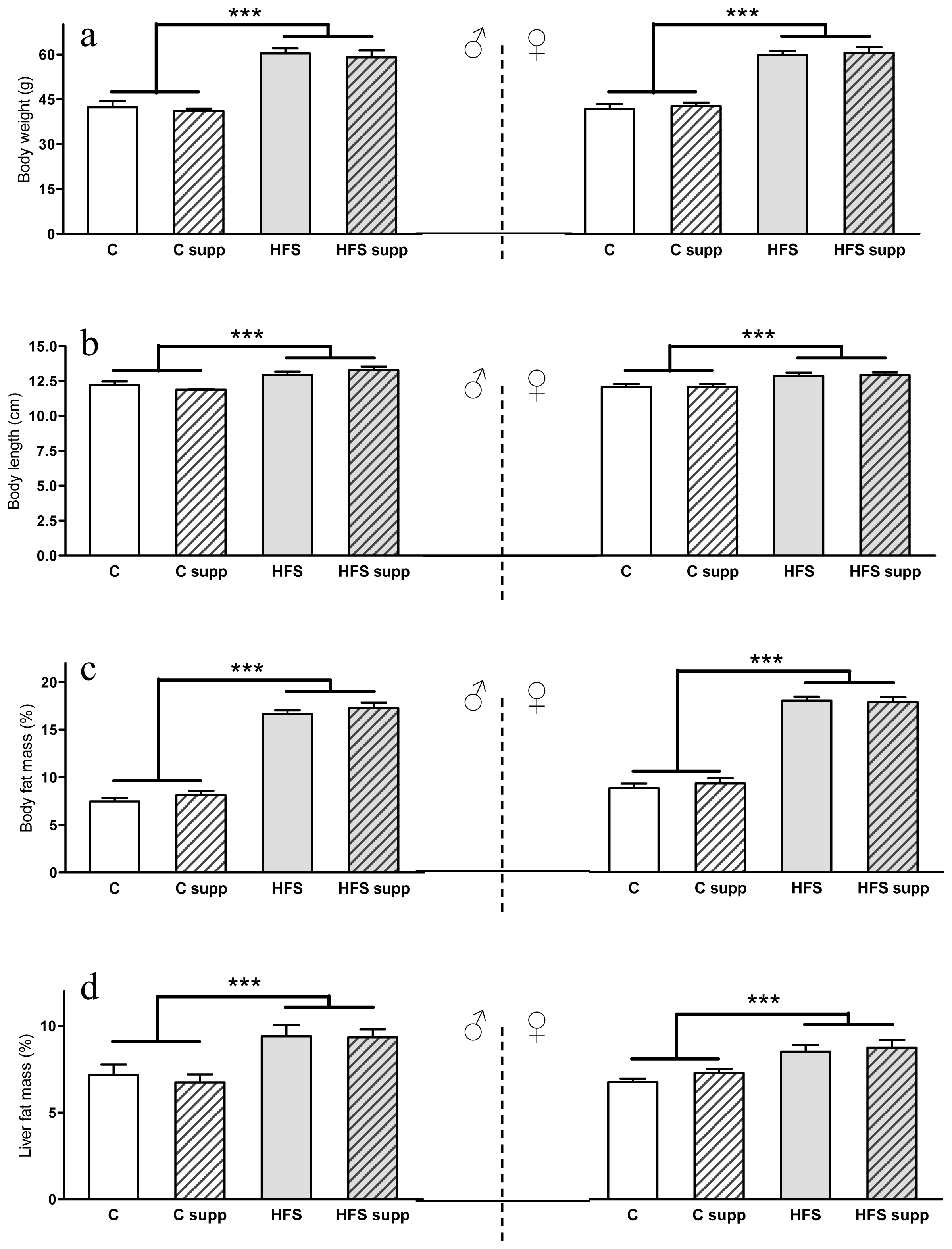
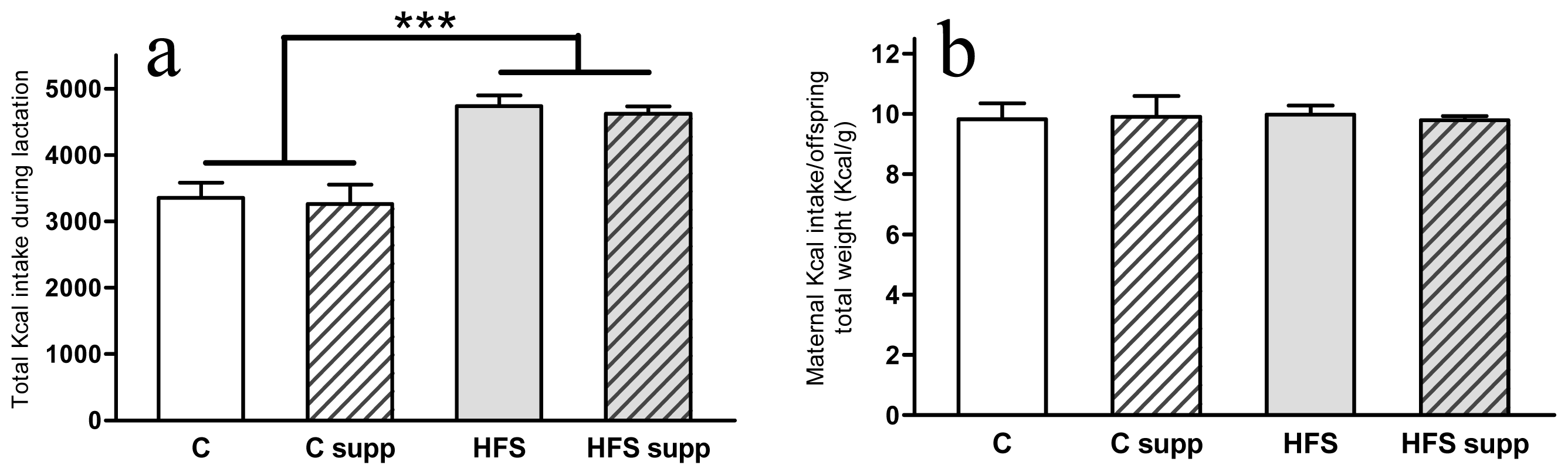
2.1. Phenotypical and Body Composition Characteristics
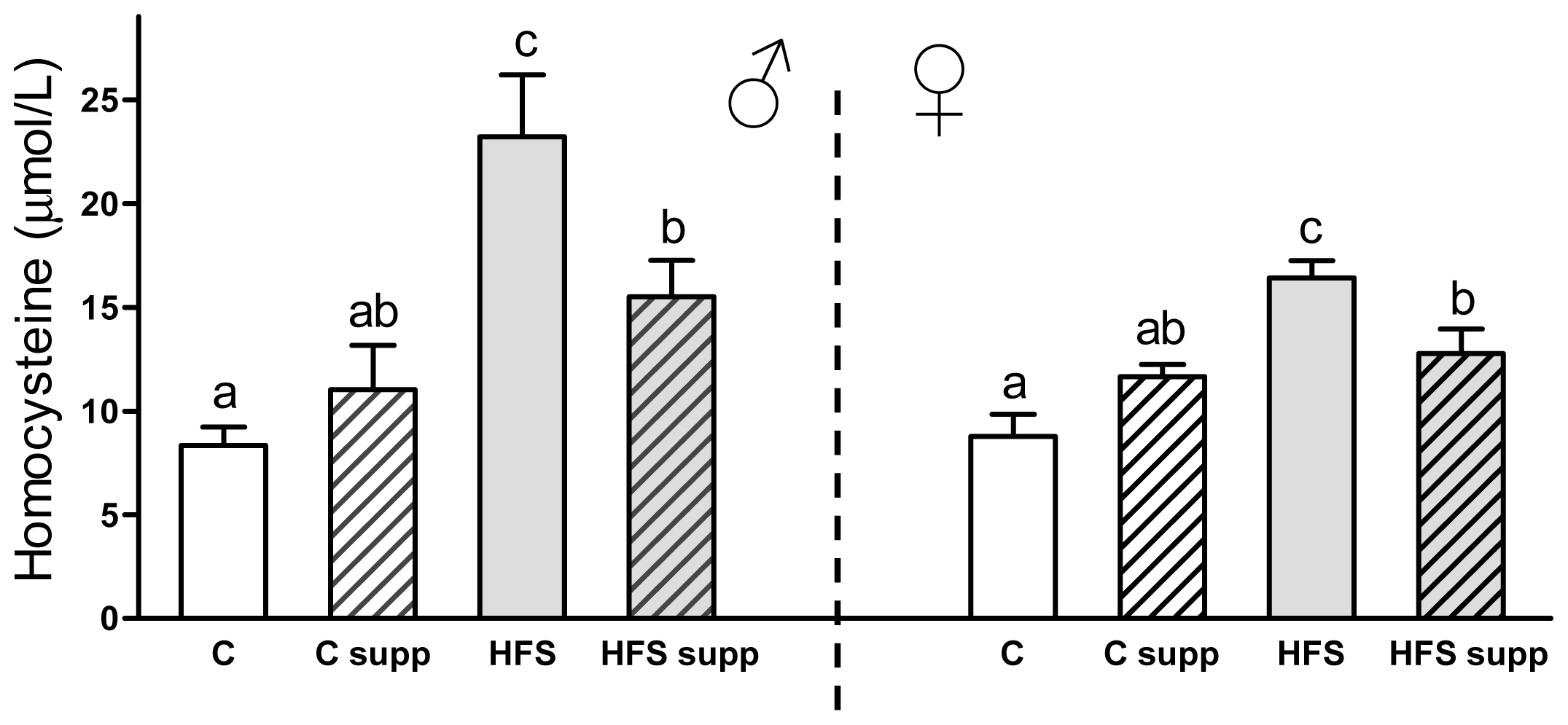
2.2. Plasma Biochemical Markers
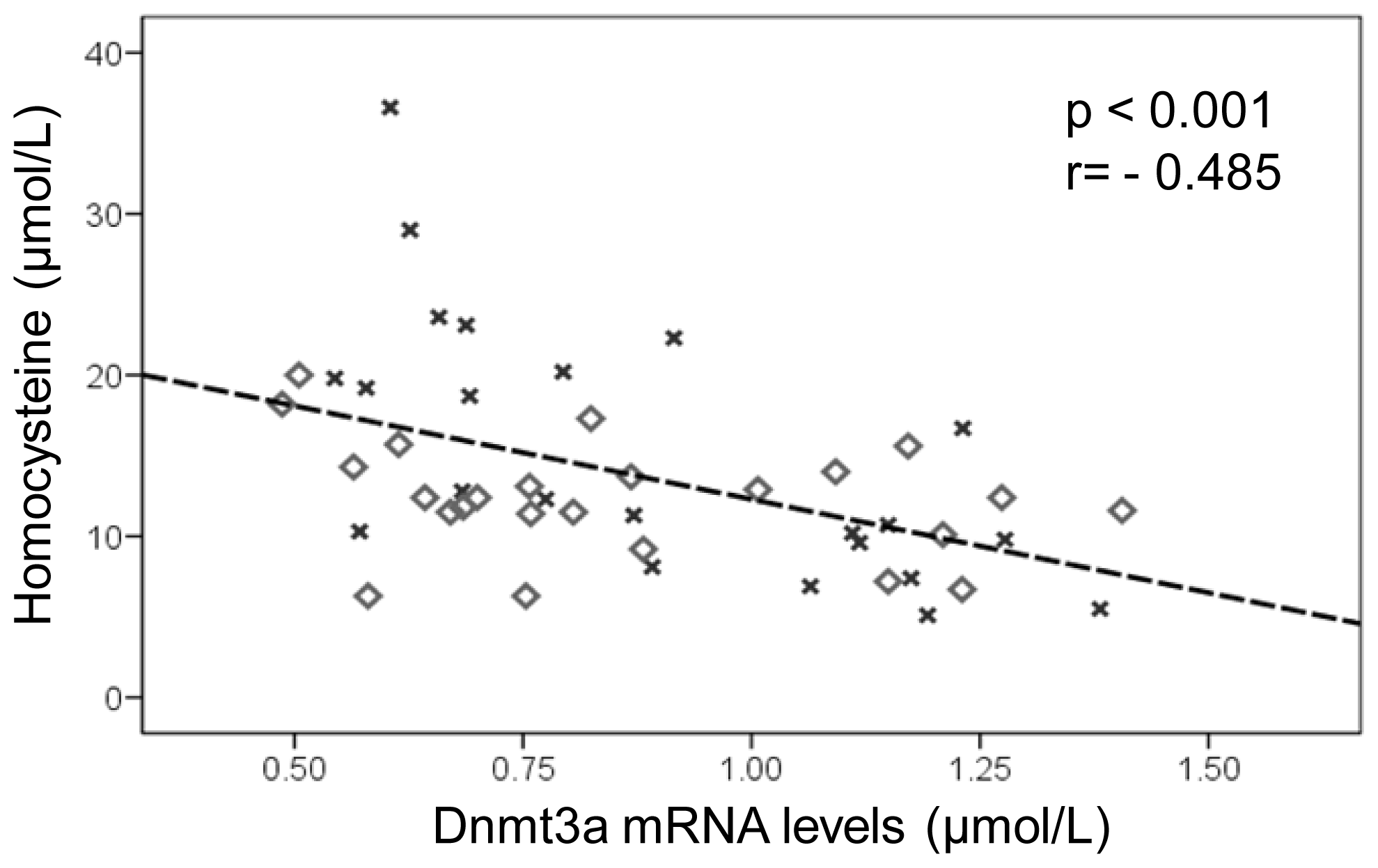
2.3. DNA Methylation Metabolism
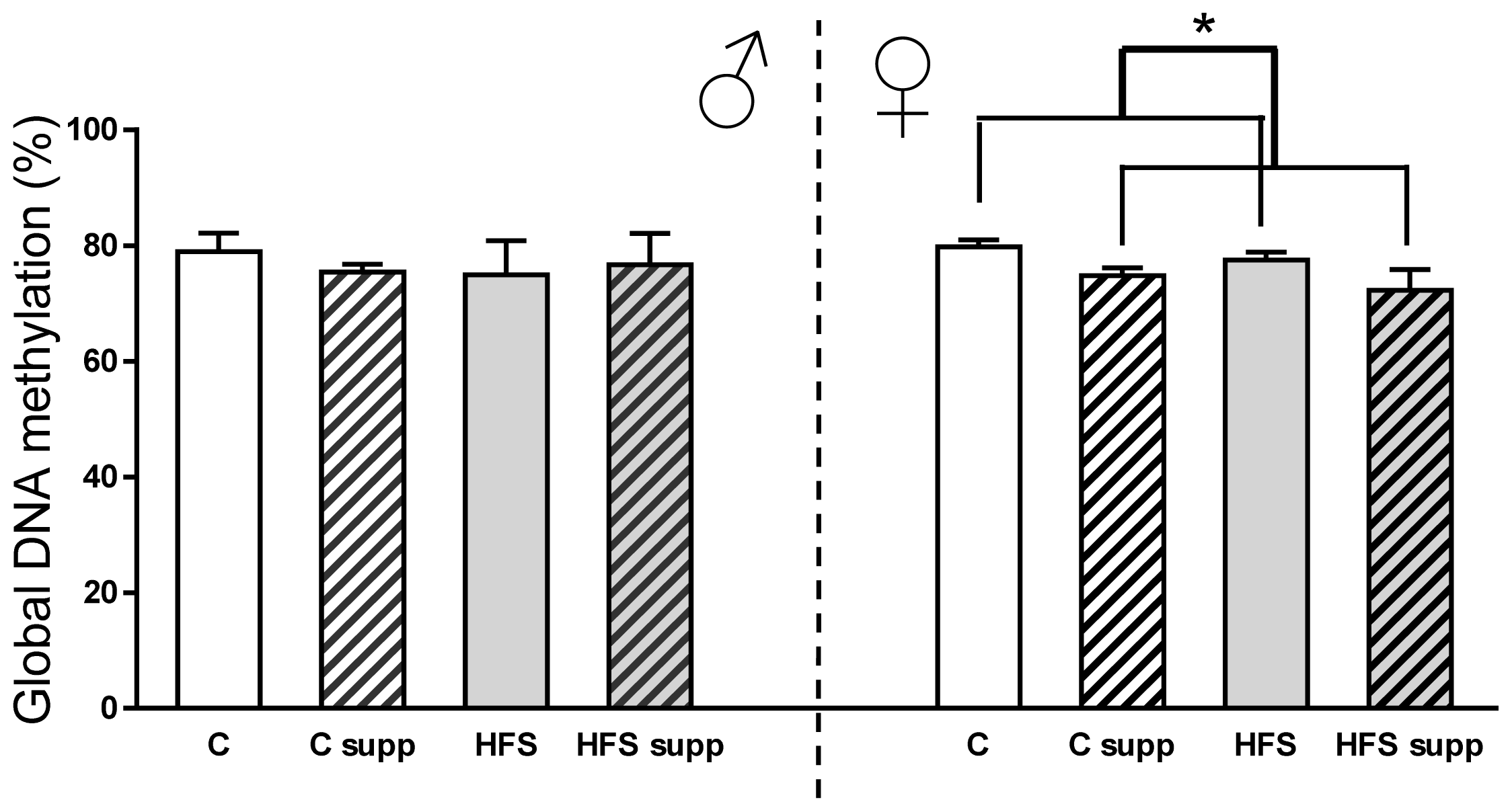
2.3.1. Hepatic Global DNA Methylation
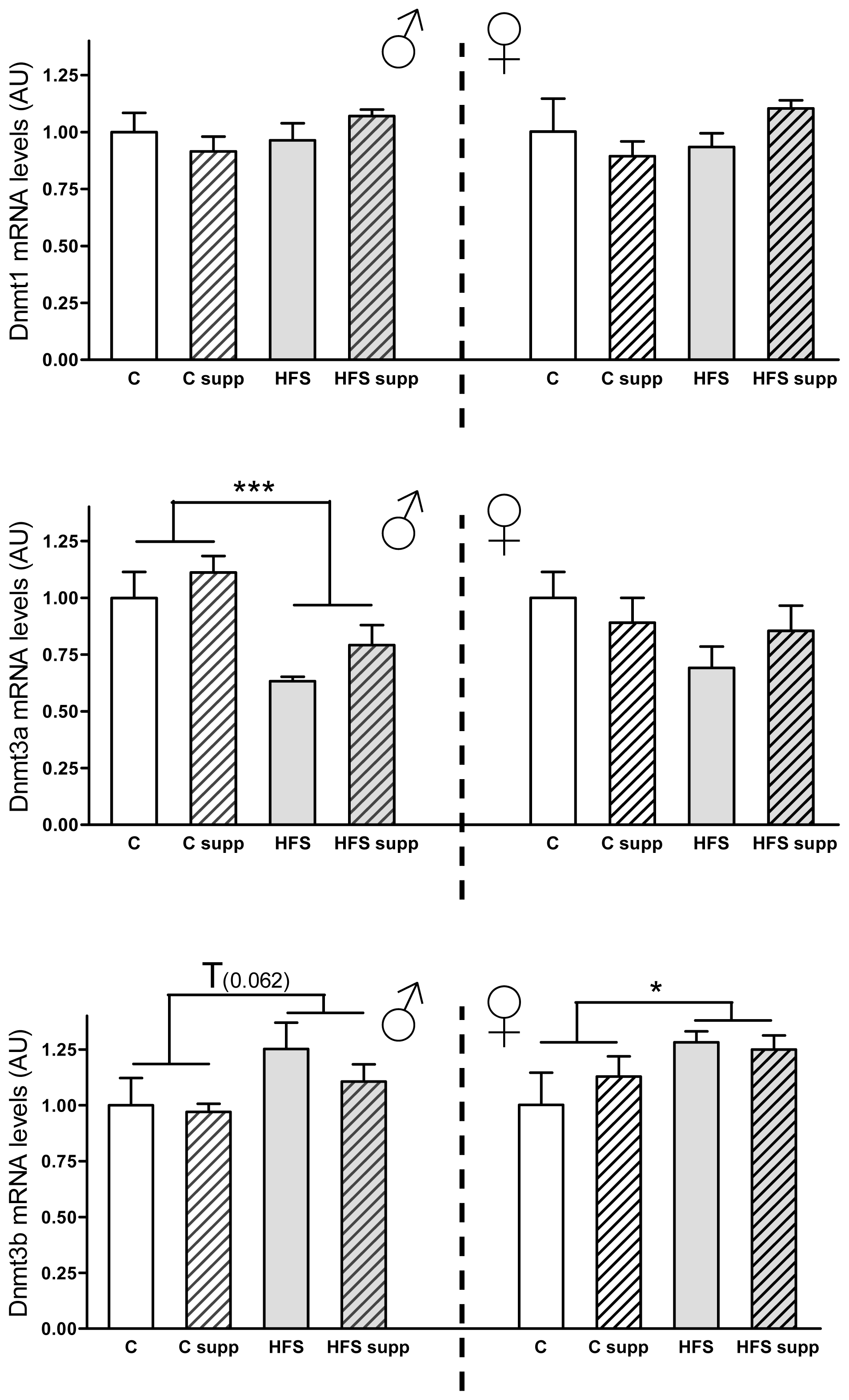
2.3.2. Hepatic DNA Methyltransferases mRNA Expression
3. Experimental Section
3.1. Animal, Diets and Experimental Design
3.2. Total Body and Liver Fat Content
3.3. Plasma Analysis
3.4. DNA and RNA Isolation
3.5. Global DNA Methylation
3.6. Real-Time qPCR
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Martinez, J.A.; Cordero, P.; Campion, J.; Milagro, F.I. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc. Nutr. Soc 2012, 71, 276–283. [Google Scholar]
- Fernandes de Abreu, D.A.; Landel, V.; Barnett, A.G.; McGrath, J.; Eyles, D.; Feron, F. Prenatal vitamin d deficiency induces an early and more severe experimental autoimmune encephalomyelitis in the second generation. Int. J. Mol. Sci 2012, 13, 10911–10919. [Google Scholar]
- Zou, M.; Arentson, E.J.; Teegarden, D.; Koser, S.L.; Onyskow, L.; Donkin, S.S. Fructose consumption during pregnancy and lactation induces fatty liver and glucose intolerance in rats. Nutr. Res 2012, 32, 588–598. [Google Scholar]
- Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; Simmers, T.A.; Osmond, C.; Barker, D.J.; Bleker, O.P.; Roseboom, T.J. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr 2006, 84, 322–327, quiz 466–327. [Google Scholar]
- Wang, P.X.; Wang, J.J.; Lei, Y.X.; Xiao, L.; Luo, Z.C. Impact of fetal and infant exposure to the Chinese Great Famine on the risk of hypertension in adulthood. PLoS One 2012, 7, e49720. [Google Scholar]
- Ozanne, S.E.; Constancia, M. Mechanisms of disease: The developmental origins of disease and the role of the epigenotype. Nat. Clin. Pract. Endocrinol. Metab 2007, 3, 539–546. [Google Scholar]
- Baumans, V. Use of animals in experimental research: An ethical dilemma? Gene Ther 2004, 11, S64–S66. [Google Scholar]
- Van Abeelen, A.F.; Veenendaal, M.V.; Painter, R.C.; de Rooij, S.R.; Thangaratinam, S.; van der Post, J.A.; Bossuyt, P.M.; Elias, S.G.; Uiterwaal, C.S.; Grobbee, D.E.; et al. The fetal origins of hypertension: A systematic review and meta-analysis of the evidence from animal experiments of maternal undernutrition. J. Hypertens 2012, 30, 2255–2267. [Google Scholar]
- Paternain, L.; Batlle, M.A.; de la Garza, A.L.; Milagro, F.I.; Martinez, J.A.; Campion, J. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology 2012, 96, 249–260. [Google Scholar]
- Sandovici, I.; Smith, N.H.; Nitert, M.D.; Ackers-Johnson, M.; Uribe-Lewis, S.; Ito, Y.; Jones, R.H.; Marquez, V.E.; Cairns, W.; Tadayyon, M.; et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc. Natl. Acad. Sci. USA 2011, 108, 5449–5454. [Google Scholar]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar]
- Glier, M.B.; Green, T.J.; Devlin, A.M. Methyl nutrients, DNA methylation, and cardiovascular disease. Mol. Nutr. Food Res 2013. [Google Scholar] [CrossRef]
- Waterland, R.A.; Travisano, M.; Tahiliani, K.G.; Rached, M.T.; Mirza, S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes. (Lond.) 2008, 32, 1373–1379. [Google Scholar]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar]
- Starlard-Davenport, A.; Tryndyak, V.; Kosyk, O.; Ross, S.R.; Rusyn, I.; Beland, F.A.; Pogribny, I.P. Dietary methyl deficiency, microRNA expression and susceptibility to liver carcinogenesis. J. Nutrigenet. Nutrigenomics 2010, 3, 259–266. [Google Scholar]
- Cordero, P.; Campion, J.; Milagro, F.I.; Martinez, J.A. Dietary supplementation with methyl donor groups could prevent nonalcoholic fatty liver. Hepatology 2011, 53, 2151–2152. [Google Scholar]
- Cordero, P.; Campion, J.; Milagro, F.I.; Martinez, J.A. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: Effect of dietary methyl donor supplementation. Mol. Genet. Metab 2013, 110, 388–395. [Google Scholar]
- Cordero, P.; Gomez-Uriz, A.M.; Campion, J.; Milagro, F.I.; Martinez, J.A. Dietary supplementation with methyl donors reduces fatty liver and modifies the Fatty Acid Synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr 2013, 8, 105–113. [Google Scholar]
- Mato, J.M.; Corrales, F.J.; Lu, S.C.; Avila, M.A. S-Adenosylmethionine: A control switch that regulates liver function. FASEB J 2002, 16, 15–26. [Google Scholar]
- Zhu, H.; Yang, W.; Lu, W.; Etheredge, A.J.; Lammer, E.J.; Finnell, R.H.; Carmichael, S.L.; Shaw, G.M. Gene variants in the folate-mediated one-carbon metabolism (FOCM) pathway as risk factors for conotruncal heart defects. Am. J. Med. Genet. A 2012, 158A, 1124–1134. [Google Scholar]
- Ciaccio, M.; Bellia, C. Hyperhomocysteinemia and cardiovascular risk: Effect of vitamin supplementation in risk reduction. Curr. Clin. Pharmacol 2010, 5, 30–36. [Google Scholar]
- Cordero, P.; Gomez-Uriz, A.M.; Milagro, F.I.; Campion, J.; Martinez, J.A. Maternal weight gain induced by an obesogenic diet affects adipose accumulation, liver weight, and insulin homeostasis in the rat offspring depending on the sex. J. Endocrinol. Invest 2012, 35, 981–986. [Google Scholar]
- Patterson, C.M.; Bouret, S.G.; Park, S.; Irani, B.G.; Dunn-Meynell, A.A.; Levin, B.E. Large litter rearing enhances leptin sensitivity and protects selectively bred diet-induced obese rats from becoming obese. Endocrinology 2010, 151, 4270–4279. [Google Scholar]
- Symonds, M.E.; Sebert, S.P.; Hyatt, M.A.; Budge, H. Nutritional programming of the metabolic syndrome. Nat. Rev. Endocrinol 2009, 5, 604–610. [Google Scholar]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull 2001, 60, 5–20. [Google Scholar]
- Nascimiento, A.R.; Machado, M.; de Jesus, N.; Gomes, F.; Lessa, M.A.; Bonomo, I.T.; Tibiriçá, E. Structural and functional microvascular alterations in a rat model of metabolic syndrome induced by a high-fat diet. Obesity (Silver Spring) 2013, 21, 2046–2054. [Google Scholar]
- Finch, J.M.; Joseph, J. Homocysteine, cardiovascular inflammation, and myocardial remodeling. Cardiovasc. Hematol. Disord. Drug Targets 2010, 10, 241–245. [Google Scholar]
- Karatela, R.A.; Sainani, G.S. Plasma homocysteine in obese, overweight and normal weight hypertensives and normotensives. Indian Heart J 2009, 61, 156–159. [Google Scholar]
- Jardine, M.J.; Kang, A.; Zoungas, S.; Navaneethan, S.D.; Ninomiya, T.; Nigwekar, S.U.; Gallagher, M.P.; Cass, A.; Strippoli, G.; Perkovic, V. The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: Systematic review and meta-analysis. BMJ 2012, 344, e3533. [Google Scholar]
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Annu. Rev. Med 2009, 60, 39–54. [Google Scholar]
- Yilmaz, H.; Sahin, S.; Sayar, N.; Tangurek, B.; Yilmaz, M.; Nurkalem, Z.; Onturk, E.; Cakmak, N.; Bolca, O. Effects of folic acid and N-acetylcysteine on plasma homocysteine levels and endothelial function in patients with coronary artery disease. Acta Cardiol 2007, 62, 579–585. [Google Scholar]
- Bhargava, S.; Tyagi, S.C. Nutriepigenetic regulation by folate-homocysteine-methionine axis: A review. Mol. Cell. Biochem 2013. [Google Scholar] [CrossRef]
- Krishna, S.M.; Dear, A.; Craig, J.M.; Norman, P.E.; Golledge, J. The potential role of homocysteine mediated DNA methylation and associated epigenetic changes in abdominal aortic aneurysm formation. Atherosclerosis 2013, 228, 295–305. [Google Scholar]
- Armstrong, K.M.; Bermingham, E.N.; Bassett, S.A.; Treloar, B.P.; Roy, N.C.; Barnett, M.P. Global DNA methylation measurement by HPLC using low amounts of DNA. Biotechnol. J 2011, 6, 113–117. [Google Scholar]
- Engeham, S.F.; Haase, A.; Langley-Evans, S.C. Supplementation of a maternal low-protein diet in rat pregnancy with folic acid ameliorates programming effects upon feeding behaviour in the absence of disturbances to the methionine-homocysteine cycle. Br. J. Nutr 2010, 103, 996–1007. [Google Scholar]
- Li, C.C.; Cropley, J.E.; Cowley, M.J.; Preiss, T.; Martin, D.I.; Suter, C.M. A sustained dietary change increases epigenetic variation in isogenic mice. PLoS Genet 2011, 7, e1001380. [Google Scholar]
- Carlin, J.; George, R.; Reyes, T.M. Methyl donor supplementation blocks the adverse effects of maternal high fat diet on offspring physiology. PLoS One 2013, 8, e63549. [Google Scholar]
- Milagro, F.I.; Campion, J.; Cordero, P.; Goyenechea, E.; Gomez-Uriz, A.M.; Abete, I.; Zulet, M.A.; Martinez, J.A. A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J 2011, 25, 1378–1389. [Google Scholar]
- Milagro, F.I.; Mansego, M.L.; de Miguel, C.; Martinez, J.A. Dietary factors, epigenetic modifications and obesity outcomes: Progresses and perspectives. Mol. Aspects Med 2013, 34, 782–812. [Google Scholar]
- Corrales, F.J.; Perez-Mato, I.; Sanchez Del Pino, M.M.; Ruiz, F.; Castro, C.; Garcia-Trevijano, E.R.; Latasa, U.; Martinez-Chantar, M.L.; Martinez-Cruz, A.; Avila, M.A.; et al. Regulation of mammalian liver methionine adenosyltransferase. J. Nutr 2002, 132, 2377S–2381S. [Google Scholar]
- Kaneda, M.; Okano, M.; Hata, K.; Sado, T.; Tsujimoto, N.; Li, E.; Sasaki, H. Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 2004, 429, 900–903. [Google Scholar]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar]
- Oka, M.; Rodic, N.; Graddy, J.; Chang, L.J.; Terada, N. CpG sites preferentially methylated by Dnmt3a in vivo. J. Biol. Chem 2006, 281, 9901–9908. [Google Scholar]
- Xu, G.L.; Bestor, T.H.; Bourc’his, D.; Hsieh, C.L.; Tommerup, N.; Bugge, M.; Hulten, M.; Qu, X.; Russo, J.J.; Viegas-Pequignot, E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature 1999, 402, 187–191. [Google Scholar]
- Hoffman, D.R.; Marion, D.W.; Cornatzer, W.E.; Duerre, J.A. S-Adenosylmethionine and S-adenosylhomocystein metabolism in isolated rat liver. Effects of l-methionine, l-homocystein, and adenosine. J. Biol. Chem 1980, 255, 10822–10827. [Google Scholar]
- Faust, I.M.; Johnson, P.R.; Hirsch, J. Long-term effects of early nutritional experience on the development of obesity in the rat. J. Nutr 1980, 110, 2027–2034. [Google Scholar]
- Nixon, J.P.; Zhang, M.; Wang, C.; Kuskowski, M.A.; Novak, C.M.; Levine, J.A.; Billington, C.J.; Kotz, C.M. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 2010, 18, 1652–1659. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem 1972, 18, 499–502. [Google Scholar]
- Pogribny, I.P.; James, S.J.; Jernigan, S.; Pogribna, M. Genomic hypomethylation is specific for preneoplastic liver in folate/methyl deficient rats and does not occur in non-target tissues. Mutat. Res 2004, 548, 53–59. [Google Scholar]
| C | C supp | HFS | HFS supp | ANOVA | |
|---|---|---|---|---|---|
| Weight before pregnancy (g) | 260.8 ± 13.1 | 269.3 ± 10.2 | 246.3 ± 10.9 | 243.8 ± 5.5 | n.s. |
| Weight gain during pregnancy (%) | 31.4 ± 1.6 | 34.0 ± 2.0 | 34.4 ± 2.4 | 32.6 ± 2.5 | n.s. |
| Weight after birth (g) | 270.5 ± 14.5 | 282.3 ± 8.7 | 267.3 ± 11.1 | 256.9 ± 8.3 | n.s. |
| Intrauterine number of pups | 10.1 ± 0.8 | 11.5 ± 0.6 | 9.9 ± 0.6 | 9.8 ± 0.6 | n.s. |
| Intrauterine number of males | 5.9 ± 0.8 | 5.5 ± 0.4 | 4.6 ± 0.8 | 4.8 ± 0.6 | n.s. |
| Intrauterine number of females | 4.3 ± 0.4 | 6.0 ± 0.6 | 5.3 ± 0.4 | 5.0 ± 0.7 | n.s. |
| C | C supp | HFS | HFS supp | 2 × 2 ANOVA | |||
|---|---|---|---|---|---|---|---|
| DIET | SUPPL | DIET × SUPPL | |||||
| Male offspring plasma values | |||||||
| Glucose (mg/dL) | 141.3 ± 6.1 | 140.9 ± 1.6 | 141.2 ± 3.6 | 151.7 ± 5.9 | n.s. | n.s. | n.s. |
| Total cholesterol (mg/dL) | 111 ± 4 | 122 ± 8 | 132 ± 7 | 155 ± 4 | *** | * | n.s. |
| HDL cholesterol (mg/dL) | 29.0 ± 1.7 | 31.5 ± 1.9 | 34.9 ± 1.2 | 38.1 ± 1.0 | *** | 0.077 | n.s. |
| LDL cholesterol (mg/dL) | 65.4 ± 4.9 | 74.6 ± 6.4 | 65.1 ± 6.1 | 88.1 ± 2.0 | n.s. | ** | n.s. |
| Triglycerides (mg/dL) | 83 ± 12 | 80 ± 13 | 109 ± 13 | 134 ± 14 | ** | n.s. | n.s. |
| Free fatty acides (mg/dL) | 0.47 ± 0.10 | 0.45 ± 0.04 | 0.81 ± 0.11 | 0.66 ± 0.05 | ** | n.s. | n.s. |
| Females offspring plasma values | |||||||
| Glucose (mg/dL) | 132.6 ± 4.6 | 148.8 ± 3.7 | 141.0 ± 5.4 | 145.8 ± 3.2 | n.s. | * | n.s. |
| Total cholesterol (mg/dL) | 110 ± 3 | 126. ± 11 | 142. ± 6 | 149 ± 4 | *** | 0.063 | n.s. |
| HDL cholesterol (mg/dL) | 28.2 ± 1.2 | 31.9 ± 2.1 | 34.1 ± 1.6 | 33.6 ± 0.8 | * | n.s. | n.s. |
| LDL cholesterol (mg/dL) | 62.8 ± 2.2 | 75.6 ± 7.3 | 80.0 ± 6.1 | 83.8 ± 7.8 | 0.051 | n.s. | n.s. |
| Triglycerides (mg/dL) | 95 ± 15 | 91 ± 23 | 137 ± 30 | 157 ± 43 | 0.092 | n.s. | n.s. |
| Free fatty acides (mg/dL) | 0.56 ± 0.05 | 0.36 ± 0.08 | 0.75 ± 0.09 | 0.73 ± 0.08 | ** | n.s. | n.s. |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cordero, P.; Milagro, F.I.; Campion, J.; Martinez, J.A. Maternal Methyl Donors Supplementation during Lactation Prevents the Hyperhomocysteinemia Induced by a High-Fat-Sucrose Intake by Dams. Int. J. Mol. Sci. 2013, 14, 24422-24437. https://doi.org/10.3390/ijms141224422
Cordero P, Milagro FI, Campion J, Martinez JA. Maternal Methyl Donors Supplementation during Lactation Prevents the Hyperhomocysteinemia Induced by a High-Fat-Sucrose Intake by Dams. International Journal of Molecular Sciences. 2013; 14(12):24422-24437. https://doi.org/10.3390/ijms141224422
Chicago/Turabian StyleCordero, Paul, Fermin I. Milagro, Javier Campion, and J. Alfredo Martinez. 2013. "Maternal Methyl Donors Supplementation during Lactation Prevents the Hyperhomocysteinemia Induced by a High-Fat-Sucrose Intake by Dams" International Journal of Molecular Sciences 14, no. 12: 24422-24437. https://doi.org/10.3390/ijms141224422






