Verifiable Hypotheses for Thymosin β4-Dependent and -Independent Angiogenic Induction of Trichinella spiralis-Triggered Nurse Cell Formation
Abstract
:1. Introduction
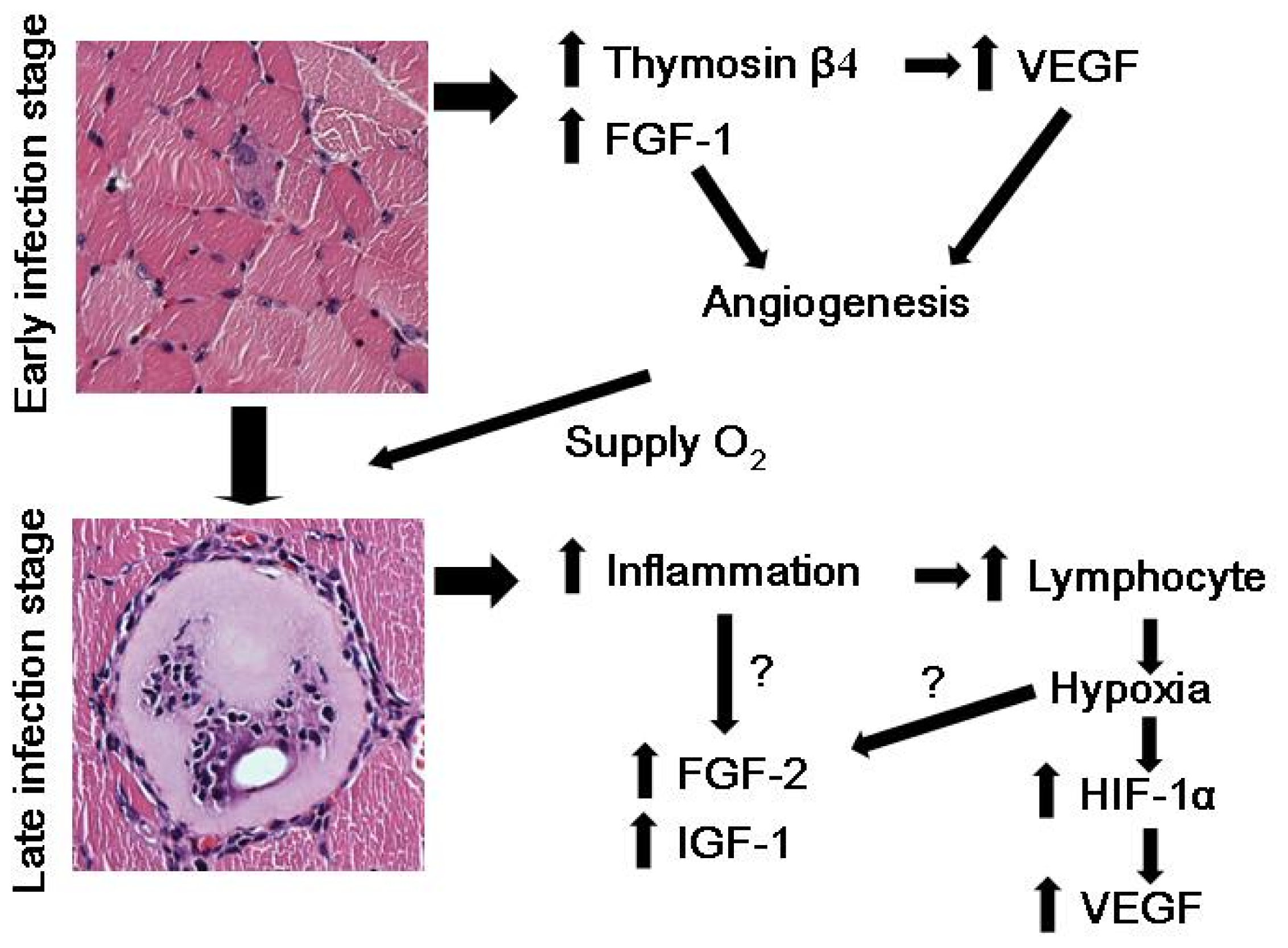
2. Nurse Cell Formation and Angiogenesis
3. Hypoxia and VEGF Induction in T. spiralis-Infected Nurse Cells
4. Thymosin β4 and T. spiralis Infection
5. Hypoxia, HIF-1α, and VEGF in T. spiralis-Infected Nurse Cells
6. Conclusions and Future Studies
Acknowledgments
Conflicts of Interest
References
- Stephenson, L.S.; Latham, M.C.; Ottesen, E.A. Malnutrition and parasitic helminth infections. Parasitology 2000, 121, S23–S38. [Google Scholar]
- Teppema, J.S.; Robinson, J.E.; Ruitenberg, E.J. Ultrastructural aspects of capsule formation in Trichinella spiralis infection in the rat. Parasitology 1973, 66, 291–296. [Google Scholar]
- Despommier, D.; Aron, L.; Turgeon, L. Trichinella spiralis: Growth of the intracellular (muscle) larva. Exp. Parasitol 1975, 37, 108–116. [Google Scholar]
- Polvere, R.I.; Kabbash, C.A.; Capó, V.A.; Kadan, I.; Despommier, D.D. Trichinella spiralis: Synthesis of type IV and type VI collagen during nurse cell formation. Exp. Parasitol 1997, 86, 191–199. [Google Scholar]
- Despommier, D.D. How does Trichinella spiralis make itself at home? Parasitol. Today 1998, 14, 318–323. [Google Scholar]
- Baruch, A.M.; Despommier, D.D. Blood vessels in Trichinella spiralis infections: A study using vascular casts. J. Parasitol 1991, 77, 99–103. [Google Scholar]
- Capó, V.A.; Despommier, D.D.; Polvere, R.I. Trichinella spiralis: Vascular endothelial growth factor is up-regulated within the nurse cell during the early phase of its formation. J. Parasitol 1998, 84, 209–214. [Google Scholar]
- Sang, N.; Fang, J.; Srinivas, V.; Leshchinsky, I.; Caro, J. Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1 α is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP. Mol. Cell. Biol 2002, 22, 2984–2992. [Google Scholar]
- Baldewijns, M.M.; van Vlodrop, I.J.; Vermeulen, P.B.; Soetekouw, P.M.; van Engeland, M.; de Bruine, A.P. VHL and HIF signalling in renal cell carcinogenesis. J. Pathol 2010, 221, 125–138. [Google Scholar]
- Ock, M.S.; Song, K.S.; Kleinman, H.; Cha, H.J. Thymosin β4 stabilizes hypoxia-inducible factor-1α protein in an oxygen-independent manner. Ann. N. Y. Acad. Sci 2012, 1269, 79–83. [Google Scholar]
- Kang, Y.J.; Jo, J.O.; Cho, M.K.; Yu, H.S.; Ock, M.S.; Cha, H.J. Trichinella spiralis infection induces angiogenic factor thymosin β4 expression. Vet. Parasitol 2011, 181, 222–228. [Google Scholar]
- Kang, Y.J.; Jo, J.O.; Cho, M.K.; Yu, H.S.; Cha, H.J.; Ock, M.S. Trichinella spiralis infection induces beta-actin co-localized with thymosin β4. Vet. Parasitol 2012, 187, 480–485. [Google Scholar]
- Cha, H.J.; Jeong, M.J.; Kleinman, H.K. Role of thymosin β4 in tumor metastasis and angiogenesis. J. Natl. Cancer Inst 2003, 95, 1674–1680. [Google Scholar]
- Malinda, K.M.; Goldstein, A.L.; Kleinman, H.K. Thymosin β4 stimulates directional migration of human umbilical vein endothelial cells. FASEB J 1997, 11, 474–481. [Google Scholar]
- Grant, D.S.; Rose, W.; Yaen, C.; Goldstein, A.; Martinez, J.; Kleinman, H. Thymosin β4 enhances endothelial cell differentiation and angiogenesis. Angiogenesis 1999, 3, 125–135. [Google Scholar]
- Huang, H.C.; Hu, C.H.; Tang, M.C.; Wang, W.S.; Chen, P.M.; Su, Y. Thymosin β4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene 2007, 26, 2781–2790. [Google Scholar]
- Kobayashi, T.; Okada, F.; Fujii, N.; Tomita, N.; Ito, S.; Tazawa, H.; Aoyama, T.; Choi, S.K.; Shibata, T.; Fujita, H.; et al. Thymosin-β4 regulates motility and metastasis of malignant mouse fibrosarcoma cells. Am. J. Pathol 2002, 160, 869–882. [Google Scholar]
- Larsson, L.I.; Holck, S. Occurrence of thymosin β4 in human breast cancer cells and in other cell types of the tumor microenvironment. Hum. Pathol 2007, 38, 114–119. [Google Scholar]
- Wang, W.S.; Chen, P.M.; Hsiao, H.L.; Wang, H.S.; Liang, W.Y.; Su, Y. Overexpression of the thymosin β4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene 2004, 23, 6666–6671. [Google Scholar]
- Malinda, K.M.; Sidhu, G.S.; Mani, H.; Banaudha, K.; Maheshwari, R.K.; Goldstein, A.L.; Kleinman, H.K. Thymosin β4 accelerates wound healing. J. Invest. Dermatol 1999, 113, 364–368. [Google Scholar]
- Philp, D.; Goldstein, A.L.; Kleinman, H.K. Thymosin β4 promotes angiogenesis, wound healing, and hair follicle development. Mech. Ageing Dev 2004, 125, 113–115. [Google Scholar]
- Philp, D.; Nguyen, M.; Scheremeta, B.; St-Surin, S.; Villa, A.M.; Orgel, A.; Kleinman, H.K.; Elkin, M. Thymosin β4 increases hair growth by activation of hair follicle stem cells. FASEB J 2004, 18, 385–387. [Google Scholar]
- Philp, D.; St-Surin, S.; Cha, H.J.; Moon, H.S.; Kleinman, H.K.; Elkin, M. Thymosin β4 induces hair growth via stem cell migration and differentiation. Ann. N. Y. Acad. Sci 2007, 1112, 95–103. [Google Scholar]
- Moon, E.Y.; Song, J.H.; Yang, K.H. Actin-sequestering protein, thymosin-β-4 (TB4), inhibits caspase-3 activation in paclitaxel-induced tumor cell death. Oncol. Res 2007, 16, 507–516. [Google Scholar]
- Niu, M.; Nachmias, V.T. Increased resistance to apoptosis in cells overexpressing thymosin β four: A role for focal adhesion kinase pp125FAK. Cell Adhes. Commun 2000, 7, 311–320. [Google Scholar]
- Sosne, G.; Qiu, P.; Christopherson, P.L.; Wheater, M.K. Thymosin β4 suppression of corneal NF-κB: A potential anti-inflammatory pathway. Exp. Eye Res 2007, 84, 663–669. [Google Scholar]
- Young, J.D.; Lawrence, A.J.; MacLean, A.G.; Leung, B.P.; McInnes, I.B.; Canas, B.; Pappin, D.J.; Stevenson, R.D. Thymosin β4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nat. Med 1999, 5, 1424–1427. [Google Scholar]
- Smart, N.; Rossdeutsch, A.; Riley, P.R. Thymosin β4 and angiogenesis: Modes of action and therapeutic potential. Angiogenesis 2007, 10, 229–241. [Google Scholar]
- Jo, J.O.; Kim, S.R.; Bae, M.K.; Kang, Y.J.; Ock, M.S.; Kleinman, H.K.; Cha, H.J. Thymosin β4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxia-inducible factor (HIF)-1α-dependent manner. Biochim. Biophys. Acta 2010, 1803, 1244–1251. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ock, M.S.; Cha, H.-J.; Choi, Y.H. Verifiable Hypotheses for Thymosin β4-Dependent and -Independent Angiogenic Induction of Trichinella spiralis-Triggered Nurse Cell Formation. Int. J. Mol. Sci. 2013, 14, 23492-23498. https://doi.org/10.3390/ijms141223492
Ock MS, Cha H-J, Choi YH. Verifiable Hypotheses for Thymosin β4-Dependent and -Independent Angiogenic Induction of Trichinella spiralis-Triggered Nurse Cell Formation. International Journal of Molecular Sciences. 2013; 14(12):23492-23498. https://doi.org/10.3390/ijms141223492
Chicago/Turabian StyleOck, Mee Sun, Hee-Jae Cha, and Yung Hyun Choi. 2013. "Verifiable Hypotheses for Thymosin β4-Dependent and -Independent Angiogenic Induction of Trichinella spiralis-Triggered Nurse Cell Formation" International Journal of Molecular Sciences 14, no. 12: 23492-23498. https://doi.org/10.3390/ijms141223492




