Diamondoid Characterization in Condensate by Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry: The Junggar Basin of Northwest China
Abstract
:1. Introduction
2. Samples and Methods
3. Results and Discussion
3.1. Identification of Adamantanes
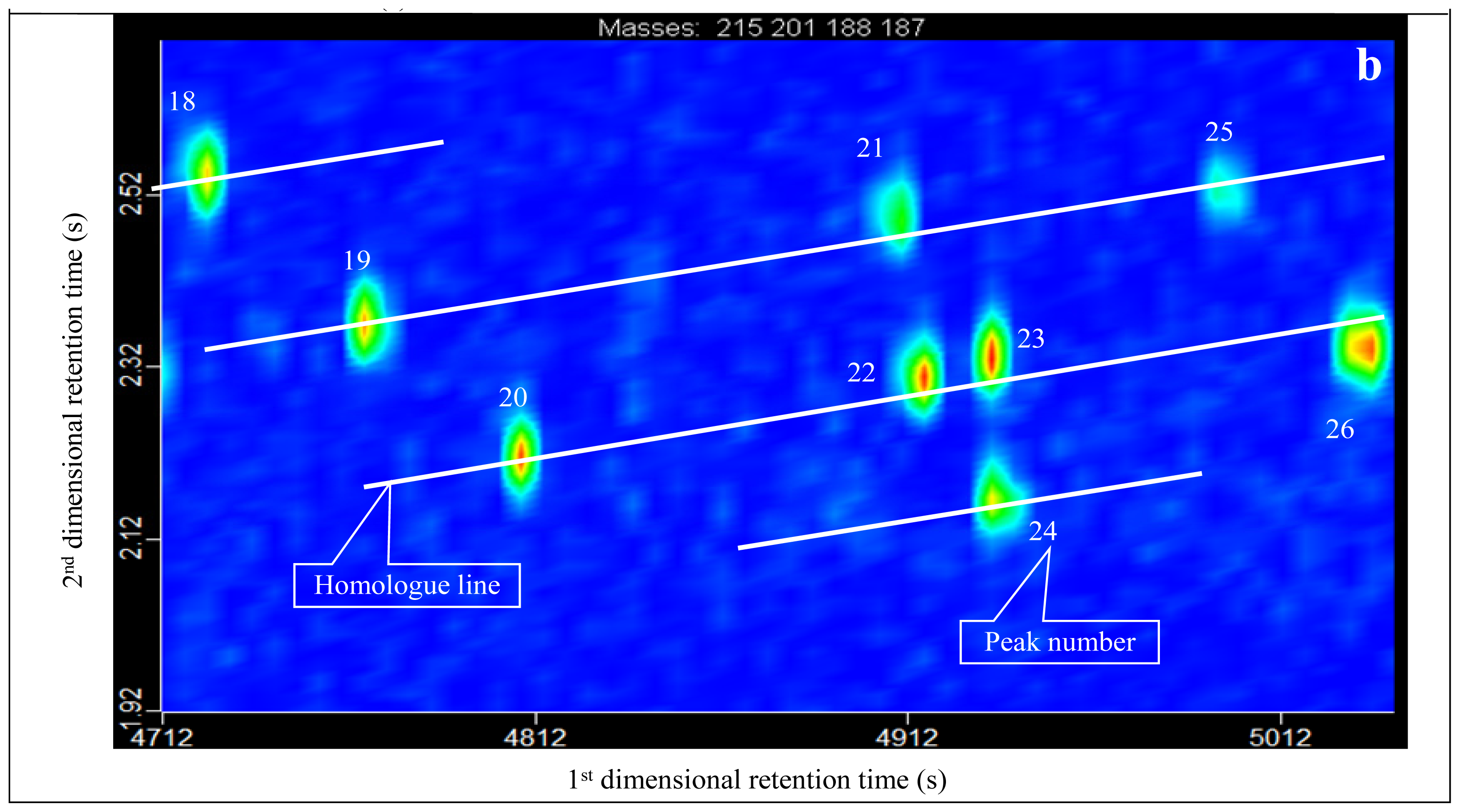
3.2. Identification of Diamantanes
3.3. Difference between Normal and Reversed Phase Column Systems
3.4. Geological and Geochemical Applications
4. Conclusions
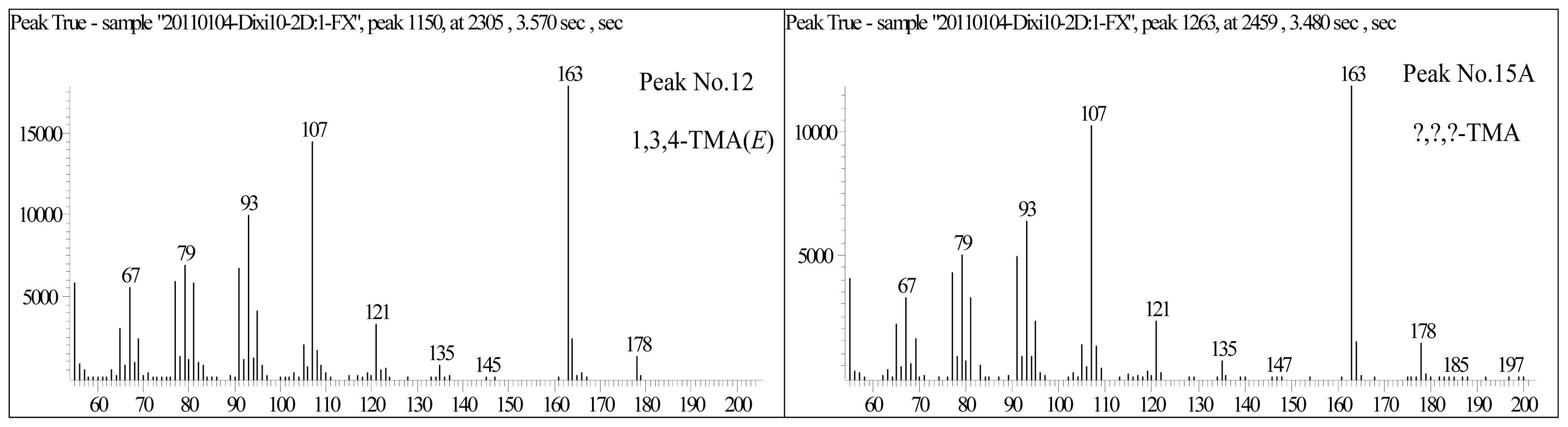
- Diamondoids can be effectively identified and characterized by GC×GC-TOFMS analysis, which is difficult to achieve using conventional GC-MS measurements. New trimethyl-adamantane 15A (the No. 15A peak in Table 1) compound was detected, which is present close to 1-methyl-3-ethyl-adamantane (the No. 15 peak in Table 1). It may be 1,2,3- or 2,3,4-trimethyladamantanes.
- Diamondoids that cannot be effectively separated in conventional GC-MS measurements can be effectively separated in GC×GC-TOFMS analysis, such as 4, 8-dimethyl-diamantane (No. 23 peak in Table 1) and trimethyl-diamantane (No. 24 peak in Table 1). This leads to more accurate quantitative analysis and calculations.
- The separation degree of diamondoids is affected by multiple factors and is generally better in a reversed phase column system than in a normal system.
- According to diamondoid indexes indicative of maturity, the condensate from Well DX 10 is highly mature with equivalent Ro being approximately 1.5%, obtaining a new effective quantitative indicator. Thus, we obtained new understanding by GC×GC-TOFMS study on diamondoids, whose prospects in the study of petroleum geochemistry were shown.
Acknowledgements
References
- Wingert, W.S. GC-MS analysis of diamondoid hydrocarbons in smackover petroleum. Fuel 1992, 71, 37–43. [Google Scholar]
- Chen, J.H.; Fu, J.M.; Sheng, G.Y.; Liu, D.H.; Zhang, J.J. Diamondoid hydrocarbon ratios: Novel maturity indices for highly mature crude oils. Org. Geochem 1996, 25, 179–190. [Google Scholar]
- Grice, K.; Alexander, R.; Kagi, R.I. Diamondoid hydrocarbon ratios as indicators of biodegradation in Australia crude oils. Org. Geochem 2000, 31, 715–725. [Google Scholar]
- Wei, Z.; Moldowan, J.M.; Paytan, A. Diamondoids and molecular biomarkers generated from modern sediments in the absence and presence of minerals during hydrous pyrolysis. Org. Geochem 2006, 37, 891–911. [Google Scholar]
- Dahl, J.E.; Moldowan, J.M.; Peters, K.E.; Claypool, G.E.; Rooney, M.A.; Michael, G.E.; Mello, M.R.; Kohnen, M.L. Diamondoid hydrocarbons as indicators of oil cracking. Nature 1999, 399, 54–56. [Google Scholar]
- Li, J.G.; Philip, P.R.; Cui, M.Z. Methyl diamantane index (MDI) as a maturity parameter for Lower Palaeozoic carbonate rocks at high maturity and overmaturity. Org. Geochem 2000, 31, 267–272. [Google Scholar]
- Lin, R.; Wilk, Z.A. Natural occurrence of tetramantane (C22H28), pentamantane (C26H32) and hexamantane (C30H36) in a deep petroleum reservoir. Fuel 1995, 74, 1512–1521. [Google Scholar]
- Azevedo, D.A.; Tamanqueira, J.B.; Dias, J.C.M.; Carmo, A.P.B.; Landau, L.; Goncalves, F.T.T. Multivariate statistical analysis of diamondoid and biomarker data from Brazilian basin oil samples. Fuel 2008, 87, 2122–2130. [Google Scholar]
- Jalees, M.I.; Bianchi, T.S.; Sassen, R.; Tahira, F. Diamondoids and biomarkers: as a tool to better define the effects of thermal cracking and microbial oxidation on oils/condensates from reservoirs of the Upper Indus Basin, Pakistan. Carbonate. Evaporite 2011, 26, 155–165. [Google Scholar]
- Schulz, L.K.; Wilhelms, A.; Rein, E.; Steen, A.S. Application of diamondoids to distinguish source rock facies. Org. Geochem 2001, 32, 365–375. [Google Scholar]
- Eiserbeck, C.; Nelson, R.; Grice, K.; Curiale, J.; Reddy, C. Comparison of GC-MS, GC-MRM-MS, and GC×GC to characterise higher plant biomarkers in Tertiary oils and rock extracts. Geochim. Cosmochim. Acta 2012, 87, 299–322. [Google Scholar]
- Ventura, G.T.; Simoneit, B.R.T.; Nelson, R.K.; Reddy, C. The composition, origin and fate of complex mixtures in the maltene fractions of hydrothermal petroleum assessed by comprehensive two-dimensional gas chromatography. Org. Geochem 2012, 45, 48–65. [Google Scholar]
- Aguiar, A.; Silva, A.I.; Azevedo, D.A.; Aquino, F.R. Application of comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry to biomarker characterization in Brazilian oils. Fuel 2010, 89, 2760–2768. [Google Scholar]
- Ventura, G.T.; Raghuraman, B.; Nelson, R.K.; Mullins, O.C.; Reddy, C.M. Compound class oil fingerprinting techniques using comprehensive two-dimensional gas chromatography (GC×GC). Org. Geochem 2010, 41, 1026–1035. [Google Scholar]
- Li, S.F.; Hu, S.Z.; He, S.; Ma, J.; Liu, G.Q.; Hou, Y.G. Analysis of common compounds in crude oils with two-dimensional gas chromatography time-of-flight mass spectrometry (GCXGC-TOFMS) (in Chinese with English abstract). Geol. Sci. Technol. Inf 2010, 29, 46–50. [Google Scholar]
- Eiserbeck, C.; Nelson, R.K.; Grice, K.; Curiale, J.; Reddy, C.M.; Raiteri, P. Separation of 18α(H)-, 18β(H)-oleanane and lupane by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2011, 1218, 5549–5553. [Google Scholar]
- Silva, R.S.F.; Aguiar, H.G.M.; Rangel, M.D.; Azevedo, D.A.; Aquino Neto, F.R. Comprehensive two-dimensional gas chromatography with time of flight mass spectrometry applied to biomarker analysis of oils form Colombia. Fuel 2011, 90, 2694–2699. [Google Scholar]
- Wang, H.T.; Weng, N.; Zhang, S.C.; Chen, J.P.; Wei, C.Y. Characteristics and identification of saturated hydrocarbons by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (in Chinese with English abstract). J. Chin. Mass Spectrom. Soc 2010, 31, 18–27. [Google Scholar]
- Philip, R.P. The Spectrum and Application of Fossil Fuel Biomarkers (in Chinese); Science Press: Beijing, China, 1987. [Google Scholar]
- Zeng, X.Z.; Jiang, J.P.; Jiang, N.H.; Chen, Z.L.; Zhou, H. The criterion of petroleum and natural gas industry, China. In The Handbook of Mass Spectrum Criterion of Petroleum Biomarkers SY5397–91 (in Chinese); Petroleum Industry Press: Beijing, China, 1992. [Google Scholar]
- Wang, P.R. The Mass Spectrum Criterion of Petroleum Biomarkers (in Chinese); Petroleum Industry Press: Beijing, China, 1993. [Google Scholar]
- Berwick, L.; Alexander, R.; Pierce, K. Formation and reactions of alkyl adamantanes in sediments: Carbon surface reactions. Org. Geochem 2011, 42, 752–761. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide, Biomarkers and Isotopes in Petroleum Exploration and Earth History; Cambridge University Press: Cambridge, UK; p. 2005.
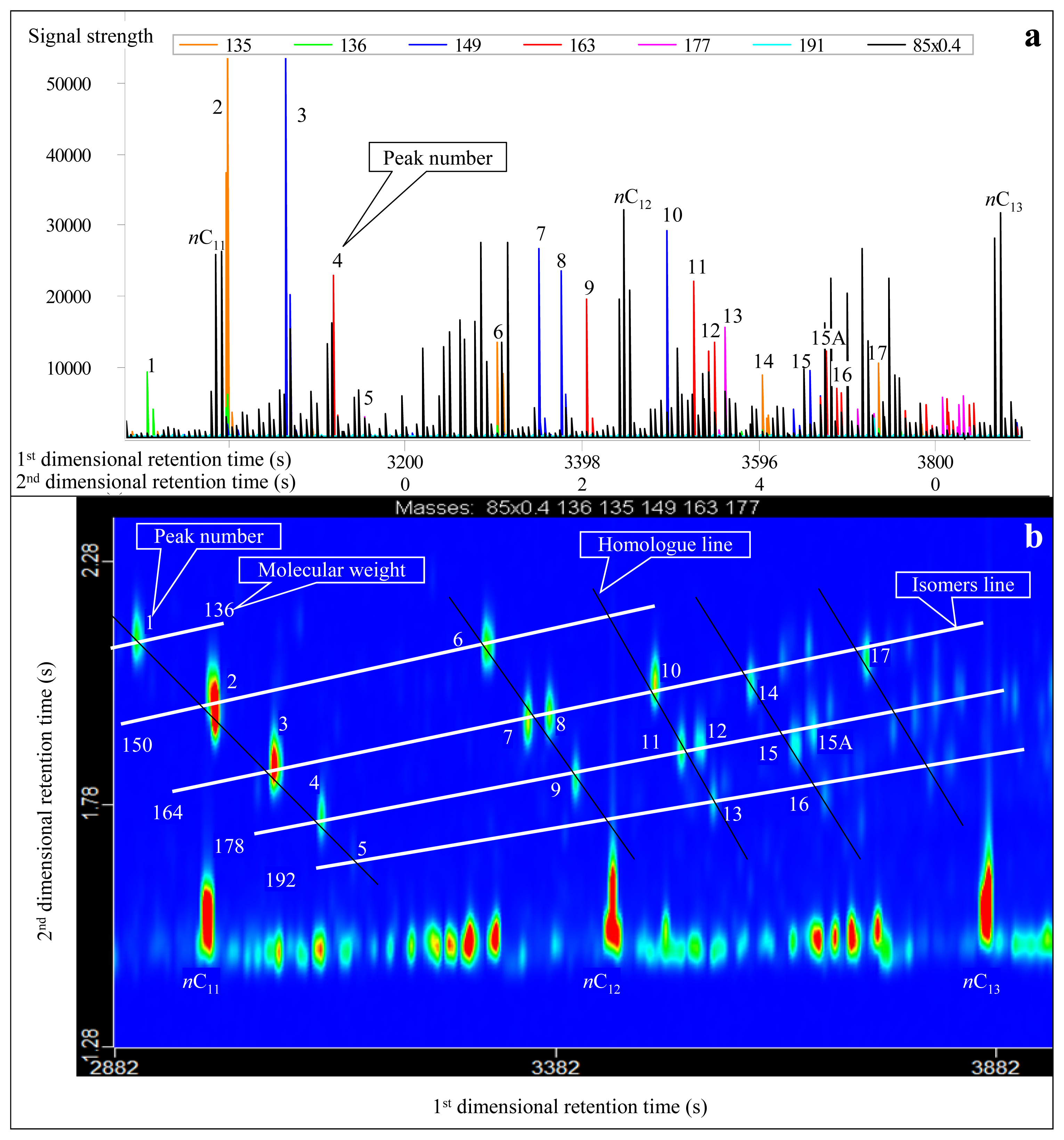
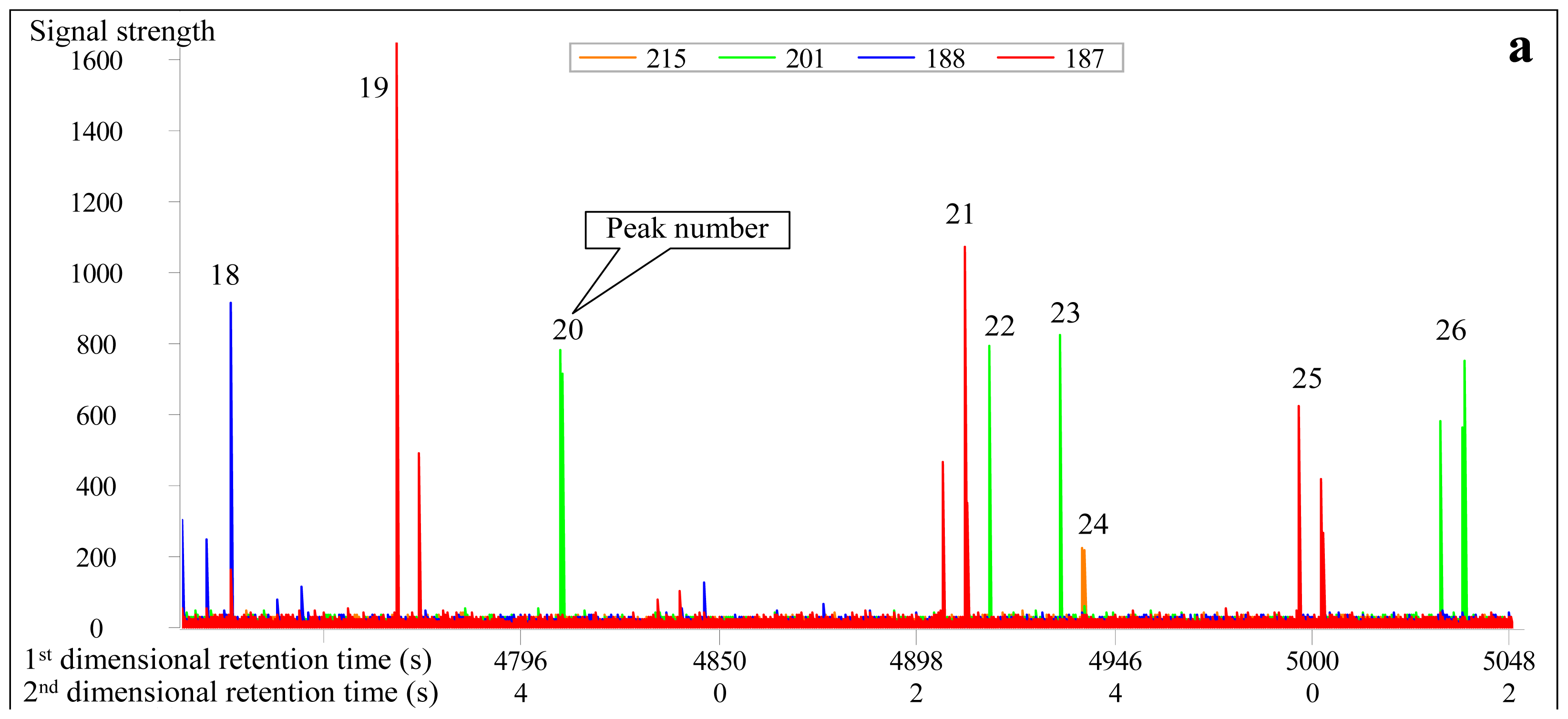
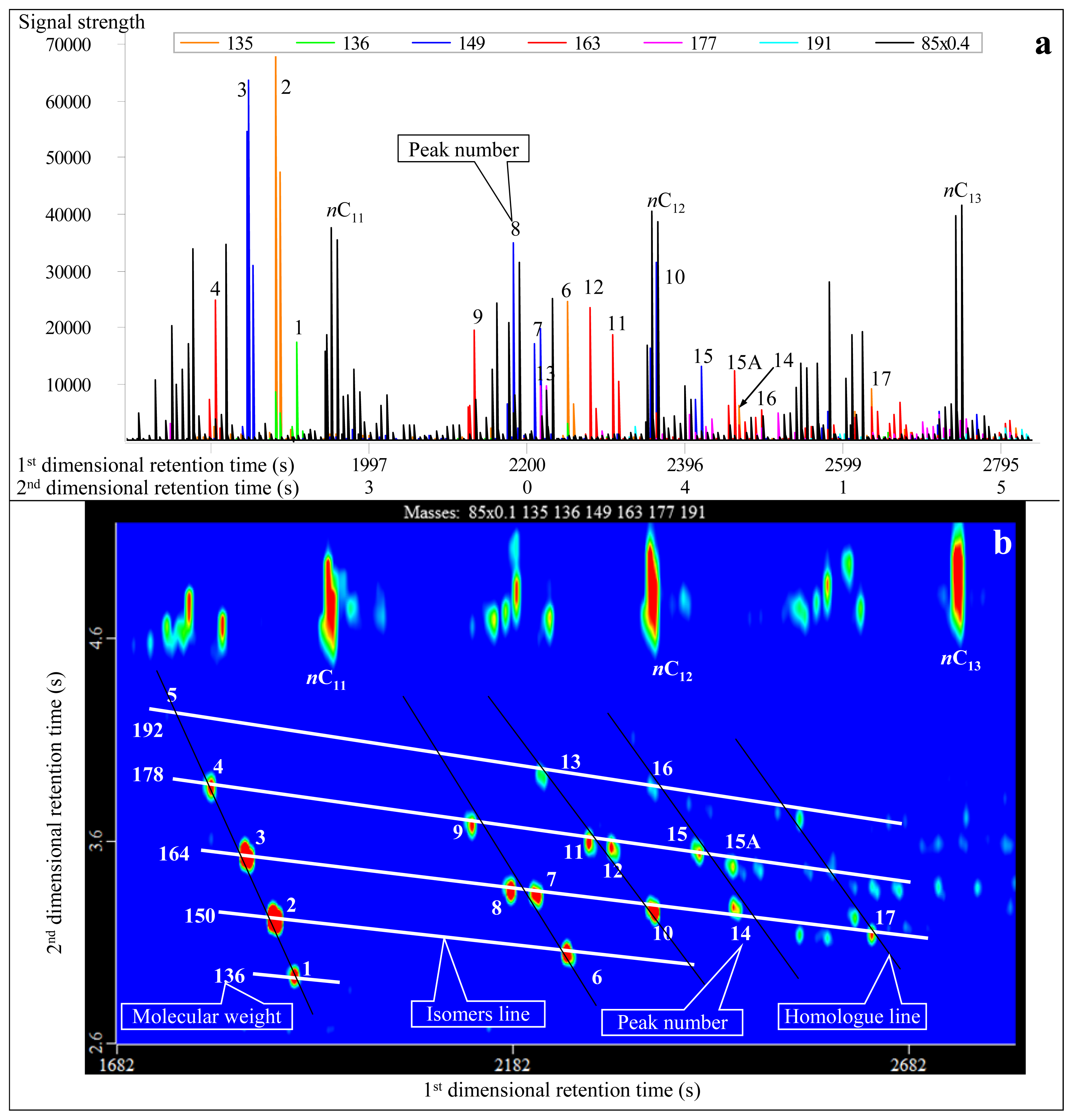
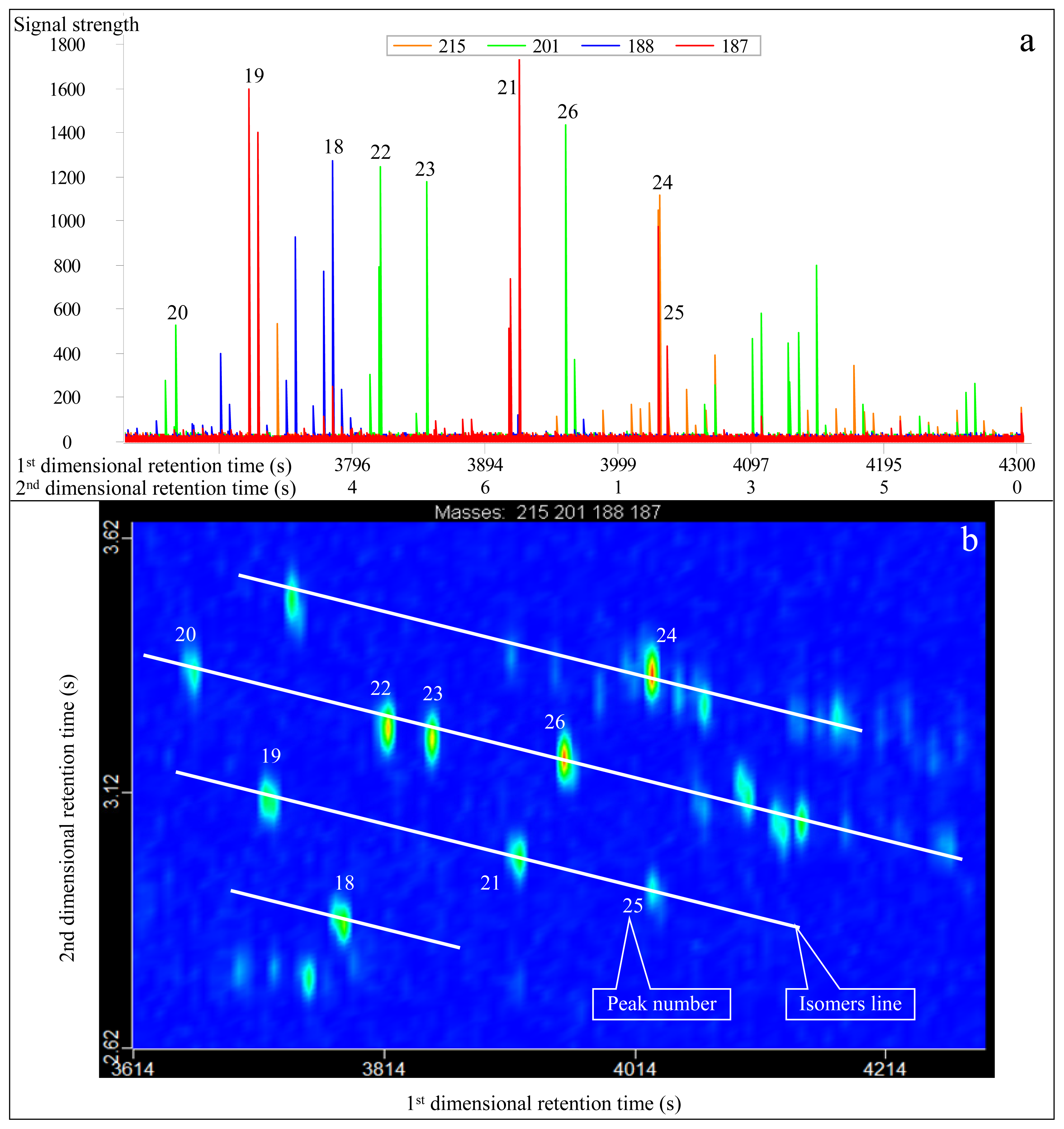
| Peak No. | Compound abbreviation | m/z | Molecular Weight | First-dimensional retention time (s) | Second-dimensional retention time (s) | Molecular Formula | Full name of compound |
|---|---|---|---|---|---|---|---|
| 1 | Adamantane (A) | 136 | 136 | 2906 (1906*) | 2.120 (2.940) | C10H16 | Adamantane |
| 2 | 1-MA | 135 | 150 | 2996 (1878) | 1.970 (3.230) | C11H18 | 1-Methyl-adamantane |
| 3 | 1,3-DMA | 149 | 164 | 3062 (1843) | 1.860 (3.540) | C12H20 | 1,3-Dimethyl-adamantane |
| 4 | 1,3,5-TMA | 163 | 178 | 3116 (1801) | 1.760 (3.870) | C13H22 | 1,3,5-Trimethyl-adamantane |
| 5 | 1,3,5,7-TeTMA | 177 | 192 | 3158 (1745) | 1.650 (4.220) | C14H24 | 1,3,5,7-Tetramethyl-adamantane |
| 6 | 2-MA | 135 | 150 | 3302 (2249) | 2.100 (3.050) | C11H18 | 2-Methyl-adamantane |
| 7 | 1,4-DMA(Z) | 149 | 164 | 3350 (2214) | 1.950 (3.330) | C12H20 | Cis-1,4-dimethyl-adamantane |
| 8 | 1,4-DMA(E) | 149 | 164 | 3374 (2179) | 1.970 (3.360) | C12H20 | Trans-1,4-dimethyl-adamantane |
| 9 | 1,3,6-TMA | 163 | 178 | 3404 (2130) | 1.830 (3.680) | C13H22 | 1,3,6-Trimethyl-adamantane |
| 10 | 1,2-DMA | 149 | 164 | 3494 (2361) | 2.030 (3.250) | C12H20 | 1,2-Dimethyl-adamantane |
| 11 | 1,3,4-TMA(Z) | 163 | 178 | 3524 (2305) | 1.890 (3.570) | C13H22 | Cis-1,3,4-Trimethyl-adamantane |
| 12 | 1,3,4-TMA(E) | 163 | 178 | 3548 (2277) | 1.900 (3.600) | C13H22 | Trans-1,3,4-Trimethyl-adamantane |
| 13 | 1,2,5,7-TeTMA | 177 | 192 | 3560 (2221) | 1.790 (3.910) | C14H24 | 1,2,5,7-Tetramethyl-adamantane |
| 14 | 1-EA | 135 | 164 | 3602 (2459) | 2.030 (3.280) | C12H20 | 1-Ethyl-adamantane |
| 15 | 1-M-3-EA | 149 | 178 | 3656 (2417) | 1.900 (3.530) | C13H22 | 1-Methyl-3-ethyl-adamantane |
| 15A | ?,?,?-TMA | 163 | 178 | 3674 (2459) | 1.930 (3.480) | C13H22 | ?,?,?-Trimethyl-adamantane |
| 16 | 1-E-3,5-DMA | 163 | 192 | 3686 (2354) | 1.800 (3.880) | C13H22 | 1-Methyl-3,5-dimethyl-adamantane |
| 17 | 2-EA | 135 | 164 | 3734 (2613) | 2.080 (3.220) | C12H20 | 2-Ethyl-adamantane |
| 18 | Diamantane (D) | 188 | 188 | 4724 (3782) | 2.539 (2.870) | C14H20 | Diamantane |
| 19 | 4-MD | 187 | 202 | 4766 (3719) | 2.370 (3.110) | C15H22 | 4-Methyl-diamantane |
| 20 | 4,9-DMD | 201 | 216 | 4808 (3663) | 2.220 (3.358) | C16H24 | 4,9-Dimethyl-diamantane |
| 21 | 1-MD | 187 | 202 | 4910 (3922) | 2.491 (2.990) | C15H22 | 1-Methyl-diamantane |
| 22 | 1,2-+2,4-DMD | 201 | 216 | 4916 (3817) | 2.309 (3.249) | C16H24 | 1,2-+2,4-Dimethyl-diamantane |
| 23 | 4,8-DMD | 201 | 216 | 4934 (3838) | 2.330 (3.230) | C16H24 | 4,8-Dimethyl-diamantane |
| 24 | TMD | 215 | 230 | 4934 (4027) | 2.164 (3.350) | C17H26 | Trimethyl-diamantane |
| 25 | 3-MD | 187 | 202 | 5000 (4027) | 2.520 (2.935) | C15H22 | 3-methyl-diamantane |
| 26 | 3,4-DMD | 201 | 216 | 5036 (3957) | 2.345 (3.190) | C16H24 | 2-Methyl-adamantane |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, S.; Hu, S.; Cao, J.; Wu, M.; Zhang, D. Diamondoid Characterization in Condensate by Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry: The Junggar Basin of Northwest China . Int. J. Mol. Sci. 2012, 13, 11399-11410. https://doi.org/10.3390/ijms130911399
Li S, Hu S, Cao J, Wu M, Zhang D. Diamondoid Characterization in Condensate by Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry: The Junggar Basin of Northwest China . International Journal of Molecular Sciences. 2012; 13(9):11399-11410. https://doi.org/10.3390/ijms130911399
Chicago/Turabian StyleLi, Shuifu, Shouzhi Hu, Jian Cao, Ming Wu, and Dongmei Zhang. 2012. "Diamondoid Characterization in Condensate by Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry: The Junggar Basin of Northwest China " International Journal of Molecular Sciences 13, no. 9: 11399-11410. https://doi.org/10.3390/ijms130911399




