The Metalloporphyrin Antioxidant, MnTE-2-PyP, Inhibits Th2 Cell Immune Responses in an Asthma Model
Abstract
:1. Introduction
2. Results and Discussion
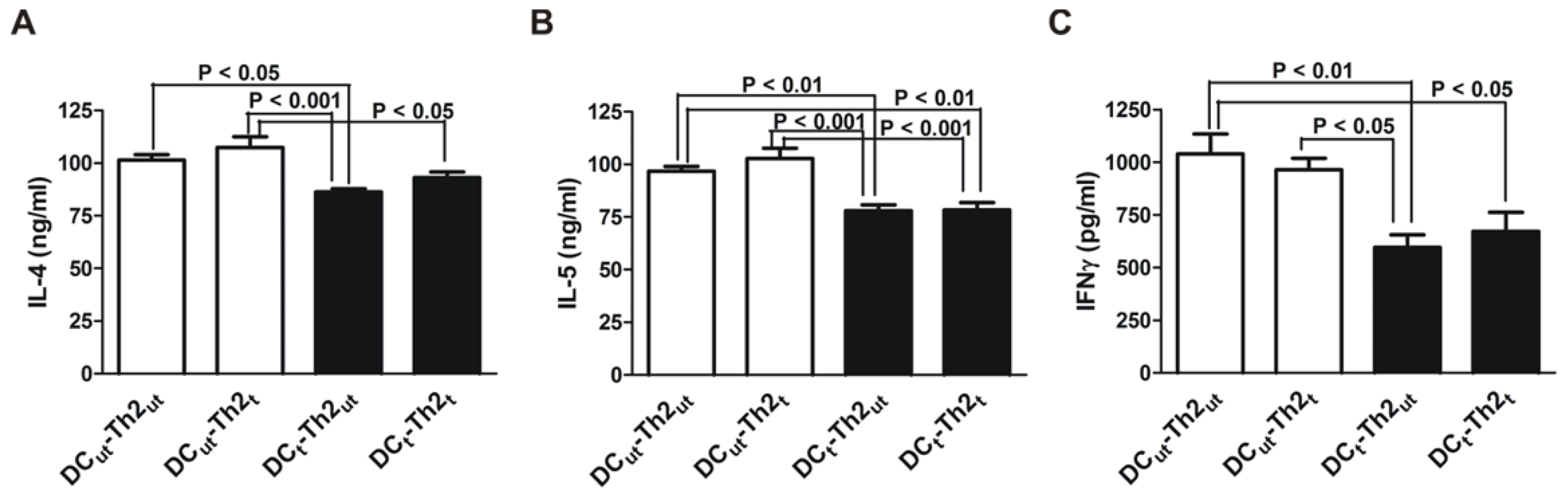
2.1. MnTE-2-PyP Reduces IL-4 and IL-5 Production in DC-T Cell Responses
2.2. MnTE-2-PyP Down-Regulates CD25 on Th2 Cells
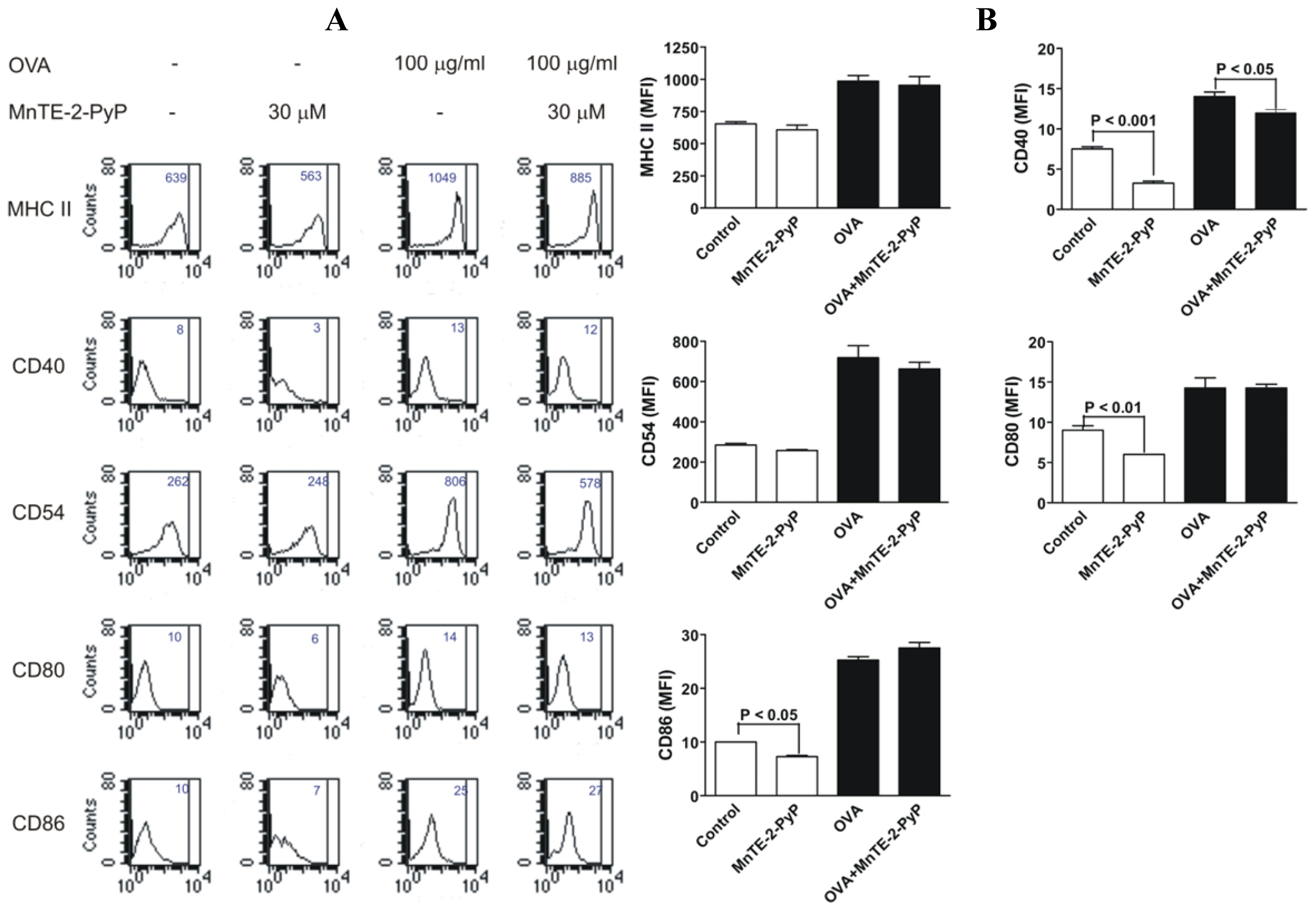
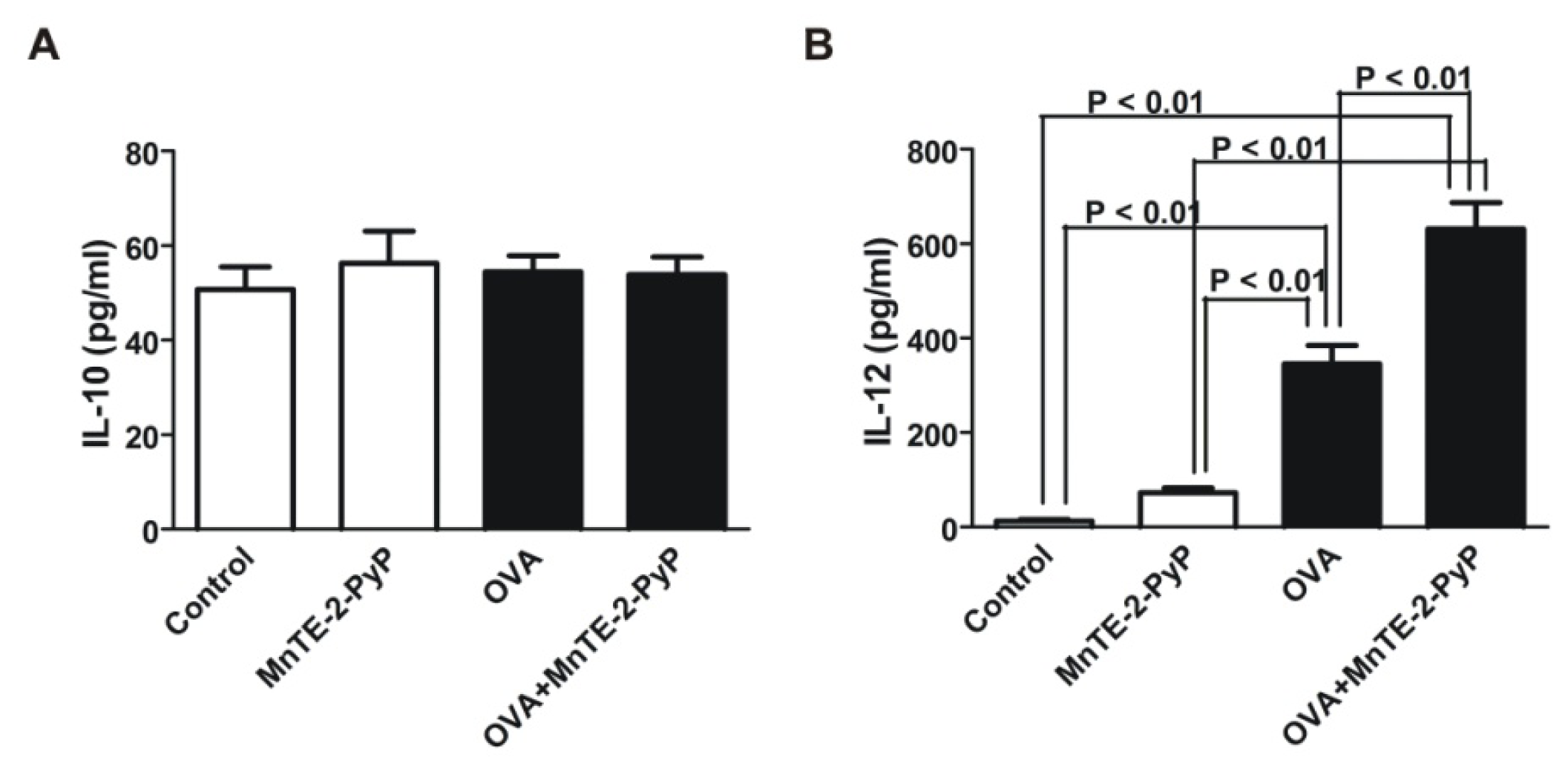
2.3. Effect of MnTE-2-PyP on DC Surface Molecule Expression and Cytokine Production
3. Experimental Section
3.1. Generation of Dendritic Cells from Bone Marrow Progenitors
3.2. Generation of OVA-Specific Th2 Cells
3.3. DC-Dependent T Cell Production of Cytokines
3.4. Antigen-Specific Th2 Cell Proliferation
3.5. FACS Analysis of Cell Surface Markers
3.6. IL-10 and IL-12 Cytokine Assays
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Chang, L.Y.; Crapo, J.D. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic. Biol. Med 2002, 33, 379–386. [Google Scholar]
- Jungsuwadee, P.; Dekan, G.; Stingl, G.; Epstein, M.M. Recurrent aerosol antigen exposure induces distinct patterns of experimental allergic asthma in mice. Clin. Immunol. (Orlando, Fla.) 2002, 102, 145–153. [Google Scholar]
- Henricks, P.A.; Nijkamp, F.P. Reactive oxygen species as mediators in asthma. Pulm. Pharmacol. Ther 2001, 14, 409–420. [Google Scholar]
- Jarjour, N.N.; Calhoun, W.J. Enhanced production of oxygen radicals in asthma. J. Lab. Clin. Med 1994, 123, 131–136. [Google Scholar]
- Vachier, I.; Damon, M.; le Doucen, C.; de Paulet, A.C.; Chanez, P.; Michel, F.B.; Godard, P. Increased oxygen species generation in blood monocytes of asthmatic patients. Am. Rev. Respir. Dis 1992, 146, 1161–1166. [Google Scholar]
- Sanders, S.P.; Zweier, J.L.; Harrison, S.J.; Trush, M.A.; Rembish, S.J.; Liu, M.C. Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. Am. J. Respir. Crit. Care Med 1995, 151, 1725–1733. [Google Scholar]
- Kelly, F.J.; Mudway, I.; Blomberg, A.; Frew, A.; Sandstrom, T. Altered lung antioxidant status in patients with mild asthma. Lancet 1999, 354, 482–483. [Google Scholar]
- Batinic-Haberle, I.; Benov, L.; Spasojevic, I.; Fridovich, I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem 1998, 273, 24521–24528. [Google Scholar]
- Day, B.J.; Batinic-Haberle, I.; Crapo, J.D. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic. Biol. Med 1999, 26, 730–736. [Google Scholar]
- Day, B.J.; Fridovich, I.; Crapo, J.D. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys 1997, 347, 256–262. [Google Scholar]
- Day, B.J.; Shawen, S.; Liochev, S.I.; Crapo, J.D. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J. Pharmacol. Exp. Ther 1995, 275, 1227–1232. [Google Scholar]
- Ferrer-Sueta, G.; Vitturi, D.; Batinic-Haberle, I.; Fridovich, I.; Goldstein, S.; Czapski, G.; Radi, R. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. J. Biol. Chem 2003, 278, 27432–27438. [Google Scholar]
- Mackensen, G.B.; Patel, M.; Sheng, H.; Calvi, C.L.; Batinic-Haberle, I.; Day, B.J.; Liang, L.P.; Fridovich, I.; Crapo, J.D.; Pearlstein, R.D.; et al. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. J. Neurosci 2001, 21, 4582–4592. [Google Scholar]
- Oury, T.D.; Thakker, K.; Menache, M.; Chang, L.Y.; Crapo, J.D.; Day, B.J. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am. J. Respir. Cell Mol. Biol 2001, 25, 164–169. [Google Scholar]
- Vujaskovic, Z.; Batinic-Haberle, I.; Rabbani, Z.N.; Feng, Q.F.; Kang, S.K.; Spasojevic, I.; Samulski, T.V.; Fridovich, I.; Dewhirst, M.W.; Anscher, M.S. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic. Biol. Med 2002, 33, 857–863. [Google Scholar]
- Tse, H.M.; Thayer, T.C.; Steele, C.; Cuda, C.M.; Morel, L.; Piganelli, J.D.; Mathews, C.E. NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. J. Immunol 2010, 185, 5247–5258. [Google Scholar]
- Piganelli, J.D.; Flores, S.C.; Cruz, C.; Koepp, J.; Batinic-Haberle, I.; Crapo, J.; Day, B.; Kachadourian, R.; Young, R.; Bradley, B.; et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 2002, 51, 347–355. [Google Scholar]
- Tse, H.M.; Milton, M.J.; Piganelli, J.D. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: Implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic. Biol. Med 2004, 36, 233–247. [Google Scholar]
- Huh, J.C.; Strickland, D.H.; Jahnsen, F.L.; Turner, D.J.; Thomas, J.A.; Napoli, S.; Tobagus, I.; Stumbles, P.A.; Sly, P.D.; Holt, P.G. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J. Exp. Med 2003, 198, 19–30. [Google Scholar]
- Labeur, M.S.; Roters, B.; Pers, B.; Mehling, A.; Luger, T.A.; Schwarz, T.; Grabbe, S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J. Immunol 1999, 162, 168–175. [Google Scholar]
- Son, Y.I.; Egawa, S.; Tatsumi, T.; Redlinger, R.E., Jr; Kalinski, P.; Kanto, T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods 2002, 262, 145–157. [Google Scholar]
- Marovich, M.A.; McDowell, M.A.; Thomas, E.K.; Nutman, T.B. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol 2000, 164, 5858–5865. [Google Scholar]
- Corry, D.B.; Folkesson, H.G.; Warnock, M.L.; Erle, D.J.; Matthay, M.A.; Wiener-Kronish, J.P.; Locksley, R.M. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med 1996, 183, 109–117. [Google Scholar]
- Koch, M.; Witzenrath, M.; Reuter, C.; Herma, M.; Schutte, H.; Suttorp, N.; Collins, H.; Kaufmann, S.H. Role of local pulmonary IFN-γ expression in murine allergic airway inflammation. Am. J. Respir. Cell Mol. Biol 2006, 35, 211–219. [Google Scholar]
- Dobis, D.R.; Sawyer, R.T.; Gillespie, M.M.; Huang, J.; Newman, L.S.; Maier, L.A.; Day, B.J. Modulation of lymphocyte proliferation by antioxidants in chronic beryllium disease. Am. J. Respir. Crit. Care Med 2008, 177, 1002–1011. [Google Scholar]
- Sklavos, M.M.; Tse, H.M.; Piganelli, J.D. Redox modulation inhibits CD8 T cell effector function. Free Radic. Biol. Med 2008, 45, 1477–1486. [Google Scholar]
- Tse, H.M.; Milton, M.J.; Schreiner, S.; Profozich, J.L.; Trucco, M.; Piganelli, J.D. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J. Immunol 2007, 178, 908–917. [Google Scholar]
- Won, H.Y.; Sohn, J.H.; Min, H.J.; Lee, K.; Woo, H.A.; Ho, Y.S.; Park, J.W.; Rhee, S.G.; Hwang, E.S. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid. Redox Signal 2010, 13, 575–587. [Google Scholar]
- Case, A.J.; McGill, J.L.; Tygrett, L.T.; Shirasawa, T.; Spitz, D.R.; Waldschmidt, T.J.; Legge, K.L.; Domann, F.E. Elevated mitochondrial superoxide disrupts normal T cell development, impairing adaptive immune responses to an influenza challenge. Free Radic. Biol. Med 2011, 50, 448–458. [Google Scholar]
- Jendrysik, M.A.; Vasilevsky, S.; Yi, L.; Wood, A.; Zhu, N.; Zhao, Y.; Koontz, S.M.; Jackson, S.H. NADPH oxidase-2 derived ROS dictates murine DC cytokine-mediated cell fate decisions during CD4 T helper-cell commitment. PLoS One 2011, 6, e28198. [Google Scholar]


© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jungsuwadee, P.; Weaver, M.R.; Gally, F.; Oberley-Deegan, R.E. The Metalloporphyrin Antioxidant, MnTE-2-PyP, Inhibits Th2 Cell Immune Responses in an Asthma Model. Int. J. Mol. Sci. 2012, 13, 9785-9797. https://doi.org/10.3390/ijms13089785
Jungsuwadee P, Weaver MR, Gally F, Oberley-Deegan RE. The Metalloporphyrin Antioxidant, MnTE-2-PyP, Inhibits Th2 Cell Immune Responses in an Asthma Model. International Journal of Molecular Sciences. 2012; 13(8):9785-9797. https://doi.org/10.3390/ijms13089785
Chicago/Turabian StyleJungsuwadee, Paiboon, Michael R. Weaver, Fabienne Gally, and Rebecca E. Oberley-Deegan. 2012. "The Metalloporphyrin Antioxidant, MnTE-2-PyP, Inhibits Th2 Cell Immune Responses in an Asthma Model" International Journal of Molecular Sciences 13, no. 8: 9785-9797. https://doi.org/10.3390/ijms13089785



