Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications
Abstract
:1. Introduction
- (a)
- Finite-size effects (single-domain or multi-domain structures and quantum confinement of the electrons);
- (b)
- Surface effects, which results from the symmetry breaking of the crystal structure at the surface of the particle, oxidation, dangling bonds, existence of surfactants, surface strain, or even different chemical and physical structures of internal “core” and surface “shell” parts of the nanoparticle.
- (a)
- The external magnetostatic energy (EMS), which increases with the volume of the particle;
- (b)
- The domain-wall energy (Edw), which increases with the interfacial area between domains.
- (a)
- Spin rotation instead of domain wall motion
- (b)
- Shape anisotropy. Coercivity is smaller when the particles are spherical.
- (a)
- The size of the particles
- (b)
- The effective anisotropy constant, Keff
- (c)
- The applied magnetic field
- (d)
- The experimental measurement time
2. Surface Effects
- (a)
- The existence of randomly oriented uncompensated surface spins.
- (b)
- The existence of canted spins.
- (c)
- The existence of a spin-glass-like behavior of the surface spins.
- (d)
- The existence of a magnetically dead layer at the surface.
- (e)
- The enhancement of the magnetic anisotropy which results from surface anisotropy.
3. Ferrite Nanoparticles
4. Applications of Magnetic Nanoparticles
4.1. Properties for Medical Applications
- (a)
- The magnetic nanoparticles should be biocompatible and non-toxic.
- (b)
- The magnetic nanoparticles are preferred to be sufficiently small (10–50 nm). This will have several advantages:
- (i)
- The nanoparticles will preserve their colloidal stability and resist aggregation if their magnetic interaction is reduced. This can be achieved if their magnetism disappears after removal of applied magnetic field. This superparamagnetic behavior is only achievable under certain particle size and above the blocking temperature.
- (ii)
- The dipole-dipole interactions scale as r6 (r is the radius of the particle). Hence, the dipolar interactions become very small when the particle size becomes very small. This will serve to minimize particle aggregation when the field is applied.
- (iii)
- Decreasing size means larger surface area for certain volume (or mass) of the particle. The efficiency of coating (and also the attachment of ligands) will improve leading to even more resistance to agglomeration, avoidance of biological clearance and better targeting.
- (iv)
- Being very small, the particles can remain in the circulation after injection and pass through the capillary systems of organs and tissues avoiding vessel embolism.
- (v)
- The magnetic particles will be stable in water at pH = 7 and in a physiological environment.
- (vi)
- Precipitation due to gravitation forces can be avoided with small particles.
- (c)
- The magnetic particles must have a high saturation magnetization. This is an important requirement for two reasons:
- (i)
- The movement of the particles in the blood can be controlled with a moderate external magnetic field.
- (ii)
- The particles can be moved close to the targeted pathologic tissue.
4.2. Biocompatibility and Toxicity of the Magnetic Nanoparticles
- (a)
- Coating the NPs with organic species (including surfactants or polymers).
- (b)
- Coating the NPs with an inorganic layer (such as silica or carbon).
4.3. Agglomeration and Particle Coating
4.4. Biomedical Applications of Magnetic Nanoparticles
4.4.1. Magnetic Hyperthermia
4.4.2. Drug and Gene Delivery
4.4.3. MNP as MRI Contrast Agents (CA)
4.4.3.1. Proton’s Relaxation Due to Paramagnetic Complexes
4.4.3.2. Proton’s Relaxation Due to MNP
4.4.3.3. Frequency Scales and Motional Regimes
5. Conclusions

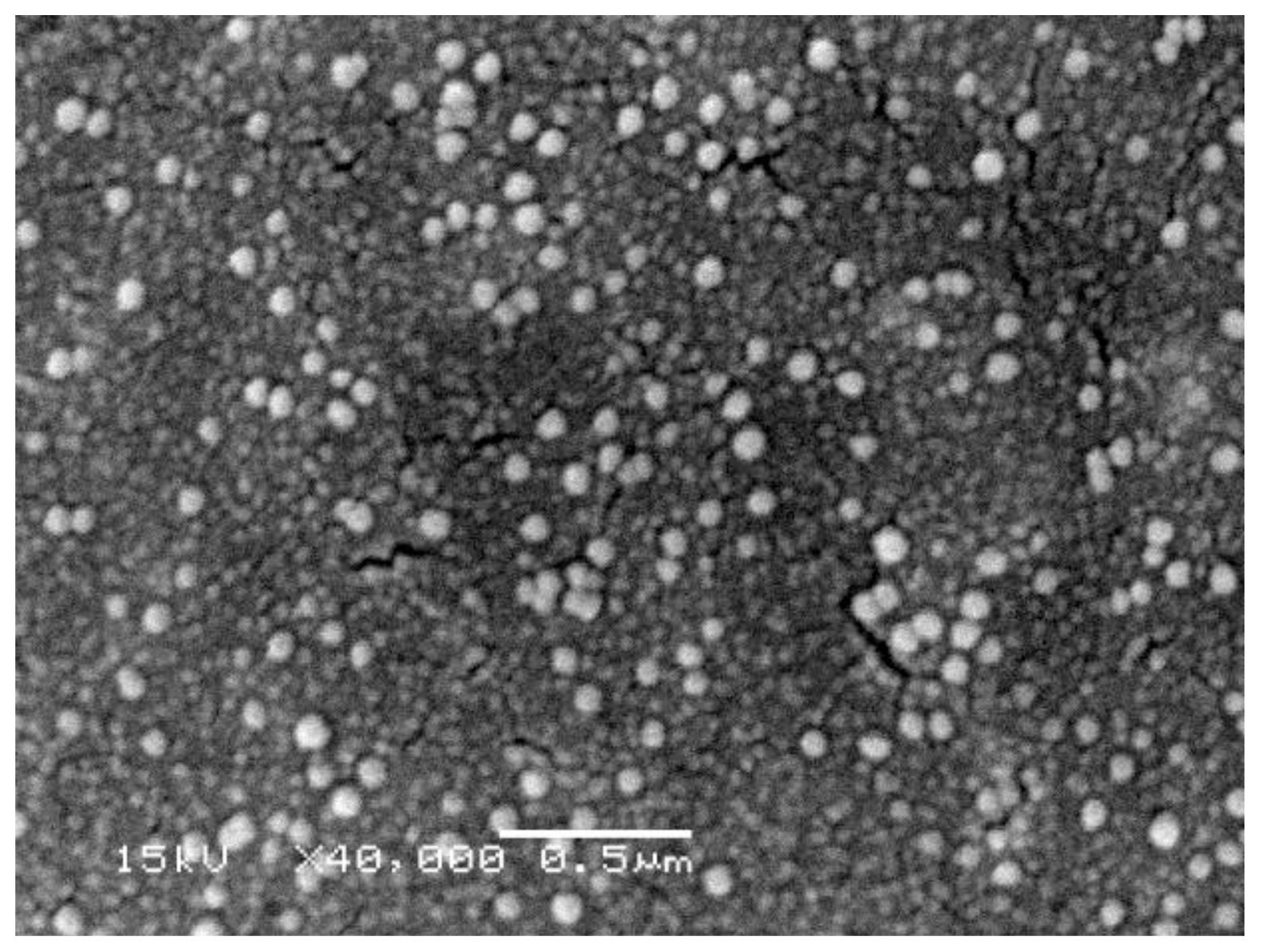

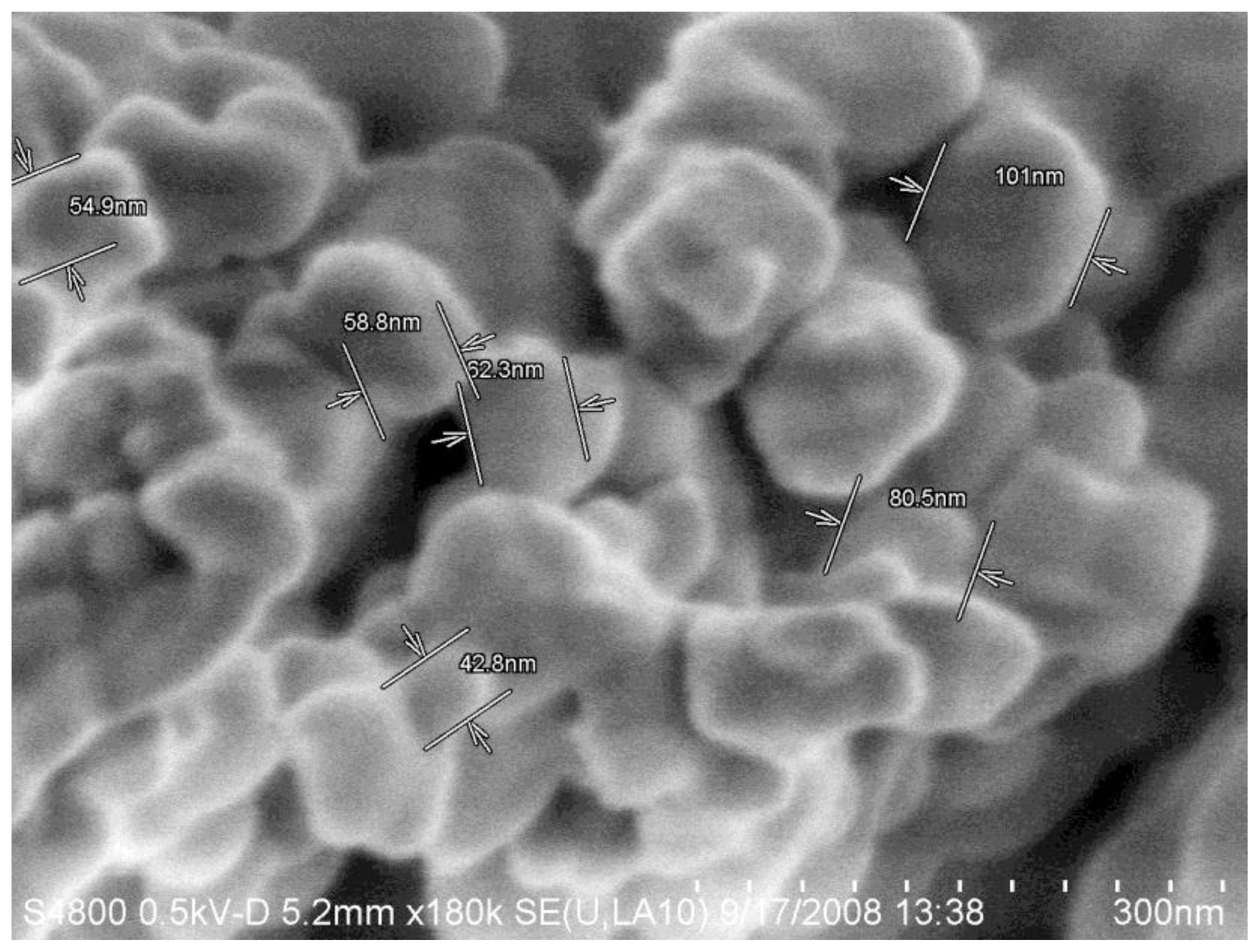
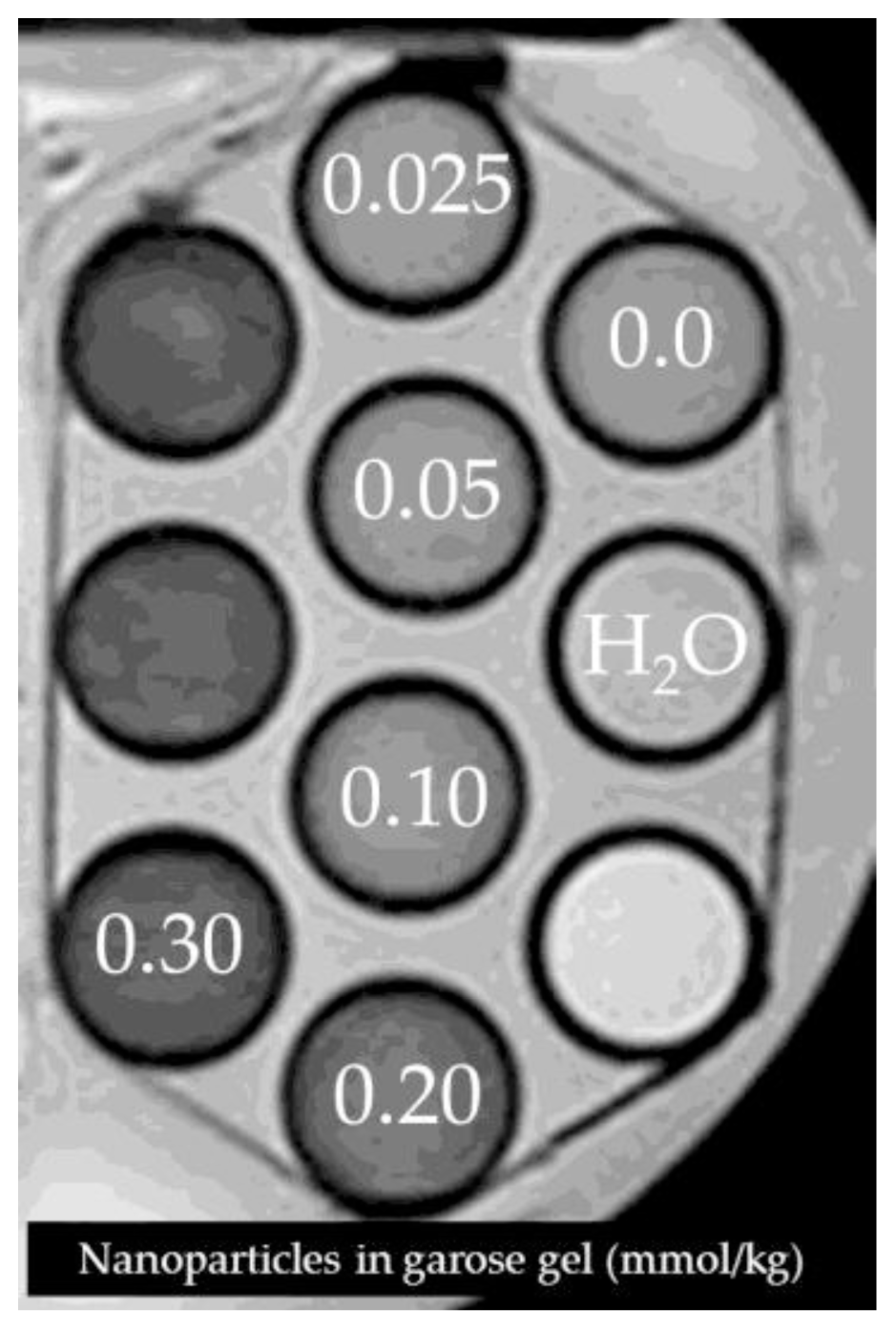
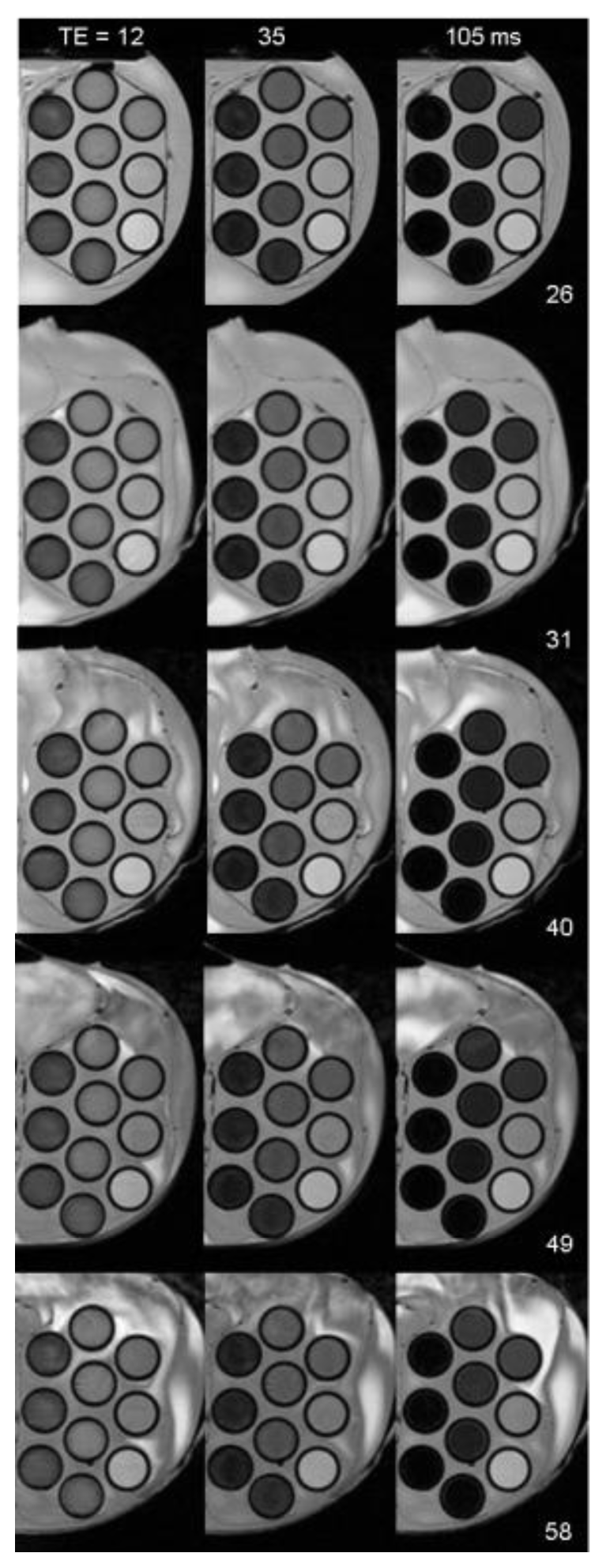

Acknowledgments
Conflicts of Interest
References
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev 2005, 74, 489–520. [Google Scholar]
- Alberto, P. Guimarães Principles of Nanomagnetism; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bertotti, G. Hysterisis in Magnetism: For Physicists, Materials Scientists, and Engineers; Academic Press-Elsevier: Waltham, MA, USA, 1998. [Google Scholar]
- Frenkel, J.; Doefman, J. Spontaneous and induced magnetisation in ferromagnetic bodies. Nature 1930, 126, 274–275. [Google Scholar]
- Kittel, C. Theory of the structure of ferromagnetic domains in films and small particles. Phys. Rev 1946, 70, 965–971. [Google Scholar]
- Mørup, S.; Hansen, M.F.; Frandsen, C. Comprehensive Nanoscience and Technology; Andrews, D., Scholes, G., Wiederrecht, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 437–491. [Google Scholar]
- Néel, L. Théorie du trainage magnétique des ferromagné tiques en grains fins avec applications aux terres cuites. Ann. Geophys 1949, 5, 99–136. [Google Scholar]
- Bean, C.P.; Livingston, J.D. Superparamagnetism. J. Appl. Phys 1959, 30, 120S–129S. [Google Scholar]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D 2009, 42, 013001. [Google Scholar]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: Magnetic and transport properties. J. Phys. D 2002, 35, R15–R42. [Google Scholar]
- Ho, C.-H.; Lai, C.-H. Size-dependent magnetic properties of PtMn nanoparticles. IEEE Trans. Magn 2006, 42, 3069–3071. [Google Scholar]
- Dobrynin, A.N.; Ievlev, D.N.; Temst, K.; Lievens, P.; Margueritat, J.; Gonzalo, J.; Afonso, C.N.; Zhou, S.Q.; Vantomme, A.; Piscopiello, E.; van Tendeloo, G. Critical size for exchange bias in ferromagnetic-antiferromagnetic particles. Appl. Phys. Lett 2005, 87, 012501. [Google Scholar] [Green Version]
- Kodama, R.H. Magnetic nanoparticles. J. Magn. Magn. Mater 1999, 200, 359–372. [Google Scholar]
- Nunes, A.C.; Yang, L. Calculated ferrite nanocrystal relaxation and its magnetic implications. Surf. Sci 1998, 399, 225–233. [Google Scholar]
- Kodama, R.H.; Berkowitz, A.E.; Mcniff, E.J.; Foner, S. Surface spin disorder in ferrite nanoparticles ( invited). J. Appl. Phys 1997, 81, 5552–5558. [Google Scholar]
- Kodama, R.; Berkowitz, A. Atomic-scale magnetic modeling of oxide nanoparticles. Phys. Rev. B 1999, 59, 6321–6336. [Google Scholar]
- Mørup, S.; Brok, E.; Frandsen, C. Spin Structures in Magnetic Nanoparticles 2013, 2013, 720629:1–720629:8.
- Billas, I.M.L.; Chatelain, A.; Heer, W.A. De magnetism from the atom to the bulk in iron, cobalt, and nickel clusters. Science 1994, 265, 1682–1684. [Google Scholar]
- Billas, I.M.L.; Chhtelain, A.; Heer, W.A. De Magnetism of Fe, Co and Ni clusters in molecular beams. J. Magn. Magn. Mater 1997, 168, 64–84. [Google Scholar]
- Kodama, R.; Berkowitz, A.; McNiff, E.; Foner, S. Surface spin disorder in NiFe2O4 nanoparticles. Phys. Rev. Lett 1996, 77, 394–397. [Google Scholar]
- Respaud, M.; Broto, J.M.; Rakoto, H.; Fert, A.R.; Thomas, L.; Barbara, B.; Verelst, M.; Snoeck, E.; Lecante, P.; Mosset, A.; et al. Surface effects on the magnetic properties of ultrafine cobalt particles. Phys. Rev. B 1998, 57, 2925–2935. [Google Scholar]
- Jamet, M.; Wernsdorfer, W.; Thirion, C.; Mailly, D.; Dupuis, V.; Mélinon, P.; Pérez, A. Magnetic anisotropy of a single cobalt nanocluster. Phys. Rev. Lett 2001, 86, 4676–4679. [Google Scholar]
- Jamet, M.; Wernsdorfer, W.; Thirion, C.; Dupuis, V.; Mélinon, P.; Pérez, L.; Mailly, D. Magnetic anisotropy in single clusters. Phys. Rev. B 2004, 69, 024401. [Google Scholar]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface effects in metallic iron nanoparticles. Phys. Rev. Lett 1994, 72, 282–285. [Google Scholar]
- Luis, F.; Torres, J.M.; García, L.M.; Bartolomé, J.; Stankiewicz, J.; Petroff, F.; Fettar, F.; Maurice, J.-L.; Vaurès, A. Enhancement of the magnetic anisotropy of nanometer-sized Co clusters: Influence of the surface and of interparticle interactions. Phys. Rev. B 2002, 65, 094409. [Google Scholar]
- Xie, Y.; Blackman, J.A. Magnetocrystalline anisotropy and orbital polarization in ferromagnetic transition metals. Phys. Rev. B 2004, 69, 172407. [Google Scholar]
- Binns, C.; Baker, S.H.; Maher, M.J.; Louch, S.; Thornton, S.C.; Edmonds, K.W.; Dhesi, S.S.; Brookes, N.B. From Isolated Particles to Nanostructured Materials. Phys. Status Solidi A 2002, 189, 339–350. [Google Scholar]
- Yanes, R.; Chubykalo-Fesenko, O.; Kachkachi, H.; Garanin, D.A.; Evans, R.; Chantrell, R.W. Effective anisotropies and energy barriers of magnetic nanoparticles with Néel surface anisotropy. Phys. Rev. B 2007, 76, 064416. [Google Scholar]
- Kiwi, M. Exchange bias theory. J. Magn. Magn. Mater 2001, 234, 584–595. [Google Scholar]
- Si, P.Z.; Li, D.; Lee, J.W.; Choi, C.J.; Zhang, Z.D.; Geng, D.Y.; Brück, E. Unconventional exchange bias in oxide-coated manganese nanoparticles. Appl. Phys. Lett 2005, 87, 133122. [Google Scholar]
- Si, P.Z.; Li, D.; Choi, C.J.; Li, Y.B.; Geng, D.Y.; Zhang, Z.D. Large coercivity and small exchange bias in Mn3O4 / MnO nanoparticles. Solid State Commun 2007, 142, 723–726. [Google Scholar]
- Berkowitz, A.E.; Rodriguez, G.F.; Hong, J.I.; An, K.; Hyeon, T.; Agarwal, N.; Smith, D.J.; Fullerton, E.E. Monodispersed MnO nanoparticles with epitaxial Mn3O4 shells. J. Phys. D 2008, 41, 34007–34007. [Google Scholar]
- Sun, X.; Huls, N.F.; Sigdel, A.; Sun, S. Tuning Exchange Bias in Core/Shell FeO/Fe3O4 Nanoparticles. Nano Lett 2012, 12, 246–251. [Google Scholar]
- Zeng, H.; Sun, S.; Li, J.; Wang, Z.L.; Liu, J.P. Tailoring magnetic properties of core/shell nanoparticles. Appl. Phys. Lett 2004, 85, 792–795. [Google Scholar]
- Smit, J.; Wijn, H.P.J. Ferrites; Philips’ Technical Library: Eindhoven The Netherlands, 1959; pp. 139–142. [Google Scholar]
- Makovec, D.; Drofenik, M. Non-stoichiometric zinc-ferrite spinel nanoparticles. J. Nanoparticle Res 2008, 10, 131–141. [Google Scholar]
- Makovec, D.; Kodre, A.; Gyergyek, S.; Arčon, I.; Jagodič, M.; Drofenik, M. Influence of synthesis method on structural and magnetic properties of cobalt ferrite nanoparticles. J. Nanoparticle Res 2010, 12, 1263–1273. [Google Scholar]
- Ammar, S.; Jouini, N.; Fiévet, F.; Stephan, O.; Marhic, C.; Richard, M.; Villain, F.; Cartier dit Moulin, C.; Brice, S.; Sainctavit, P. Influence of the synthesis parameters on the cationic distribution of ZnFe2O4 nanoparticles obtained by forced hydrolysis in polyol medium. J. Non. Cryst. Solids 2004, 345–346, 658–662. [Google Scholar]
- Sato, T.; Haneda, K.; Seki, M.; Iijima, T. Morphology and magnetic properties of ultrafine ZnFe2O4 particles. Appl. Phys. A 1990, 50, 13–16. [Google Scholar]
- Kamiyama, T.; Haneda, K.; Sato, T.; Ikeda, S.; Asano, H. Cation distribution in ZnFe2O4 fine particles studied by neutron powder diffraction. Solid State Commun 1992, 81, 563–566. [Google Scholar]
- Jeyadevan, B.; Tohji, K.; Nakatsuka, K. Fine structure. J. Appl. Phys 1994, 76, 6325–6327. [Google Scholar]
- Hamdeh, H.H.; Ho, J.C.; Oliver, S.A.; Willey, R.J.; Oliveri, G.; Busca, G.; Introduction, I. Magnetic properties of partially-inverted zinc ferrite aerogel powders. J. Appl. Phys 1997, 81, 1851–1858. [Google Scholar]
- Makovec, D.; Kodre, A.; Arčon, I.; Drofenik, M. Structure of manganese zinc ferrite spinel nanoparticles prepared with co-precipitation in reversed microemulsions. J. Nanoparticle Res 2009, 11, 1145–1158. [Google Scholar]
- Makovec, D.; Kodre, A.; Arčon, I.; Drofenik, M. The structure of compositionally constrained zinc-ferrite spinel nanoparticles. J. Nanoparticle Res 2011, 13, 1781–1790. [Google Scholar]
- Bellouard, C.; Mirebeau, I.; Hennion, M. Magnetic correlations of fine ferromagnetic particles studied by small-angle neutron scattering. Phys. Rev. B 1996, 53, 5570–5578. [Google Scholar]
- Lin, D.; Nunes, A.C.; Majkrzak, C.F.; Berkowitz, A.E. Polarized neutron study of the magnetization density distribution within a CoFe2O4 colloidal particle II. J. Magn. Magn. Mater 1995, 145, 343–348. [Google Scholar]
- Hendriksen, P.V.; Bødker, F.; Linderoth, S.; Wells, S.; Mørup, S. Ultrafine maghemite particles. I. Studies of induced magnetic texture. J. Phys. Condens. Matter 1994, 6, 3081–3090. [Google Scholar]
- Linderoth, S.; Hendriksen, P.V.; Bødker, F.; Wells, S.; Davies, K.; Charles, S.W.; Mørup, S. On spin-canting in maghemite particles. J. Appl. Phys 2004, 75, 6583–6585. [Google Scholar]
- Xuan, Y.; Li, Q.; Yang, G. Synthesis and magnetic properties of Mn–Zn ferrite nanoparticles. J. Magn. Magn. Mater 2007, 312, 464–469. [Google Scholar]
- Wang, J.; Zeng, C.; Peng, Z.; Chen, Q. Synthesis and magnetic properties of Zn1-xMnxFe2O4 nanoparticles. Phys. B Condens. Matter 2004, 349, 124–128. [Google Scholar]
- Mathur, P.; Thakur, A.; Singh, M. Effect of nanoparticles on the magnetic properties of Mn-Zn soft ferrite. J. Magn. Magn. Mater 2008, 320, 1364–1369. [Google Scholar]
- Del Bianco, L.; Hernando, A.; Multigner, M.; Prados, C.; Sánchez-López, J.C.; Fernández, A.; Conde, C.F.; Conde, A. Evidence of spin disorder at the surface–core interface of oxygen passivated Fe nanoparticles. J. Appl. Phys 1998, 84, 71–76. [Google Scholar]
- Gazeau, F.; Dubois, E.; Hennion, M.; Perzynski, R.; Raikher, Y. Quasi-elastic neutron scattering on γ-Fe2O3 nanoparticles. Eur. Lett 1997, 40, 575–580. [Google Scholar]
- Hennion, M.; Bellouard, C.; Mirebeau, I.; Dormann, J.L.; Nogues, M. Dual Spin Dynamics of Small Fe Particles. Eur. Lett 1994, 25, 43–48. [Google Scholar]
- Millan, A.; Urtizberea, A.; Silva, N.J.O.; Palacio, F.; Amaral, V.S.; Snoeck, E.; Serin, V. Surface effects in maghemite nanoparticles. J. Magn. Magn. Mater 2007, 312, L5–L9. [Google Scholar]
- Gaharwar, A.K.; Wong, J.E.; Müller-Schulte, D.; Bahadur, D.; Richtering, W. Magnetic Nanoparticles Encapsulated Within a Thermoresponsive Polymer. J. Nanosci. Nanotechnol 2009, 9, 5355–5361. [Google Scholar]
- Iida, H.; Takayanagi, K.; Nakanishi, T.; Osaka, T. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J. Colloid Interface Sci 2007, 314, 274–280. [Google Scholar]
- Brockhouse, B.N.; Corliss, L.M.; Hastings, J.M. Multiple scattering of slow neutrons by flat specimens and magnetic scattering by zinc ferrite. Phys. Rev 1955, 98, 1721–1727. [Google Scholar]
- Lotgering, F.K. The influence of Fe3+ ions at tetrahedral sites on the magnetic properties of ZnFe2O4. J. Phys. Chem. Solids 1966, 27, 139–145. [Google Scholar]
- Ligenza, S. A study of the 6S5/2-term splitting of an Fe3+ ion in zinc ferrite by neutron spectroscopy. Phys. Status Solidi 1976, 75, 315–326. [Google Scholar]
- O’Neill, H.S.C. Temperature dependence of the cation distribution in zinc ferrite (ZnFe2O4) from powder XRD structural refinements. Eur. J. Miner 1992, 4, 571–580. [Google Scholar]
- Makovec, D.; Košak, A.; Drofenik, M. The preparation of MnZn–ferrite nanoparticles in water-CTAB-hexanol microemulsions. Nanotechnology 2004, 15, S160–S166. [Google Scholar]
- Sivakumar, N.; Narayanasamy, A.; Shinoda, K.; Chinnasamy, C.N.; Jeyadevan, B.; Greneche, J.M. Electrical and magnetic properties of chemically derived nanocrystalline cobalt ferrite. J. Appl. Phys 2007, 102, 013916. [Google Scholar]
- Tirosh, E.; Shemer, G.; Markovich, G. Optimizing cobalt ferrite nanocrystal synthesis using a magneto-optical probe. Chem. Mater 2006, 18, 465–470. [Google Scholar]
- Li, S.; John, V.T.; O’Connor, C.; Harris, V.; Carpenter, E. Cobalt-ferrite nanoparticles: Structure, cation distributions, and magnetic properties. J. Appl. Phys 2000, 87, 6223–6225. [Google Scholar]
- Berkowitz, A.E.; Shuele, W.J.; Flanders, P.J. Influence of crystallite size on the magnetic properties of acicular γ-Fe2O3 particles. J. Appl. Phys 1968, 39, 1261–1263. [Google Scholar]
- Jacob, J.; Khadar, M.A. Investigation of mixed spinel structure of nanostructured nickel ferrite. J. Appl. Phys 2010, 107, 114310. [Google Scholar]
- Obaidat, I.M.; Issa, B.; Albiss, B.A.; Qabaja, G.; Al Khaili, N.E.; Karam, Z.A.; Hefaity, A.M.; Qadri, S.; Al-Okour, A.; Haik, Y. Finite size and surface effects in ferrite nanoparticles. J. Nanoeng. Nanomanufacturing 2012, 2, 325–331. [Google Scholar]
- Auzans, E.; Zins, D.; Blums, E.; Massart, R. Synthesis and properties of Mn-Zn ferrite ferrofluids. J. Mater. Sci 1999, 34, 1253–1260. [Google Scholar]
- Kubo, R.; Nagamiya, T. Solid State Physics; McGraw Hill: New York NY, USA, 1969. [Google Scholar]
- Antoniak, C.; Farle, M.; Physik, F. Magnetism at the nanoscale: The case of FePt. Mod. Phys. Lett. B 2007, 21, 1111–1131. [Google Scholar]
- Upadhyay, R.V.; Mehta, R.V.; Parekh, K.; Srinivas, D.; Pant, R.P. Gd-substituted ferrite ferrofluid: A possible candidate to enhance pyromagnetic coefficient. J. Magn. Magn. Mater 1999, 201, 129–132. [Google Scholar]
- Hemeda, O.M.; Said, M.Z.; Barakat, M.M. Spectral and transport phenomena in Ni ferrite-substituted Gd O. J. Magn. Magn. Mater 2001, 224, 132–142. [Google Scholar]
- Brusentsova, T.N.; Brusentsov, N.A.; Kuznetsov, V.D.; Nikiforov, V.N. Synthesis and investigation of magnetic properties of Gd-substituted Mn–Zn ferrite nanoparticles as a potential low-TC agent for magnetic fluid hyperthermia. J. Magn. Magn. Mater 2005, 293, 298–302. [Google Scholar]
- Du, A.; Xu, Y. Effect of the surface anisotropy and the shape on the magnetization behavior in an egg-shaped nanoparticle. J. Magn. Magn. Mater 2008, 320, 2567–2570. [Google Scholar]
- De Biasi, E.; Ramos, C.A.; Zysler, R.D.; Fiorani, D. Metropolis algorithm for simulating hysteresis in ferromagnetic nanoparticles. Phys. B 2006, 372, 345–349. [Google Scholar]
- Labarta, A.; Batle, X.; Iglesias, O. Surface Effects in Magnetic Nanoparticles; Fiorani, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 105–140. [Google Scholar]
- Mamiya, H.; Nakatani, I.; Furubayashi, T. Blocking and freezing of magnetic moments for iron nitride fine particle systems. Phys. Rev. Lett 1998, 80, 177–180. [Google Scholar]
- Mørup, S. Superparamagnetism and spin glass ordering in magnetic nanocomposites. Eur. Lett 1994, 28, 671–676. [Google Scholar]
- Mørup, S.; Bodker, F.; Hendriksen, P.V.; Linderoth, S. Spin-glass-like ordering of the magnetic moments of interacting nanosized maghemite particles. Phys. Rev. B 1995, 52, 287–294. [Google Scholar]
- Taylor, R.; Coulombe, S.; Otanicar, T.; Phelan, P.; Gunawan, A.; Lv, W.; Rosengarten, G.; Prasher, R.; Tyagi, H. Small particles, big impacts: A review of the diverse applications of nanofluids. J. Appl. Phys 2013, 113, 011301. [Google Scholar]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl 2007, 46, 1222–44. [Google Scholar]
- Vallejo-Fernandez, G.; Whear, O.; Roca, A.G.; Hussain, S.; Timmis, J.; Patel, V.; O’Grady, K. Mechanisms of hyperthermia in magnetic nanoparticles. J. Phys. D 2013, 46, 312001. [Google Scholar]
- Thomas, R.; Park, I.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci 2013, 14, 15910–15930. [Google Scholar]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci 2013, 14, 15977–16009. [Google Scholar]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev 2009, 38, 2532–2542. [Google Scholar]
- Singamaneni, S.; Bliznyuk, V.N.; Binekc, C.; Tsymbalc, E.Y. Magnetic nanoparticles: Recent advances in synthesis, self-assembly and applications. J. Mater. Chem 2011, 21, 16819–16845. [Google Scholar]
- Skumryev, V.; Stoyanov, S.; Zhang, Y.; Hadjipanayis, G.; Givord, D.; Nogués, J. Beating the superparamagnetic limit with exchange bias. Nature 2003, 423, 850–853. [Google Scholar]
- Larsen, B.A.; Haag, M.A.; Serkova, N.J.; Shroyer, K.R.; Stoldt, C.R. Controlled aggregation of superparamagnetic iron oxide nanoparticles for the development of molecular magnetic resonance imaging probes. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed 2004, 17, 484–499. [Google Scholar]
- Zhu, D.; Liu, F.; Ma, L.; Liu, D.; Wang, Z. Nanoparticle-based systems for T1-weighted magnetic resonance imaging contrast agents. Int. J. Mol. Sci 2013, 14, 10591–10607. [Google Scholar]
- Papaefthymiou, G.C. Nanoparticle magnetism. Nano Today 2009, 4, 438–447. [Google Scholar]
- Ito, A.; Honda, H.; Kobayashi, T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol. Immunother 2006, 55, 320–328. [Google Scholar]
- Lübbe, A.S.; Bergemann, C.; Riess, H.; Lãbbe, A.S.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dã, B.; Herrinann, F.; et al. Clinical experiences with magnetic drug targeting: A Phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors in 14 patients with advanced solid tumors1. Cancer Res 1996, 56, 4686–4693. [Google Scholar]
- Nobuto, H.; Sugita, T.; Kubo, T.; Shimose, S.; Yasunaga, Y.; Murakami, T.; Ochi, M. Evaluation of systemic chemotherapy with magnetic liposomal doxorubicin and a dipole external electromagnet. Int. J. Cancer 2004, 109, 627–635. [Google Scholar]
- Ruysschaert, T.; Paquereau, L.; Winterhalter, M.; Fournier, D. Stabilization of liposomes through enzymatic polymerization of DNA. Nano Lett 2006, 6, 2755–2757. [Google Scholar]
- Zhang, L.; Granick, S. How to stabilize phospholipid liposomes (using nanoparticles). Nano Lett 2006, 6, 694–698. [Google Scholar]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar]
- Di Marco, M.; Guilbert, I.; Port, M.; Robic, C.; Couvreur, P.; Dubernet, C. Colloidal stability of ultrasmall superparamagnetic iron oxide (USPIO) particles with different coatings. Int. J. Pharm 2007, 331, 197–203. [Google Scholar]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar]
- Batlle, X.; Pérez, N.; Guardia, P.; Iglesias, O.; Labarta, A.; Bartolomé, F.; Garcıa, L.M.; Bartolomé, J.; Roca, A.G.; Morales, M.P.; Serna, C.J. Magnetic nanoparticles with bulk-like properties. J. Appl. Phys 2011, 109, 07B254. [Google Scholar]
- Nitin, N.; LaConte, L.E.W.; Zurkiya, O.; Hu, X.; Bao, G. Functionalization and peptide-based delivery of magnetic nanoparticles for intracellular MRI contrast. J. Biol. Inorg Chem 2004, 9, 706–712. [Google Scholar]
- Nafee, N.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Relevance of the colloidal stability of chitosan/PLGA nanoparticles on their cytotoxicity profile. Int. J. Pharm 2009, 381, 130–139. [Google Scholar]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar]
- Frankel, R.B.; Blakemore, R.P.; Wolfe, R.S. Magnetite in freshwater magnetotactic bacteria. Science 1979, 203, 1355–1356. [Google Scholar]
- Mann, S.; Frankel, R.B.; Blakemore, R. Structure, morphology and crystal growth of bacterial magnetite. Nature 1984, 310, 405–407. [Google Scholar]
- Gould, J.L.; Kirschvink, J.L.; Deffeyes, K.S. Bees have magnetic remanence. Science 1978, 201, 1026–1028. [Google Scholar]
- Zoeger, J.; Dunn, J.R.; Fuller, M. Magnetic material in the head of the common Pacific dolphin. Science 1981, 213, 892–894. [Google Scholar]
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev 2002, 54, 459–476. [Google Scholar]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm 2006, 307, 93–102. [Google Scholar]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev 2001, 53, 283–318. [Google Scholar]
- Shenoy, D.B.; Amiji, M.M. Poly(ethylene oxide)-modified poly(epsilon-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int. J. Pharm 2005, 293, 261–70. [Google Scholar]
- Briley-Saebo, K.; Bjørnerud, A.; Grant, D.; Ahlstrom, H.; Berg, T.; Kindberg, G.M. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: Implications for magnetic resonance imaging. Cell Tissue Res 2004, 316, 315–323. [Google Scholar]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene Glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int. J. Mol. Sci 2012, 13, 5554–5570. [Google Scholar]
- Zaitsev, V.S.; Filimonov, D.S.; Presnyakov, I.A.; Gambino, R.J.; Chu, B. Physical and chemical properties of magnetite and magnetite-polymer nanoparticles and their colloidal dispersions. J. Colloid Interface Sci 1999, 212, 49–57. [Google Scholar]
- Orive, G.; Ali, O.A.; Anitua, E.; Pedraz, J.L.; Emerich, D.F. Biomaterial-based technologies for brain anti-cancer therapeutics and imaging. Biochim. Biophys. Acta 2010, 1806, 96–107. [Google Scholar]
- Kateb, B.; Chiu, K.; Black, K.L.; Yamamoto, V.; Khalsa, B.; Ljubimova, J.Y.; Ding, H.; Patil, R.; Portilla-Arias, J.A.; Modo, M.; et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: What should be the policy? Neuroimage 2011, 54, S106–S124. [Google Scholar]
- Butterworth, M.D.; Illum, L.; Davis, S.S. Preparation of ultrafine silica- and PEG-coated magnetite particles. Colloids Surf. A 2001, 179, 93–102. [Google Scholar]
- Josephson, L.; Tung, C.H.; Moore, A.; Weissleder, R.; Josephson, L.; Tung, C.H.; Moore, A.; Weissleder, R. High efficiency intracellular magnetic labeling is possible with novel superparamagnetic tat peptide conjugates. Bioconjug. Chem 1999, 10, 186–191. [Google Scholar]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; Muhammed, M. Protective coating of superparamagnetic iron oxide nanoparticles. Chem. Mater 2003, 15, 1617–1627. [Google Scholar]
- Liu, Q.; Xu, Z. Self-assembled monolayer coatings on nanosized magnetic particles using 16-mercaptohexadecanoic acid. Langmuir 1995, 11, 4617–4622. [Google Scholar]
- Bautista, M.C.; Bomati-Miguel, O.; Zhao, X.; Morales, M.P.; González-Carreño, T.; Alejo, R.P.; De Ruiz-Cabello, J.; Veintemillas-Verdaguer, S. Comparative study of ferrofluids based on dextran-coated iron oxide and metal nanoparticles for contrast agents in magnetic resonance imaging. Nanotechnology 2004, 15, S154–S159. [Google Scholar]
- Berry, C.C.; Wells, S.; Charles, S.; Aitchison, G.; Curtis, A.S.G. Cell response to dextran-derivatised iron oxide nanoparticles post internalisation. Biomaterials 2004, 25, 5405–5413. [Google Scholar]
- Portet, D.; Denizot, B.; Rump, E.; Lejeune, J.-J.; Jallet, P. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J. Colloid Interface Sci 2001, 238, 37–42. [Google Scholar]
- Paul, K.G.; Frigo, T.B.; Groman, J.Y.; Groman, E.V. Synthesis of ultrasmall superparamagnetic iron oxides using reduced polysaccharides. Bioconjug. Chem 2004, 15, 394–401. [Google Scholar]
- LaConte, L.E.W.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O’Connor, C.J.; Hu, X.; Bao, G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J. Magn. Reson. Imaging 2007, 26, 1634–41. [Google Scholar]
- Ma, N.; Xu, L.; Wang, Q.; Zhang, X.; Zhang, W.; Li, Y.; Jin, L.; Li, S. Development and evaluation of new sustained-release floating microspheres. Int. J. Pharm 2008, 358, 82–90. [Google Scholar]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. In J. Phys. D; 2009; Volume 42, p. 224001. [Google Scholar]
- Krishnan, K.M. Advances in magnetics biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Treans. Magn 2010, 46, 2523–2558. [Google Scholar]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 2003, 36, R167–R181. [Google Scholar]
- Fortin, J.P.; Gaxeau, G.; Wilhelm, C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys 2008, 37, 223–228. [Google Scholar]
- Christophi, C.; Winkworth, A.; Muralihdaran, V. The treatment of malignancy by hyperthermia. Surg. Oncol 1999, 7, 83–90. [Google Scholar]
- Wust, P.; Gneveckow, U.; Johannsen, M.; Böhmer, D.; Henkel, T.; Kahmann, F.; Sehouli, J.; Felix, R.; Ricke, J.; Jordan, A. Magnetic nanoparticles for interstitial thermotherapy—feasibility, tolerance and achieved temperatures. Int. J. Hyperth 2006, 22, 673–685. [Google Scholar]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater 2007, 311, 187–192. [Google Scholar]
- Barry, S.E. Challenges in the development of magnetic particles for therapeutic applications. Int. J. Hyperth 2008, 24, 451–66. [Google Scholar]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth 2008, 24, 467–474. [Google Scholar]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D 2003, 36, R198–R206. [Google Scholar]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; Jordan, A. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol 2007, 81, 53–60. [Google Scholar]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; WaldÖFner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth 2005, 21, 637–647. [Google Scholar]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldöfner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol 2007, 52, 1653–1662. [Google Scholar]
- Koneracka, M.; Kopc, P.; Antalmh, M.; Timko, M.; Ramchand, C.N.; Lobo, D.; Mehta, R.V.; Upadhyay, R.V. Immobilization of proteins and enzymes to fine magnetic particles. J. Magn. Magn. Mater 1999, 201, 427–430. [Google Scholar]
- Koneracka, M.; Kopcansky, P.; Timko, M.; Ramchand, C.N.; de Sequeira, A.; Trevan, M. Direct binding procedure of proteins and enzymes to fine magnetic particles. J. Mol. Catal. B 2002, 18, 13–18. [Google Scholar]
- Senyei, A.; Widder, K.; Czerlinski, C. Magnetic guidance of drug carrying microspheres. J. Appl. Phys 1978, 49, 3578–3583. [Google Scholar]
- Mosbach, K.; Schröder, U. Preparation and application of magnetic polymers for targeting of drugs. FEBS Lett 1979, 102, 112–116. [Google Scholar]
- Alexiou, C.; Arnold, W.; Klein, R.J.; Parak, F.G.; Hulin, P.; Bergemann, C.; Erhardt, W.; Wagenpfeil, S.; Lübb, A.S. Locoregional cancer treatment with magnetic drug targeting. Cancer Res 2000, 60, 6641–6648. [Google Scholar]
- Lübbe, A.S.; Alexiou, C.; Bergemann, C. Clinical applications of magnetic drug targeting. J. Surg. Res 2001, 95, 200–206. [Google Scholar]
- Häfeli, U.O.; Gilmour, K.; Zhou, A.; Lee, S.; Hayden, M.E. Modeling of magnetic bandages for drug targeting: Button versus Halbach arrays. J. Magn. Magn. Mater 2007, 311, 323–329. [Google Scholar]
- Häfeli, U.; Pauer, G.; Failing, S.; Tapolsky, G. Radiolabeling of magnetic particles with rhenium-188 for cancer therapy. J. Magn. Magn. Mater 2001, 225, 73–78. [Google Scholar]
- Mah, C.; Fraites, T.J.; Zolotukhin, I.; Song, S.; Flotte, T.R.; Dobson, J.; Batich, C.; Byrne, B.J. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther 2002, 6, 106–112. [Google Scholar]
- Caplen, N. Cystic fibrosis gene therapy trials and tribulations. Trends Mol. Med 2001, 7, 488. [Google Scholar]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar]
- Plank, C.; Schillinger, U.; Scherer, F.; Bergemann, C.; Rémy, J.-S.; Krötz, F.; Anton, M.; Lausier, J.; Rosenecker, J. The magnetofection method: Using magnetic force to enhance gene delivery. Biol. Chem 2003, 384, 737–747. [Google Scholar]
- Mykhaylyk, O.; Zelphati, O.; Rosenecker, J.; Plank, C. siRNA delivery by magnetofection. Curr. Opin. Mol. Ther 2003, 10, 493–505. [Google Scholar]
- Stride, E.; Porter, C.; Prieto, A.G.; Pankhurst, Q. Enhancement of microbubble mediated gene delivery by simultaneous exposure to ultrasonic and magnetic fields. Ultrasound Med. Biol 2009, 35, 861–868. [Google Scholar]
- Hüttinger, C.; Hirschberger, J.; Jahnke, A.; Köstlin, R.; Brill, T.; Plank, C.; Küchenhoff, H.; Krieger, S.; Schillinger, U. Neoadjuvant gene delivery of feline granulocyte-macrophage colony-stimulating factor using magnetofection for the treatment of feline fibrosarcomas: A phase I trial. J. Gene Med 2008, 10, 655–667. [Google Scholar]
- Dobson, J.; St Pierre, T.G. Application of the ferromagnetic transduction model to DC and pulsed magnetic fields: Effects on epileptogenic tissue and implications for cellular phone safety. Biochem. Biophys. Res. Commun 1996, 227, 718–723. [Google Scholar]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell-surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar]
- Wang, N.; Ingber, D.E. Probing transmembrane mechanical coupling and cytomechanics using magnetic twisting cytometry. Biochem. Cell Biol 1995, 73, 327–335. [Google Scholar]
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. SENSE: Sensitivity encoding for fast MRI. Magn. Reson. Med 1999, 42, 952–962. [Google Scholar]
- Pouliquen, D.; Perroud, H.; Calza, F.; Jallet, P.; Le Jeune, J.J. Investigation of the magnetic properties of iron oxide nanoparticles used as contrast agent for MRI. Magn. Reson. Med 1992, 24, 75–84. [Google Scholar]
- Wang, Y.X.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol 2001, 11, 2319–2331. [Google Scholar]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest. Radiol 2005, 40, 715–724. [Google Scholar]
- Engström, M.; Klasson, A.; Pedersen, H.; Vahlberg, C.; Käll, P.-O.; Uvdal, K. High proton relaxivity for gadolinium oxide nanoparticles. MAGMA 2006, 19, 180–186. [Google Scholar]
- Yan, G.-P.; Robinson, L.; Hogg, P. Magnetic resonance imaging contrast agents: Overview and perspectives. Radiography 2007, 13, e5–e19. [Google Scholar]
- Corti, M.; Lascialfari, A.; Micotti, E.; Castellano, A.; Donativi, M.; Quarta, A.; Cozzoli, P.D.; Manna, L.; Pellegrino, T.; Sangregorio, C. Magnetic properties of novel superparamagnetic MRI contrast agents based on colloidal nanocrystals. J. Magn. Magn. Mater 2008, 320, e320–e323. [Google Scholar]
- Geraldes, C.F.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging Mol. Imaging 2009, 4, 1–23. [Google Scholar]
- Lauffer, R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: Theory and design. Chem. Rev 1987, 87, 901–927. [Google Scholar]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev 1999, 99, 2293–2352. [Google Scholar]
- Ayant, Y.; Belorizky, E.; Alizon, J.; Gallice, J. Calcul des densit s spectrales r sultant d’un mouvement aléatoire de translation en relaxation par interaction dipolaire magnétique dans les liquides. In J. Phys. A; 1975; Volume 36, pp. 991–1004. [Google Scholar]
- Freed, J.H. Dynamic effects of pair correlation functions on spin relaxation by translational diffusion in liquids. II. Finite jumps and independent T1 processes. J. Chem. Phys 1978, 68, 4034–4037. [Google Scholar]
- Hwang, L.-P.; Freed, J.H. Dynamic effects of pair correlation functions on spin relaxation by translational diffusion in liquids. J. Chem. Phys 1975, 63, 4017–4025. [Google Scholar]
- Gueron, M. Nuclear relaxation in macromolecules by paramagnetic ions: A novel mechnism. J. Magn. Reson 1975, 19, 58–66. [Google Scholar]
- Gillis, P.; Roch, A.; Brooks, R.A. Corrected equations for susceptibility-induced T2-shortening. J. Magn. Reson 1999, 137, 402–407. [Google Scholar]
- Roch, A.; Muller, R.N.; Gillis, P. Water relaxation by SPM particles: Neglecting the magnetic anisotropy? A caveat. J. Magn. Reson. Imaging 2001, 14, 94–96. [Google Scholar]
- Koenig, S.H.; Kellar, K.E. Theory of 1/T1 and 1/T2 NMRD profiles of solutions of magnetic nanoparticles. Magn. Reson. Med 1995, 34, 227–233. [Google Scholar]
- Kellar, K.E.; Fujii, D.K.; Gunther, W.H.; Briley-Saebø, K.; Spiller, M.; Koenig, S.H. NC100150 a preparation of iron oxide nanoparticles ideal for positive-contrast MR angiography. Magn. Reson. Mater. Phys. Biol. Med 1999, 8, 207–213. [Google Scholar]
- Kellar, K.E.; Fujii, D.K.; Gunther, W.H.; Briley-Saebø, K.; Bjørnerud, A.; Spiller, M.; Koenig, S.H. NC100150 Injection, a preparation of optimized iron oxide nanoparticles for positive-contrast MR angiography. J. Magn. Reson. Imaging 2000, 11, 488–494. [Google Scholar]
- Roch, A.; Muller, R.N.; Gillis, P. Theory of proton relaxation induced by superparamagnetic particles. J. Chem. Phys 1999, 110, 5403–5411. [Google Scholar]
- Roch, A.; Gillis, P.; Ouakssim, A.; Muller, R.N. Proton magnetic relaxation in superparamagnetic aqueous colloids: A new tool for the investigation of ferrite crystal anisotropy. J. Magn. Magn. Mater 1999, 201, 77–79. [Google Scholar]
- Roch, A.; Muller, R.N. Longitudinal Relaxation of Water Protons in Colloidal Suspensions of Superparamagnetic Crystals. Proceedings of the 11th Annual Meeting of the Society of Magnetic Resonance in Medicine, Works in Progress, Berlin, Germany, Aug 8–14, 1992; p. 1447.
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev 2008, 108, 2064–2110. [Google Scholar]
- Noginova, N.; Weaver, T.; King, M.; Bourlinos, A.B.; Giannelis, E.P.; Atsarkin, V.A. NMR and spin relaxation in systems with magnetic nanoparticles. J. Phys. Condens. Matter 2007, 19, 076210. [Google Scholar]
- Gillis, P.; Koenig, S.H. Transverse relaxation of solvent protons induced by magnetized spheres: Application to ferritin, erythrocytes, and magnetite. Magn. Reson. Med 1987, 5, 323–345. [Google Scholar]
- Gillis, P.; Moiny, F.; Brooks, R.A. On T2—shortening by strongly magnetized spheres: A partial refocusing model. Magn. Reson. Med 2002, 263, 257–263. [Google Scholar]
- Issa, B.; Obaidat, I.M.; Hejasee, R.H.; Qadri, S.; Haik, Y. NMR relaxation in systems with magnetic nanoparticles: A temperature study. J. Magn. Reson. Imaging 2013. [Google Scholar] [CrossRef]
- Kennan, R.P.; Zhong, J.; Gore, J.C. Intravascular susceptibility contrast mechanisms in tissues. Magn. Reson. Med 1994, 31, 9–21. [Google Scholar]
- Brown, R.J.S. Distribution of fields from randomly displaced dipoles: Free-precession signal decay as a result of magnetic grains. Phys. Rev 1961, 121, 1379–1382. [Google Scholar]
- Yablonskiy, D.A.; Haacke, E.M. Theory of NMR signal behavior in magnetic ally inhomogeneous tissues : The static dephasing regime. Magn. Reson. Med 1994, 32, 749–763. [Google Scholar]
- Roch, A.; Gossuin, Y.; Muller, R.N.; Gillis, P. Superparamagnetic colloid suspensions: Water magnetic relaxation and clustering. J. Magn. Magn. Mater 2005, 293, 532–539. [Google Scholar]
- Bomatí-Miguel, O.; Morales, M.P.; Tartaj, P.; Ruiz-Cabello, J.; Bonville, P.; Santos, M.; Zhao, X.; Veintemillas-Verdaguer, S. Fe-based nanoparticulate metallic alloys as contrast agents for magnetic resonance imaging. Biomaterials 2005, 26, 5695–5703. [Google Scholar]
- Elias, A.; Tsourkas, A. Imaging circulating cells and lymphoid tissues with iron oxide nanoparticles. Hematology 2009, 2009, 720–726. [Google Scholar]
- Hejase, H.; Hayek, S.S.; Qadri, S.; Haik, Y. MnZnFe nanoparticles for self-controlled magnetic hyperthermia. J. Magn. Magn. Mater 2012, 324, 3620–3628. [Google Scholar]
- Issa, B.; Obaidat, I.M.; Qadri, S.M.; Abdulrehman, T.; Haik, Y. Thermally reversible nanoparticle aggregation explains magnetic moment increase with temperature. Curr. Nanosci 2013, 9, 381–386. [Google Scholar]
- Kobayashi, Y.; Imai, J.; Nagao, D.; Takeda, M.; Ohuchi, N.; Kasuya, A.; Konno, M. Preparation of multilayered silica–Gd–silica core-shell particles and their magnetic resonance images. Colloids Surf. A 2007, 308, 14–19. [Google Scholar]
- Heitsch, A.T.; Smith, D.K.; Patel, R.E.; Ress, D.; Korgel, B.A. Multifunctional particles: Magnetic nanocrystals and gold nanorods coated with fluorescent dye-doped silica shells. J. Solid State Chem 2008, 181, 1590–1599. [Google Scholar]
- Brooks, R.A.; Vymazal, J.; Baumgarner, C.D.; Tran, B.S.V. Comparison of T2 relaxation in. J. Magn. Reson. Imaging 1995, 4, 446–450. [Google Scholar]
- Lisitza, N.V.; Warren, W.S.; Song, Y.-Q. Study of diffusion in erythrocyte suspension using internal magnetic field inhomogeneity. J. Magn. Reson 2007, 187, 146–154. [Google Scholar]
- Gossuin, Y.; Roch, A.; Lo Bue, F.; Muller, R.N.; Gillis, P. Nuclear magnetic relaxation dispersion of ferritin and ferritin-like magnetic particle solutions: A pH-effect study. Magn. Reson. Med 2001, 46, 476–481. [Google Scholar]
- Anand, V.; Hirasaki, G.J. Paramagnetic relaxation in sandstones: Distinguishing T1 and T2 dependence on surface relaxation, internal gradients and dependence on echo spacing. J. Magn. Reson 2008, 190, 68–85. [Google Scholar]
- Jensen, J.H.; Chandra, R. NMR relaxation in tissues with weak magnetic inhomogeneities. Magn. Reson. Med 2000, 44, 144–156. [Google Scholar]
- Jensen, J.H.; Chandra, R. Strong field behavior of the NMR signal from magnetically heterogeneous tissues. Magn. Reson. Med 2000, 43, 226–236. [Google Scholar]
- Yung, K.-T. Empirical models of transverse relaxation for spherical magnetic perturbers. Magn. Reson. Imaging 2003, 21, 451–463. [Google Scholar]
- Weisskoff, R.M.; Zuo, C.S.; Boxerman, J.L.; Rosen, B.R. Microscopic susceptibility variation and transverse relaxation : Theory and experiment. Magn. Reson. Med 1994, 31, 601–610. [Google Scholar]
- Matsumoto, Y.; Jasanoff, A. T2 relaxation induced by clusters of superparamagnetic nanoparticles: Monte Carlo simulations. Magn. Reson. Imaging 2008, 26, 994–998. [Google Scholar]
- Issa, B.; Qadri, S.; Obaidat, I.M.; Bowtell, R.W.; Haik, Y. PEG coating reduces NMR relaxivity of Mn0.5Zn0.5Gd0.02Fe1.98O4 hyperthermia nanoparticles. J. Magn. Reson. Imaging 2011, 34, 1192–1198. [Google Scholar]
- Ouakssim, A.; Fastrez, S.; Roch, A.; Laurent, S.; Gossuin, Y.; Piérart, C.; Vander Elst, L.; Muller, R.N. Control of the synthesis of magnetic fluids by relaxometry and magnetometry. J. Magn. Magn. Mater 2004, 272–276, E1711–E1713. [Google Scholar]
- Noginova, N.; Weaver, T.; Andreyev, A.; Radocea, A.; Atsarkin, V. A NMR and spin relaxation in systems with magnetic nanoparticles: Effects of size and molecular motion. J. Phys. Condens. Matter 2009, 21, 255301. [Google Scholar]
- Carroll, M.R.J.; Huffstetler, P.P.; Miles, W.C.; Goff, J.D.; Davis, R.M.; Riffle, J.S.; House, M.J.; Woodward, R.C.; St Pierre, T.G. The effect of polymer coatings on proton transverse relaxivities of aqueous suspensions of magnetic nanoparticles. Nanotechnology 2011, 22, 325702. [Google Scholar]
- Carroll, M.R.J.; Woodward, R.C.; House, M.J.; Teoh, W.Y.; Amal, R.; Hanley, T.L.; St Pierre, T.G. Experimental validation of proton transverse relaxivity models for superparamagnetic nanoparticle MRI contrast agents. Nanotechnology 2010, 21, 035103. [Google Scholar]
- Ouakssim, A.; Roch, A.; Pierart, C.; Muller, R. Characterization of polydisperse superparamagnetic particles by nuclear magnetic relaxation dispersion (NMRD) profiles. J. Magn. Magn. Mater 2002, 252, 49–52. [Google Scholar]
- Bulte, J.W.; Brooks, R.A.; Moskowitz, B.M.; Bryant, L.H.; Frank, J.A. Relaxometry and magnetometry of the MR contrast agent MION-46L. Magn. Reson. Med 1999, 42, 379–384. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266-21305. https://doi.org/10.3390/ijms141121266
Issa B, Obaidat IM, Albiss BA, Haik Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. International Journal of Molecular Sciences. 2013; 14(11):21266-21305. https://doi.org/10.3390/ijms141121266
Chicago/Turabian StyleIssa, Bashar, Ihab M. Obaidat, Borhan A. Albiss, and Yousef Haik. 2013. "Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications" International Journal of Molecular Sciences 14, no. 11: 21266-21305. https://doi.org/10.3390/ijms141121266




