Silencing the SpMPK1, SpMPK2, and SpMPK3 Genes in Tomato Reduces Abscisic Acid—Mediated Drought Tolerance
Abstract
:1. Introduction
2. Results
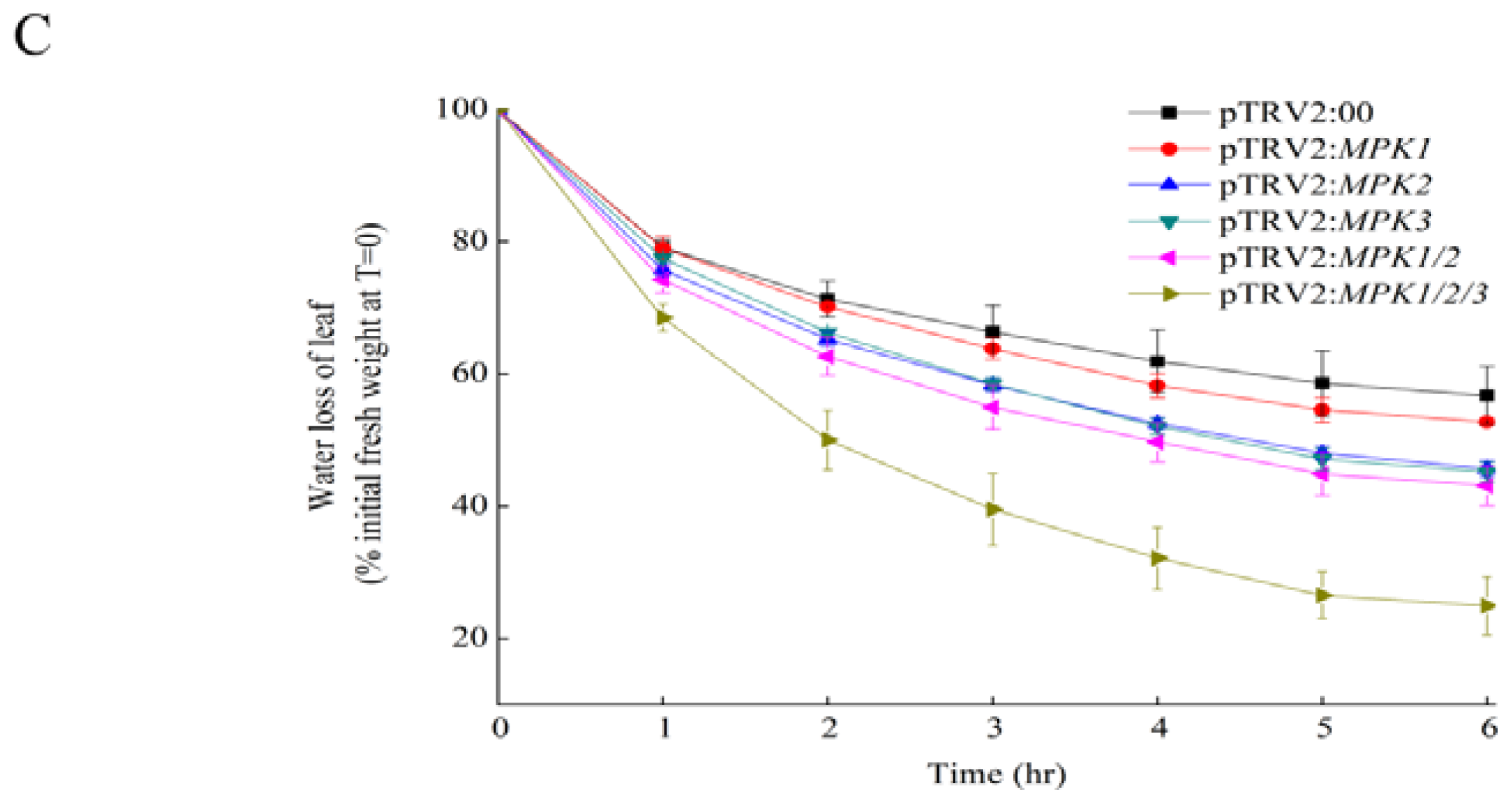
2.1. Silencing SpMPK1, SpMPK2, and SpMPK3 Reduced the Drought Tolerance of Tomato Plants
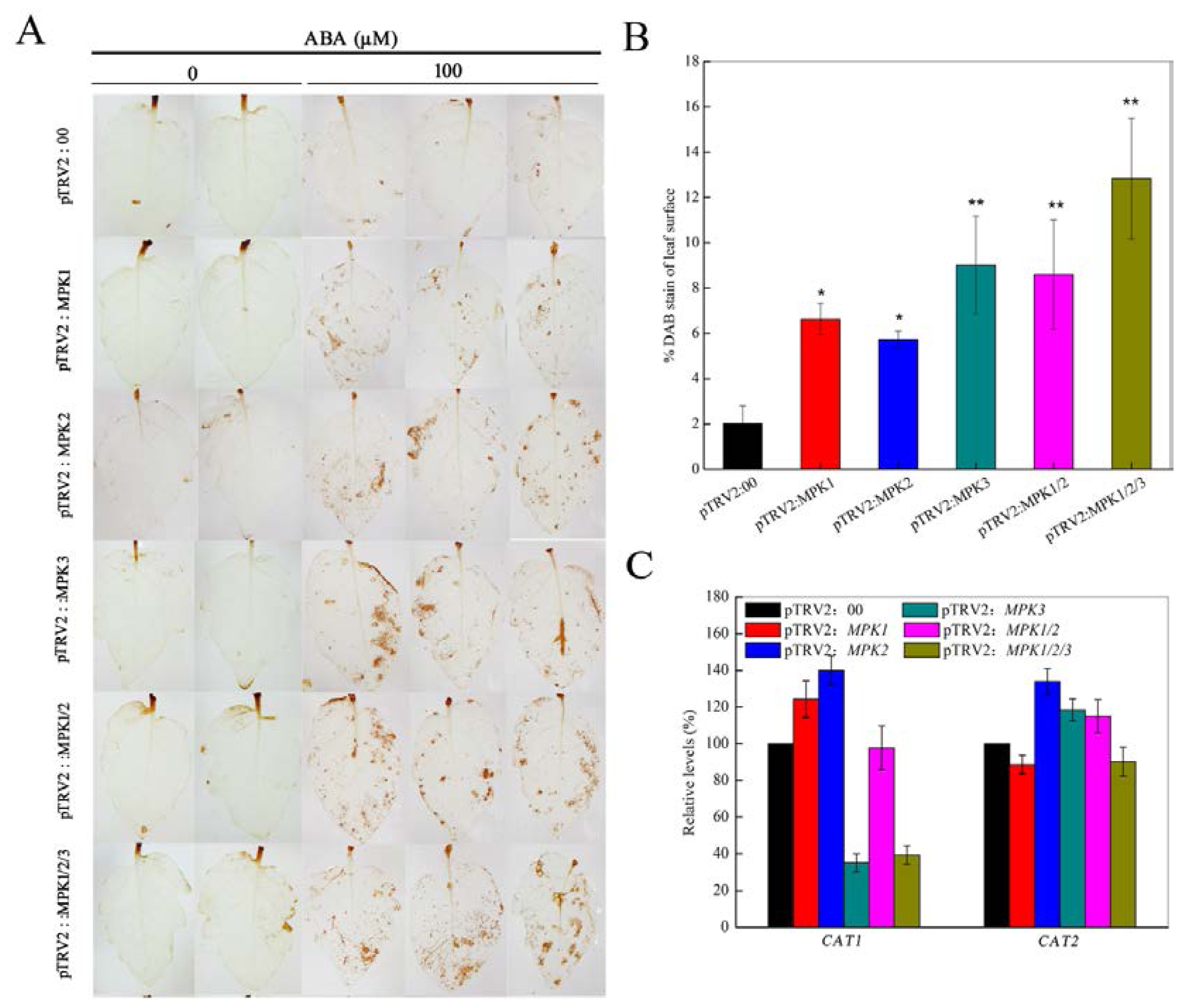
2.2. Silencing SpMPK1, SpMPK2, and SpMPK3 Impaired Stomatal Closure in Response to Abscisic Acid (ABA) and Hydrogen Peroxide (H2O2)
2.3. SpMPK3 is Involved in Abscisic Acid (ABA)-Induced Hydrogen Peroxide (H2O2) Production by Regulating the Expression of CAT1
3. Discussion
4. Experimental Section
4.1. Plant Material and Growth Conditions
4.2. VIGS Constructs
4.3. Infiltration of pTRV-Containing A. tumefaciens Cultures into Cotyledons
4.4. RNA Isolation and Quantitative RT-PCR
4.5. Measurement of Stomatal Aperture
4.6. DAB Staining
4.7. Drought Tolerance Assay
4.8. Tissue Sampling for Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Information
ijms-14-21983-s001.pdf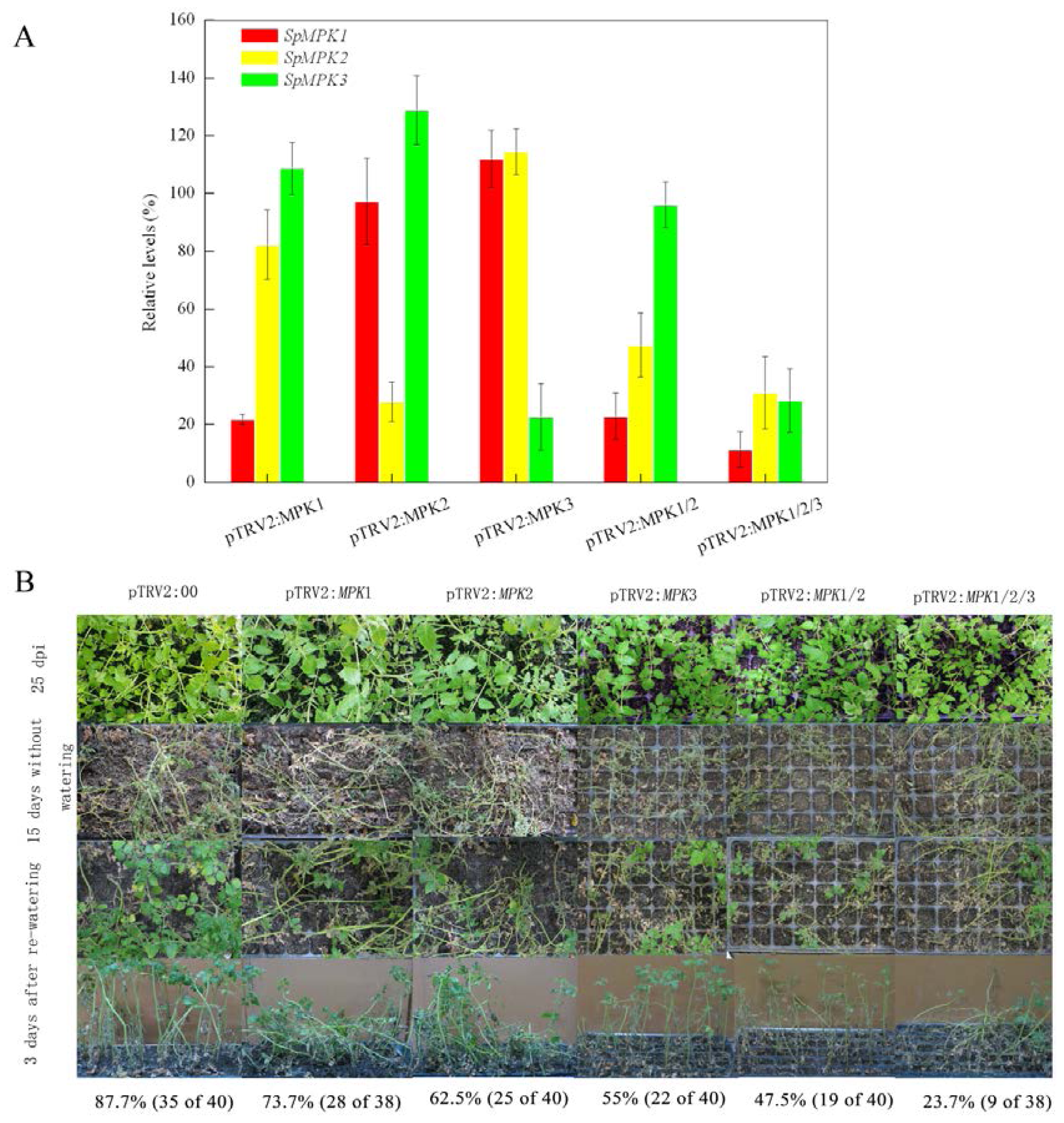

Acknowledgments
Conflicts of Interest
References
- Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y.; Champion, A.; Kreis, M.; Zhang, S.; Hirt, H.; Wilson, C. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci 2002, 7, 301–308. [Google Scholar]
- Tena, G.; Asai, T.; Chiu, W.-L.; Sheen, J. Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol 2001, 4, 392–400. [Google Scholar]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci 2001, 6, 520–527. [Google Scholar]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol 2009, 12, 421–426. [Google Scholar]
- Komis, G.; Illés, P.; Beck, M.; Šamaj, J. Microtubules and mitogen-activated protein kinase signalling. Curr. Opin. Plant Biol 2011, 14, 650–657. [Google Scholar]
- Jonak, C.; Ökrész, L.; Bögre, L.; Hirt, H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol 2002, 5, 415–424. [Google Scholar]
- Lampard, G.R.; Lukowitz, W.; Ellis, B.E.; Bergmann, D.C. Novel and expanded roles for MAPK signaling in Arabidopsis stomatal cell fate revealed by cell type-specific manipulations. Plant Cell Online 2009, 21, 3506–3517. [Google Scholar]
- Wang, H.; Ngwenyama, N.; Liu, Y.; Walker, J.C.; Zhang, S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell Online 2007, 19, 63–73. [Google Scholar]
- Li, Q.; Xie, Q.-G.; Smith-Becker, J.; Navarre, D.A.; Kaloshian, I. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol. Plant Microbe Interact 2006, 19, 655–664. [Google Scholar]
- Kandoth, P.K.; Ranf, S.; Pancholi, S.S.; Jayanty, S.; Walla, M.D.; Miller, W.; Howe, G.A.; Lincoln, D.E.; Stratmann, J.W. Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc. Natl. Acad. Sci. USA 2007, 104, 12205–12210. [Google Scholar]
- Melech-Bonfil, S.; Sessa, G. The SlMKK2 and SlMPK2 genes play a role in tomato disease resistance to Xanthomonas campestris pv. vesicatoria. Plant Signal. Behav. 2011, 6, 154–156. [Google Scholar]
- Hirayama, T.; Shinozaki, K. Perception and transduction of abscisic acid signals: Keys to the function of the versatile plant hormone ABA. Trends Plant Sci 2007, 12, 343–351. [Google Scholar]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 2001, 27, 325–333. [Google Scholar]
- Alzwiy, I.A.; Morris, P.C. A mutation in the Arabidopsis MAP kinase kinase 9 gene results in enhanced seedling stress tolerance. Plant Sci 2007, 173, 302–308. [Google Scholar]
- Xing, Y.; Jia, W.; Zhang, J. AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J. Exp. Bot 2007, 58, 2969–2981. [Google Scholar]
- Hwa, C.-M.; Yang, X.-C. The AtMKK3 pathway mediates ABA and salt signaling in Arabidopsis. Acta Physiol. Plant 2008, 30, 277–286. [Google Scholar]
- Xing, Y.; Jia, W.; Zhang, J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J 2008, 54, 440–451. [Google Scholar]
- Wang, X.-J.; Zhu, S.-Y.; Lu, Y.-F.; Zhao, R.; Xin, Q.; Wang, X.-F.; Zhang, D.-P. Two coupled components of the mitogen-activated protein kinase cascade MdMPK1 and MdMKK1 from apple function in ABA signal transduction. Plant Cell Physiol 2010, 51, 754–766. [Google Scholar]
- Xing, Y.; Jia, W.; Zhang, J. AtMKK1 and AtMPK6 are involved in abscisic acid and sugar signaling in Arabidopsis seed germination. Plant Mol. Biol 2009, 70, 725–736. [Google Scholar]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E. Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar]
- Nakagami, H.; Soukupová, H.; Schikora, A.; Zárský, V.; Hirt, H. A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J. Biol. Chem 2006, 281, 38697–38704. [Google Scholar]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 2008, 18, 1190–1198. [Google Scholar]
- Pitzschke, A.; Djamei, A.; Bitton, F.; Hirt, H. A major role of the MEKK1–MKK1/2–MPK4 pathway in ROS signalling. Mol. Plant 2009, 2, 120–137. [Google Scholar]
- Gudesblat, G.E.; Iusem, N.D.; Morris, P.C. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol 2007, 173, 713–721. [Google Scholar]
- Liu, P.F.; Chang, W.C.; Wang, Y.K.; Chang, H.Y.; Pan, R.L. Signaling pathways mediating the suppression of Arabidopsis thaliana Ku gene expression by abscisic acid. Biochim. Biophys. Acta-Gene Regul. Mech 2008, 1779, 164–174. [Google Scholar]
- Jammes, F.; Song, C.; Shin, D.; Munemasa, S.; Takeda, K.; Gu, D.; Cho, D.; Lee, S.; Giordo, R.; Sritubtim, S. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20520–20525. [Google Scholar]
- Desikan, R.; Cheung, M.K.; Bright, J.; Henson, D.; Hancock, J.T.; Neill, S.J. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J. Exp. Bot 2004, 55, 205–212. [Google Scholar]
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol 2010, 61, 561–591. [Google Scholar]
- Wang, P.; Song, C.P. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 2008, 178, 703–718. [Google Scholar]
- Cho, D.; Shin, D.; Jeon, B.W.; Kwak, J.M. ROS-mediated ABA signaling. J. Plant Biol 2009, 52, 102–113. [Google Scholar]
- MacRobbie, E. Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. Lond. Ser. B 1998, 353, 1475–1488. [Google Scholar]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—Powdery mildew interaction. Plant J 1997, 11, 1187–1194. [Google Scholar]
- Ichimura, K.; Casais, C.; Peck, S.C.; Shinozaki, K.; Shirasu, K. MEKK1 is required for MPK4 activation and regulates tissue-specific and temperature-dependent cell death in Arabidopsis. J. Biol. Chem 2006, 281, 36969–36976. [Google Scholar]
- Kong, F.; Wang, J.; Cheng, L.; Liu, S.; Wu, J.; Peng, Z.; Lu, G. Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum. Gene 2012, 499, 108–120. [Google Scholar]
- Brock, A.K.; Willmann, R.; Kolb, D.; Grefen, L.; Lajunen, H.M.; Bethke, G.; Lee, J.; Nürnberger, T.; Gust, A.A. The Arabidopsis mitogen-activated protein kinase phosphatase PP2C5 affects seed germination, stomatal aperture, and abscisic acid-inducible gene expression. Plant Physiol 2010, 153, 1098–1111. [Google Scholar]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar]
- Zhu, D.; Scandalios, J.G. Differential accumulation of manganese-superoxide dismutase transcripts in maize in response to abscisic acid and high osmoticum. Plant Physiol 1994, 106, 173–178. [Google Scholar]
- Desikan, R.; Reynolds, A.; Hancock, J.; Neill, S. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J 1998, 330, 115–120. [Google Scholar]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot 2010, 61, 2979–2990. [Google Scholar]
- Pei, Z.-M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S. Virus-induced gene silencing in tomato. Plant J 2002, 31, 777–786. [Google Scholar]
- Velásquez, A.C.; Chakravarthy, S.; Martin, G.B. Virus-induced gene silencing (VIGS) in Nicotiana benthamian a and tomato. J. Vis. Exp 2009. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Gong, P.; Zhang, J.; Li, H.; Yang, C.; Zhang, C.; Zhang, X.; Khurram, Z.; Zhang, Y.; Wang, T.; Fei, Z. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot 2010, 61, 3563–3575. [Google Scholar]
- Lee, S.; Choi, H.; Suh, S.; Doo, I.-S.; Oh, K.-Y.; Choi, E.J.; Taylor, A.T.S.; Low, P.S.; Lee, Y. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 1999, 121, 147–152. [Google Scholar]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell Online 2012, 24, 275–287. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, C.; Yan, J.-M.; Li, Y.-Z.; Zhang, Z.-C.; Wang, Q.-L.; Liang, Y. Silencing the SpMPK1, SpMPK2, and SpMPK3 Genes in Tomato Reduces Abscisic Acid—Mediated Drought Tolerance. Int. J. Mol. Sci. 2013, 14, 21983-21996. https://doi.org/10.3390/ijms141121983
Li C, Yan J-M, Li Y-Z, Zhang Z-C, Wang Q-L, Liang Y. Silencing the SpMPK1, SpMPK2, and SpMPK3 Genes in Tomato Reduces Abscisic Acid—Mediated Drought Tolerance. International Journal of Molecular Sciences. 2013; 14(11):21983-21996. https://doi.org/10.3390/ijms141121983
Chicago/Turabian StyleLi, Cui, Jian-Min Yan, Yun-Zhou Li, Zhen-Cai Zhang, Qiao-Li Wang, and Yan Liang. 2013. "Silencing the SpMPK1, SpMPK2, and SpMPK3 Genes in Tomato Reduces Abscisic Acid—Mediated Drought Tolerance" International Journal of Molecular Sciences 14, no. 11: 21983-21996. https://doi.org/10.3390/ijms141121983





