A Computational Drug Metabolite Detection Using the Stable Isotopic Mass-Shift Filtering with High Resolution Mass Spectrometry in Pioglitazone and Flurbiprofen
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metabolite Detection Using Isotopic Mass-Shift Filtering
2.2. Calculation of Mass and Estimation of Formula of Metabolites
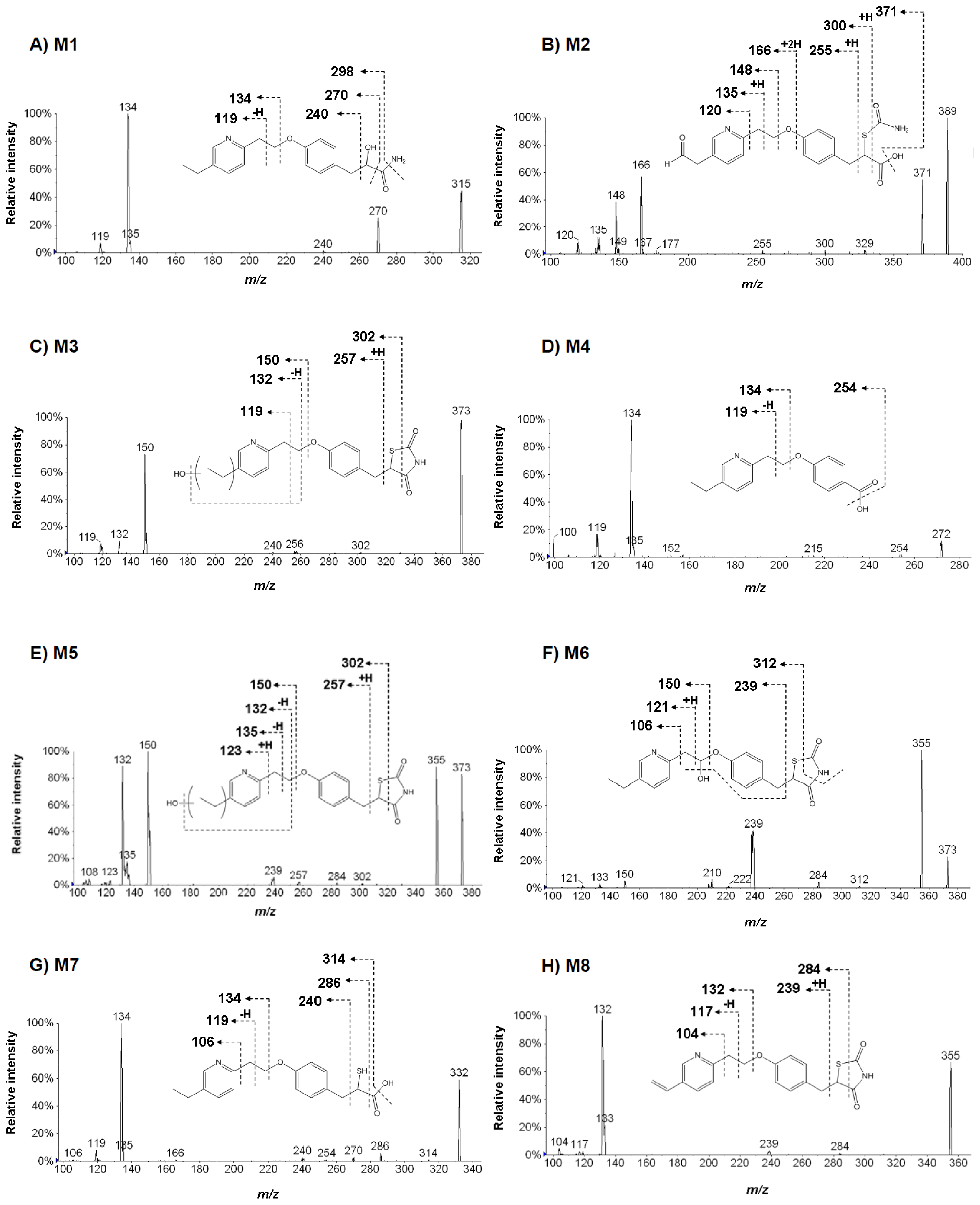
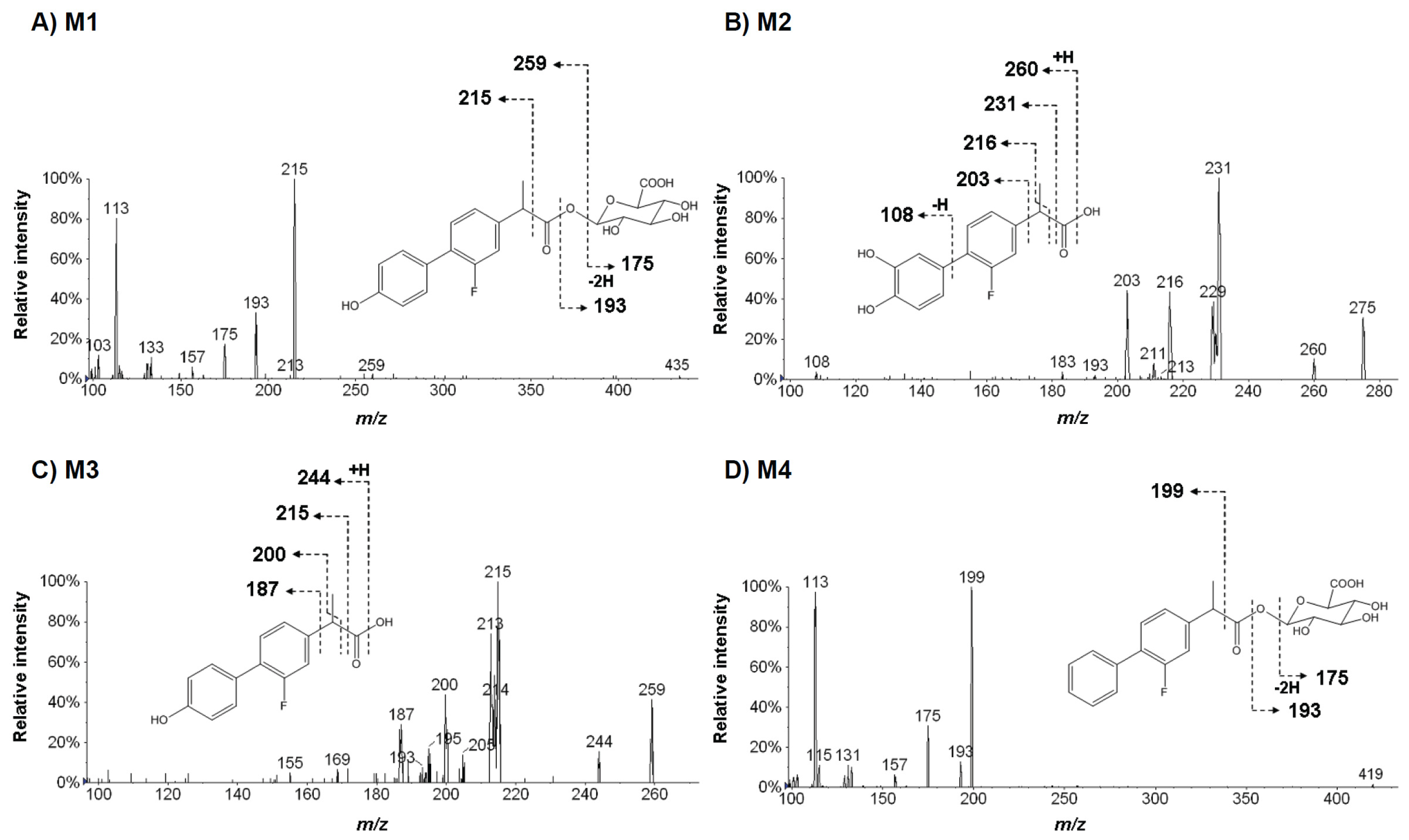
2.3. Structural Analysis of Metabolites
3. Experimental Section
3.1. Chemical and Reagents
3.2. In Vitro Metabolism Using Liver Microsomes
3.3. Liquid Chromatography
3.4. Time of Flight Mass Spectrometry
3.5. Triple Quadrupole Mass Spectrometry
3.6. Alignment and Filtering of Stable Isotope Mass-Shifts between Labeled and Un-Labeled Compound Data Sets
4. Conclusions

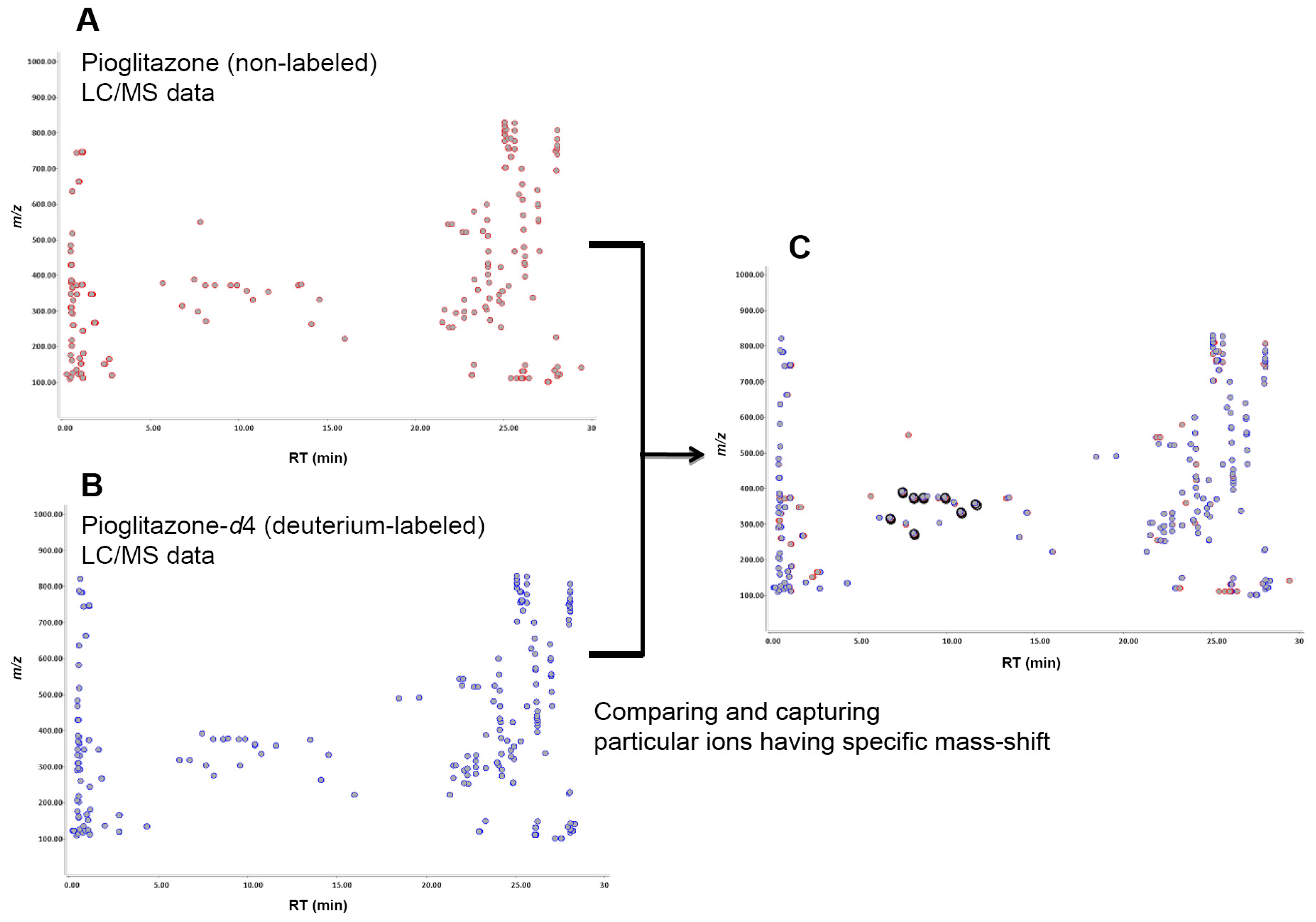

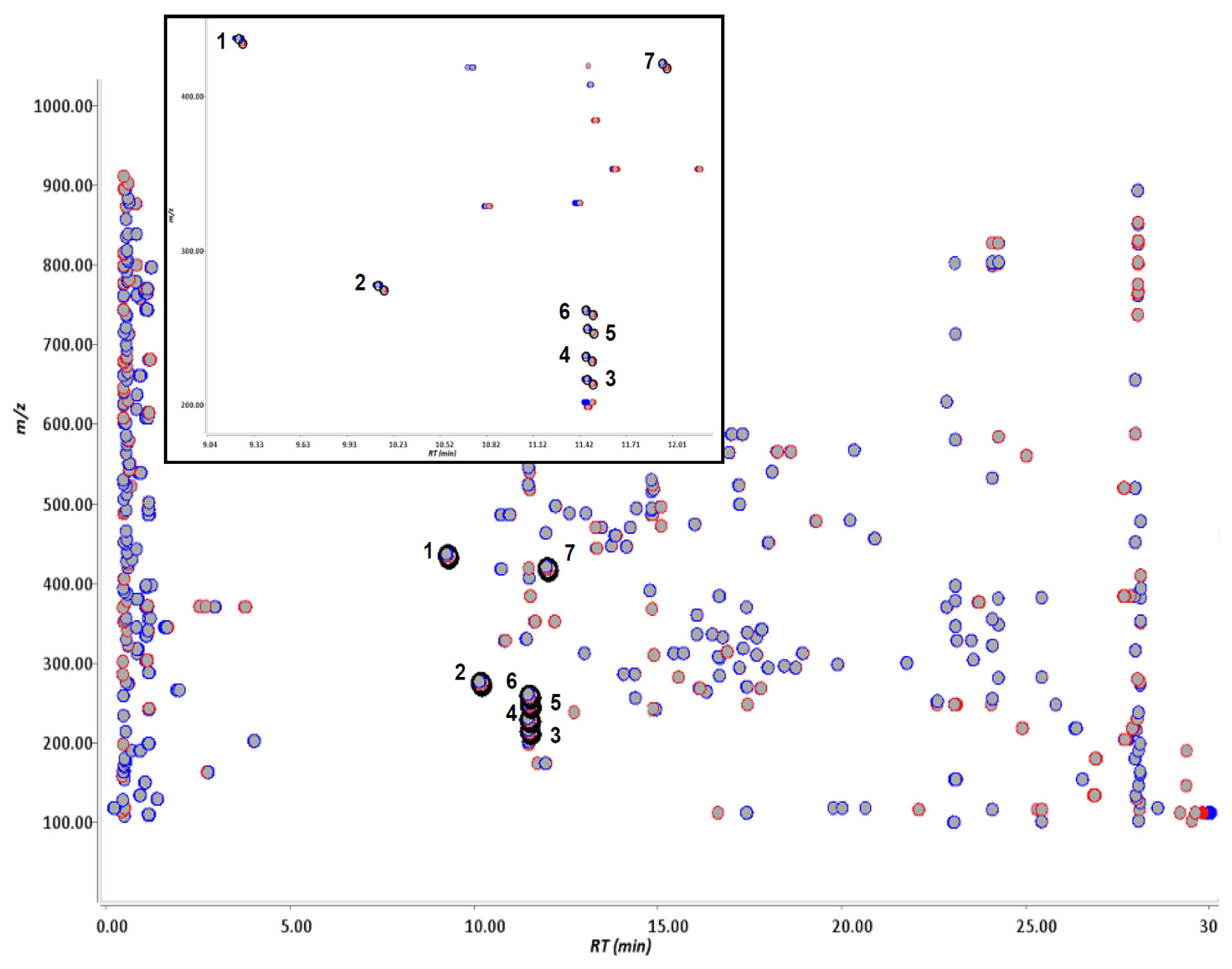
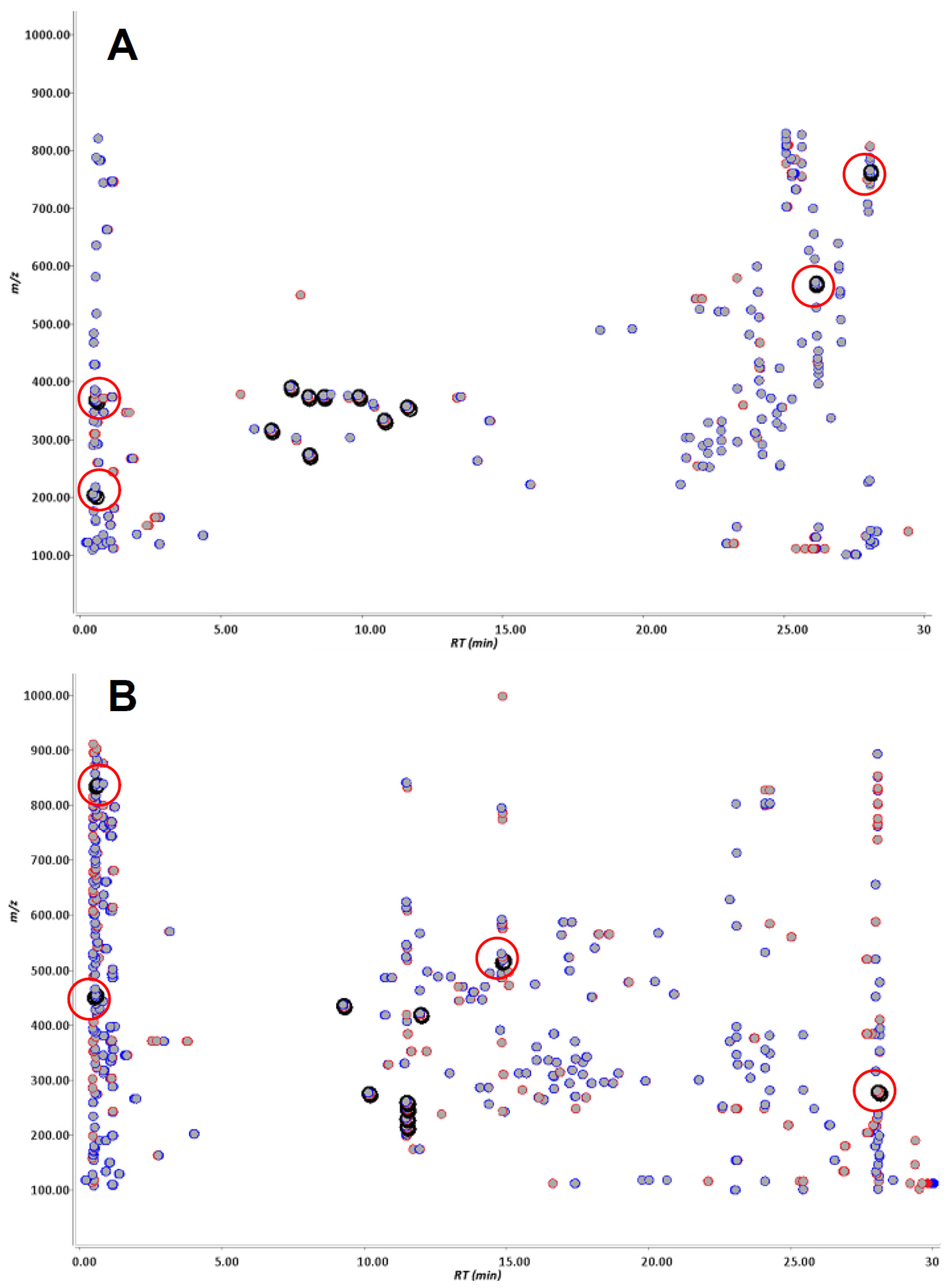
| Parent compound (a) | Number of ion pairs | Retention time (min) | Δ Retention time (min) (b) | Intensity (%) (c) | Δ intensity (%) (d) | [M + H]+//[M − H]−m/z (amu) (e) | Mass-shift Δ m/z (amu) (f) |
|---|---|---|---|---|---|---|---|
| Pioglitazone Pioglitazone-d4 | 1 | 6.78 | 1.71 | 315.17 | |||
| 6.74 | −0.04 | 1.74 | 2 | 319.19 | +4.02 | ||
| 2 | 7.46 | 10.83 | 389.11 | ||||
| 7.44 | −0.02 | 6.59 | −39 | 393.14 | +4.03 | ||
| 3 | 8.09 | 15.21 | 373.12 | ||||
| 8.06 | −0.03 | 13.65 | −10 | 377.14 | +4.02 | ||
| 4 | 8.12 | 2.01 | 272.13 | ||||
| 8.09 | −0.03 | 1.89 | −6 | 276.15 | +4.02 | ||
| 5 | 8.64 | 100.00 | 373.12 | ||||
| 8.61 | −0.03 | 67.07 | −33 | 377.14 | +4.02 | ||
| 6 | 9.89 | 11.96 | 373.12 | ||||
| 9.85 | −0.04 | 7.07 | −41 | 377.14 | +4.02 | ||
| 7 | 10.79 | 5.05 | 332.13 | ||||
| 10.75 | −0.04 | 4.66 | −8 | 336.15 | +4.02 | ||
| 8 | 11.66 | 1.35 | 355.11 | ||||
| 11.57 | −0.09 | 1.28 | −5 | 359.13 | +4.02 | ||
| Flurbiprofen Flurbiprofen-d3 | 1 | 9.26 | 4.28 | 435.10 | |||
| 9.24 | −0.02 | 4.12 | −4 | 438.12 | +3.02 | ||
| 2 | 10.16 | 5.30 | 275.07 | ||||
| 10.13 | −0.03 | 4.94 | −7 | 278.08 | +3.01 | ||
| 3 | 11.50 | 59.97 | 214.08 | ||||
| 11.46 | −0.04 | 64.26 | 7 | 217.09 | +3.01 | ||
| 4 | 11.49 | 20.50 | 229.06 | ||||
| 11.45 | −0.04 | 18.76 | −8 | 232.08 | +3.02 | ||
| 5 | 11.50 | 1.96 | 247.07 | ||||
| 11.46 | −0.04 | 2.21 | 13 | 250.09 | +3.02 | ||
| 6 | 11.50 | 7.17 | 259.07 | ||||
| 11.45 | −0.05 | 7.01 | −2 | 262.09 | +3.02 | ||
| 7 | 11.97 | 94.39 | 419.11 | ||||
| 11.94 | −0.03 | 99.25 | 5 | 422.12 | +3.01 | ||
| Parent compound | Number of ions | Retention time (min) | Observed accurate mass m/z (amu) (a) | Estimated ion formula (b) (mass, amu) (c) | Diff (ppm) (d) |
|---|---|---|---|---|---|
| Pioglitazone | 1 | 6.78 | 315.17012 | C18H23N2O3 (315.17032) | 0.63 |
| 2 | 7.46 | 389.11569 | C19H21N2O5S (389.11657) | 2.26 | |
| 3 | 8.09 | 373.12111 | C19H21N2O4S (373.12165) | 1.46 | |
| 4 | 8.12 | 272.12734 | C16H18NO3 (272.12812) | 2.09 | |
| 5 | 8.64 | 373.12123 | C19H21N2O4S (373.12165) | 1.14 | |
| 6 | 9.89 | 373. 12106 | C19H21N2O4S (373.12165) | 1.49 | |
| 7 | 10.79 | 332.13076 | C18H22NO3S (332.13149) | 2.22 | |
| 8 | 11.66 | 355.11028 | C22H15N2O3 (355.11109) | 2.28 | |
| Flurbiprofen | 1 | 9.26 | 435.10972 | C21H20FO9 (435.10968) | −0.07 |
| 2 | 10.16 | 275.07257 | C15H12FO4 (275.07251) | −0.21 | |
| 3 | 11.50 | 214.07995 | N.E. (e) | - | |
| 4 | 11.50 | 229.06721 | C14H11FO2 (229.06703) | −0.79 | |
| 5 | 11.50 | 247.07723 | C14H13FO2 (247.0776) | 1.45 | |
| 6 | 11.50 | 259.07753 | C15H12FO3 (259.07760) | 0.26 | |
| 7 | 11.97 | 419.11524 | C21H20FO8 (419.11477) | −1.11 | |
| Parent compound | Metabolite ID | Estimated formula (a) (mass, amu) (b) | Observed major production | Estimated metabolite form (c) | Mass defect (mDa) (d) |
|---|---|---|---|---|---|
| Pioglitazone (PIO) | M1 | C18H22N2O3 (314.163043) | 298, 270, 240, 134, 119 | PIO hydroxypropanamide | 43.6 |
| M2 | C19H20N2O5S (388.109295) | 371, 300, 255, 166, 148, 135, 120 | Aldehyde PIO hydrolysis | −10.2 | |
| M3 | C19H20N2O4S (372.114380) | 302, 256, 150, 132, 119 | Hydroxy PIO | −5.1 | |
| M4 | C16H17NO3 (271.120844) | 254, 134, 119 | PIO benzoic acid | 1.4 | |
| M5 | C19H20N2O4S (372.114380) | 302, 257, 150, 135, 132, 123 | Hydroxy PIO | −5.1 | |
| M6 | C19H20N2O4S (372.114380) | 312, 239, 150, 121, 106 | Hydroxy PIO | −5.1 | |
| M7 | C18H21NO3S (331.124216) | 314, 286, 240, 134, 119, 106 | PIO mercaptopropanoic acid | 4.8 | |
| M8 | C22H14N2O3 (354.100443) | 284, 239, 132, 117, 104 | Dehydro PIO | −19.0 | |
| parent | C19H20N2O3S (356.119465) | - (e) | PIO | - | |
| Flurbiprofen (FBP) | M1 | C21H21FO9 (436.116963) | 259, 215, 193, 175 | Hydroxyl FBP glucuronide | 27.0 |
| M2 | C15H13FO4 (276.079788) | 260, 231, 216, 203, 108 | Di-hydroxy FBP | −10.2 | |
| M3 | C15H13FO3 (260.084873) | 244, 215, 200, 187 | Hydroxy FBP | −5.1 | |
| M4 | C21H21FO8 (420.122048) | 199, 195, 175 | FBP glucuronide | 32.1 | |
| parent | C15H13FO2 (244.089958) | - (e) | FBP | - | |
Acknowledgments
Conflicts of Interest
References
- Edward, B.; Neville, B. Molecular and Cellular Pharmacology. In Principals of Medical Biology; Covent Garden: London, UK, 1997; pp. 1–1111. [Google Scholar]
- Food and Drug Administration (FDA), Guidance for Industry: Safety Testing of Drug Metabolites; FDA: Rockville, MD, USA, 2008.
- Kyung, S.P.; Sun, T.K.; Young, M.K.; Yunje, K.; Won, L. The matrix effect of biological concomitant element on the signal intensity of Ge, As, and Se in inductively coupled plasma/mass spectrometry. Bull. Korean Chem. Soc 2002, 23, 1389–1393. [Google Scholar]
- Liu, X.; Jia, L. The conduct of drug metabolism studies considered good practice (I): Analytical systems and in vivo studies. Curr. Drug Metab 2007, 8, 815–821. [Google Scholar]
- Nagele, E.; Fandino, A.S. Simultaneous determination of metabolic stability and identification of buspirone metabolites using multiple column fast liquid chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1156, 196–200. [Google Scholar]
- Liu, T.; Du, F.; Wan, Y.; Zhu, F.; Xing, J. Rapid identification of phase I and II metabolites of artemisinin antimalarials using LTQ-Orbitrap hybrid mass spectrometer in combination with online hydrogen/deuterium exchange technique. J. Mass Spectrom 2011, 46, 725–733. [Google Scholar]
- Holcapek, M.; Kolarova, L.; Nobilis, M. High-performance liquid chromatography-tandem mass spectrometry in the identification and determination of phase I and phase II drug metabolites. Anal. Bioanal. Chem 2008, 391, 59–78. [Google Scholar]
- Castro-Perez, J.; Plumb, R.; Granger, J.H.; Beattie, I.; Joncour, K.; Wright, A. Increasing throughput and information content for in vitro drug metabolism experiments using ultra-performance liquid chromatography coupled to a quadrupole time-of-flight mass spectrometer. Rapid Commun. Mass Spectrom 2005, 19, 843–848. [Google Scholar]
- Peterman, S.M.; Duczak, N., Jr.; Kalgutkar, A.S.; Lame, M.E.; Soglia, J.R. Application of a linear ion trap/orbitrap mass spectrometer in metabolite characterization studies: Examination of the human liver microsomal metabolism of the non-tricyclic anti-depressant nefazodone using data-dependent accurate mass measurements. J. Am. Soc. Mass Spectrom 2006, 17, 363–375. [Google Scholar]
- Ruan, Q.; Zhu, M. Investigation of bioactivation of ticlopidine using linear ion trap/orbitrap mass spectrometry and an improved mass defect filtering technique. Chem. Res. Toxicol 2010, 23, 909–917. [Google Scholar]
- Zhu, M.; Ma, L.; Zhang, D.; Ray, K.; Zhao, W.; Humphreys, W.G.; Skiles, G.; Sanders, M.; Zhang, H. Detection and characterization of metabolites in biological matrices using mass defect filtering of liquid chromatography/high resolution mass spectrometry data. Drug Metab. Dispos 2006, 34, 1722–1733. [Google Scholar]
- Bastani, N.E.; Gundersen, T.E.; Blomhoff, R. Determination of 8-epi PGF(2alpha) concentrations as a biomarker of oxidative stress using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom 2009, 23, 2885–2890. [Google Scholar]
- Taylor, E.W.; Jia, W.; Bush, M.; Dollinger, G.D. Accelerating the drug optimization process: Identification, structure elucidation, and quantification of in vivo metabolites using stable isotopes with LC/MSn and the chemiluminescent nitrogen detector. Anal. Chem 2002, 74, 3232–3238. [Google Scholar]
- Ma, S.; Chowdhury, S.K.; Alton, K.B. Application of mass spectrometry for metabolite identification. Curr. Drug Metab 2006, 7, 503–523. [Google Scholar]
- Baillie, A.T. The use of stable isotopes in pharmacological research. Pharmacol. Res 1981, 33, 81–132. [Google Scholar]
- Shibasaki, H.; Furuta, T.; Kasuya, Y. Quantification of corticosteroids in human plasma by liquid chromatography-thermospray mass spectrometry using stable isotope dilution. J. Chromatogr. B 1997, 692, 7–14. [Google Scholar]
- Wang, S.; Cyronak, M.; Yang, E. Does a stable isotopically labeled internal standard always correct analyte response? A matrix effect study on a LC/MS/MS method for the determination of carvedilol enantiomers in human plasma. J. Pharm. Biomed. Anal 2007, 43, 701–707. [Google Scholar]
- Shukla, U.A.; Marathe, P.H.; Pittman, K.A.; Barbhaiya, R.H. Pharmacokinetics, absolute bioavailability, and disposition of [14C]nefazodone in the dog. Drug Metab. Dispos 1993, 21, 502–507. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Uchida, M.; Kanazawa, M.; Ogiwara, A.; Sezaki, H.; Ando, A.; Miyamoto, Y. A Computational Drug Metabolite Detection Using the Stable Isotopic Mass-Shift Filtering with High Resolution Mass Spectrometry in Pioglitazone and Flurbiprofen. Int. J. Mol. Sci. 2013, 14, 19716-19730. https://doi.org/10.3390/ijms141019716
Uchida M, Kanazawa M, Ogiwara A, Sezaki H, Ando A, Miyamoto Y. A Computational Drug Metabolite Detection Using the Stable Isotopic Mass-Shift Filtering with High Resolution Mass Spectrometry in Pioglitazone and Flurbiprofen. International Journal of Molecular Sciences. 2013; 14(10):19716-19730. https://doi.org/10.3390/ijms141019716
Chicago/Turabian StyleUchida, Masashi, Mitsuhiro Kanazawa, Atsushi Ogiwara, Hiroshi Sezaki, Akihiro Ando, and Yohei Miyamoto. 2013. "A Computational Drug Metabolite Detection Using the Stable Isotopic Mass-Shift Filtering with High Resolution Mass Spectrometry in Pioglitazone and Flurbiprofen" International Journal of Molecular Sciences 14, no. 10: 19716-19730. https://doi.org/10.3390/ijms141019716




