Preparation of Porous Scaffolds from Silk Fibroin Extracted from the Silk Gland of Bombyx mori (B. mori)
Abstract
:1. Introduction
2. Results and Discussion
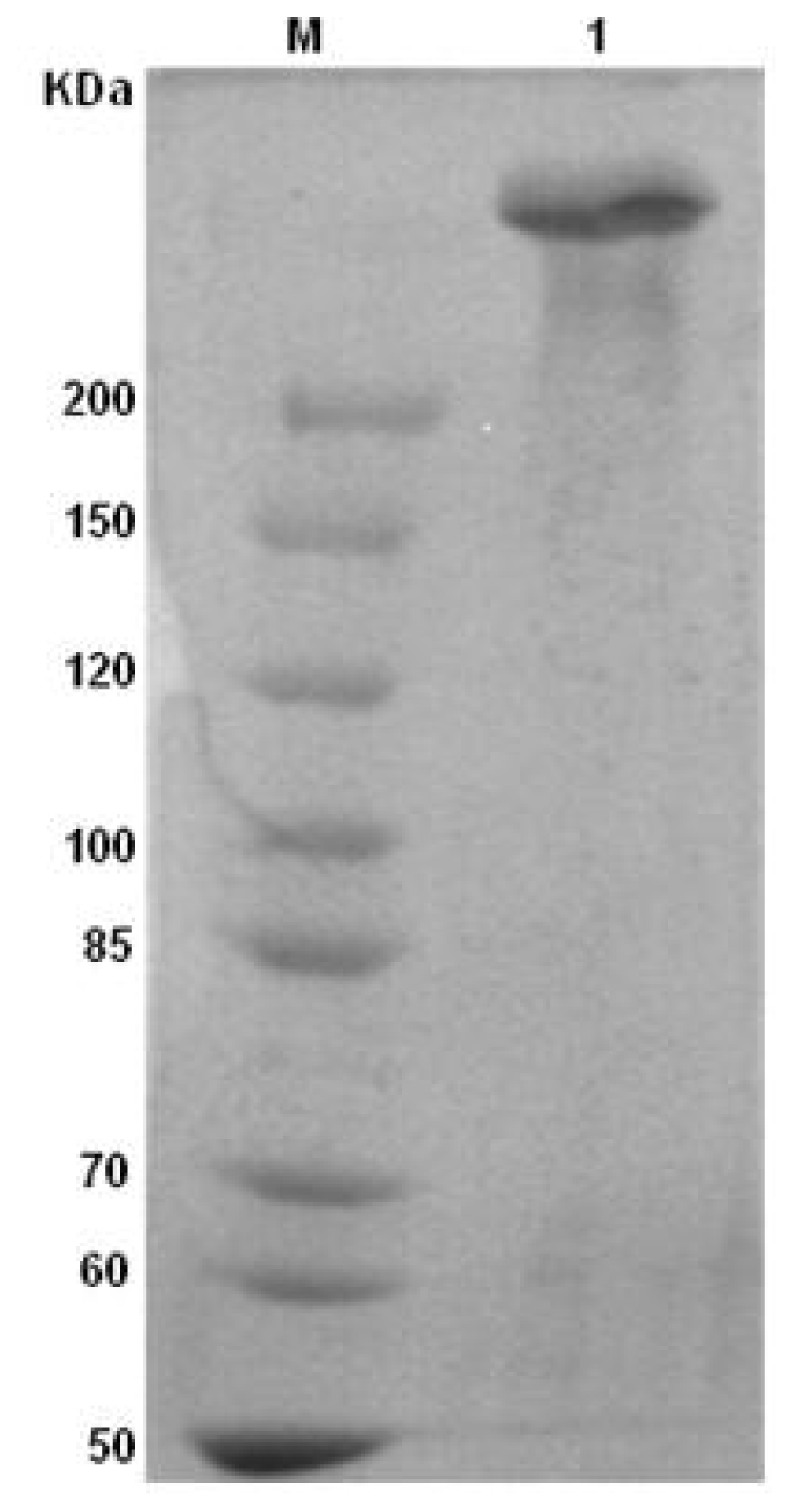
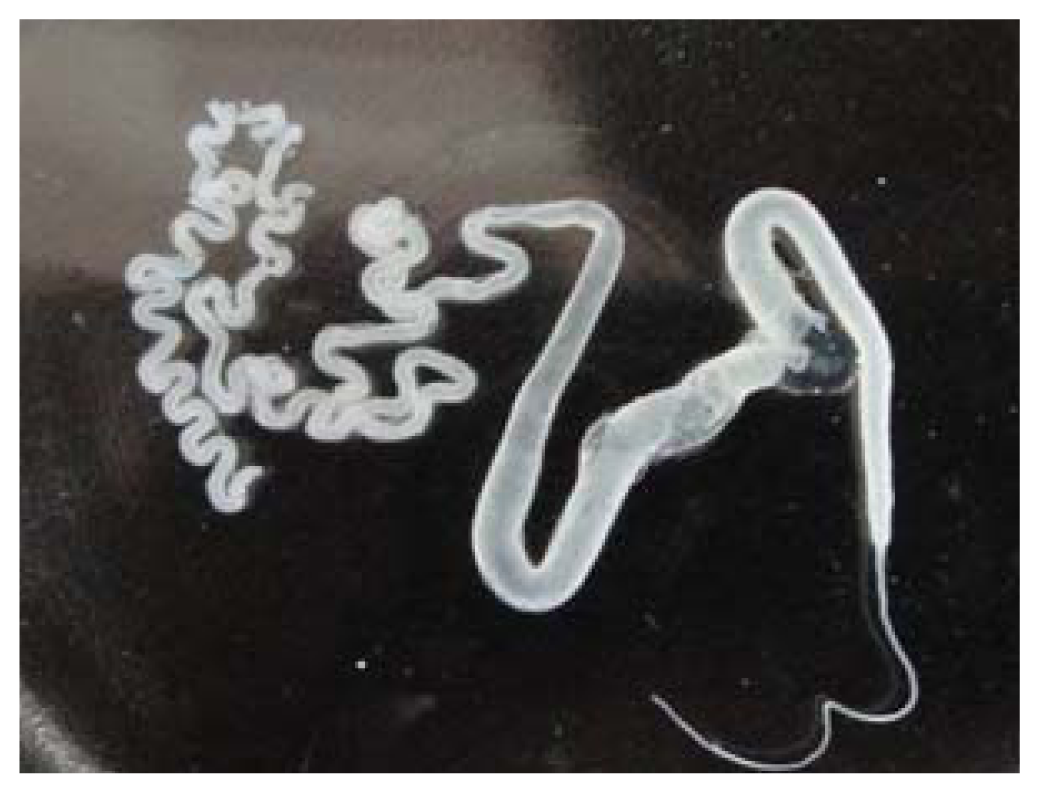
2.1. Identification of ASF
2.2. Morphology of Porous Scaffolds
2.3. The Mechanical Properties of Porous Scaffolds
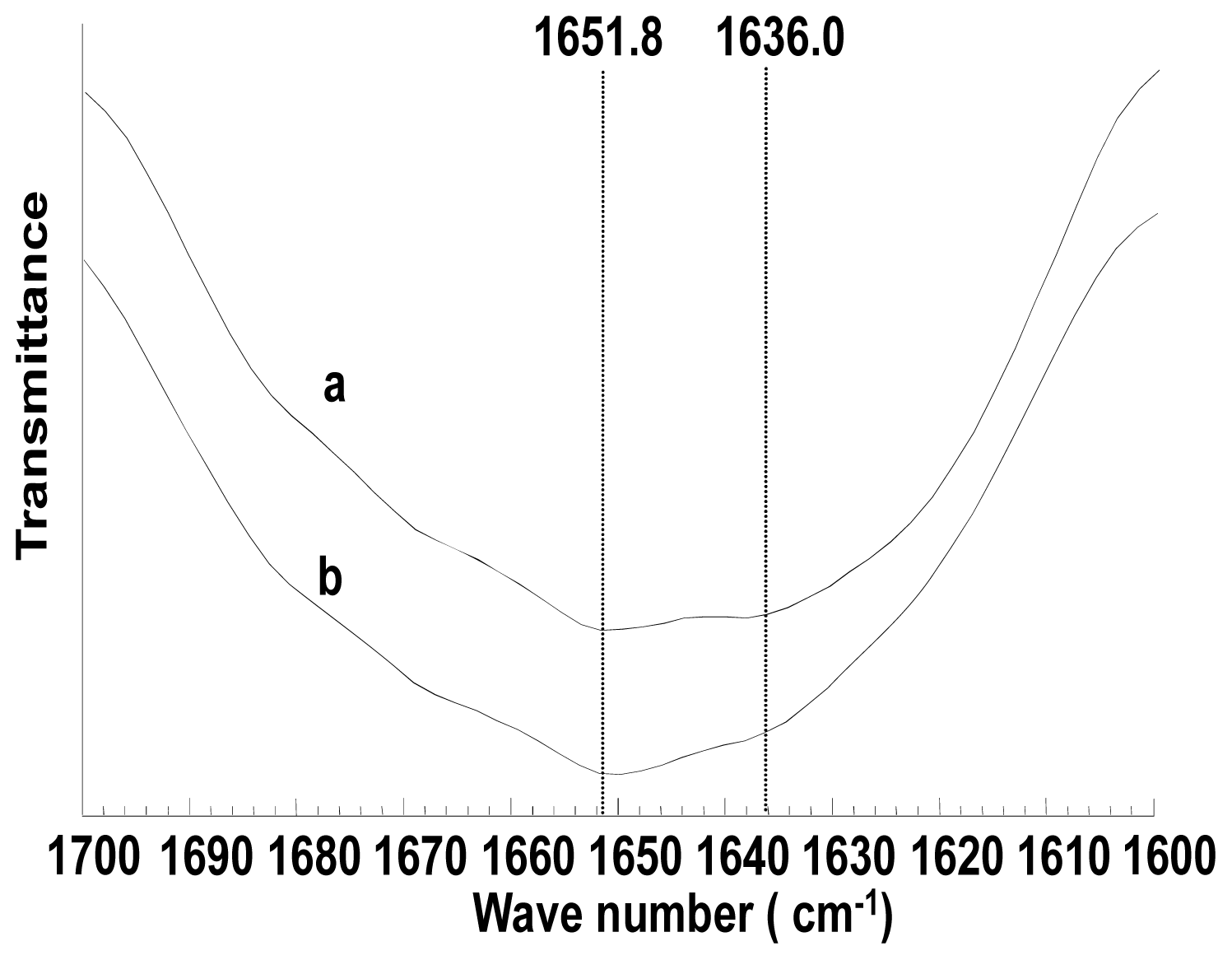
2.4. Structure Characterization of Porous Scaffolds
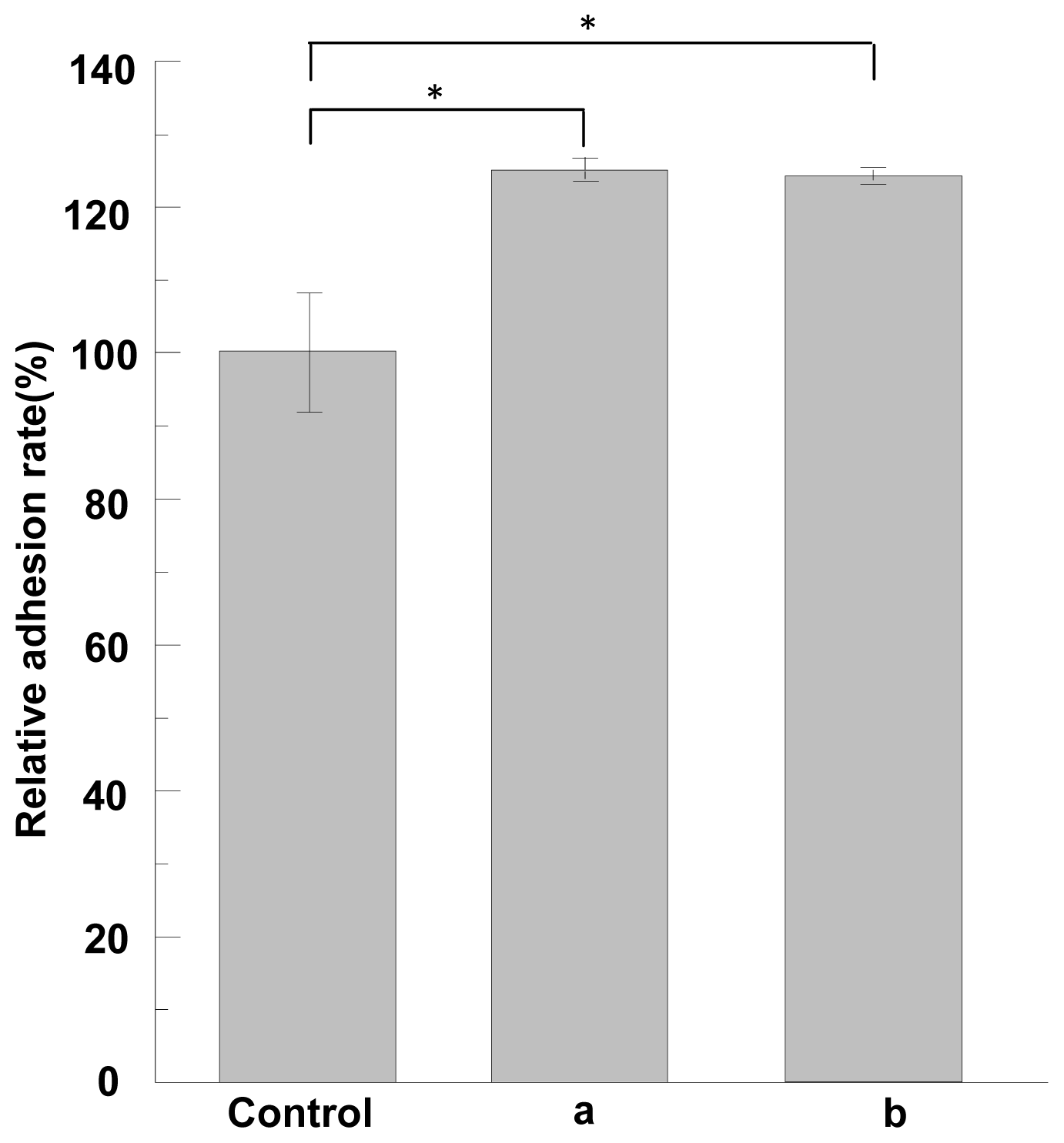
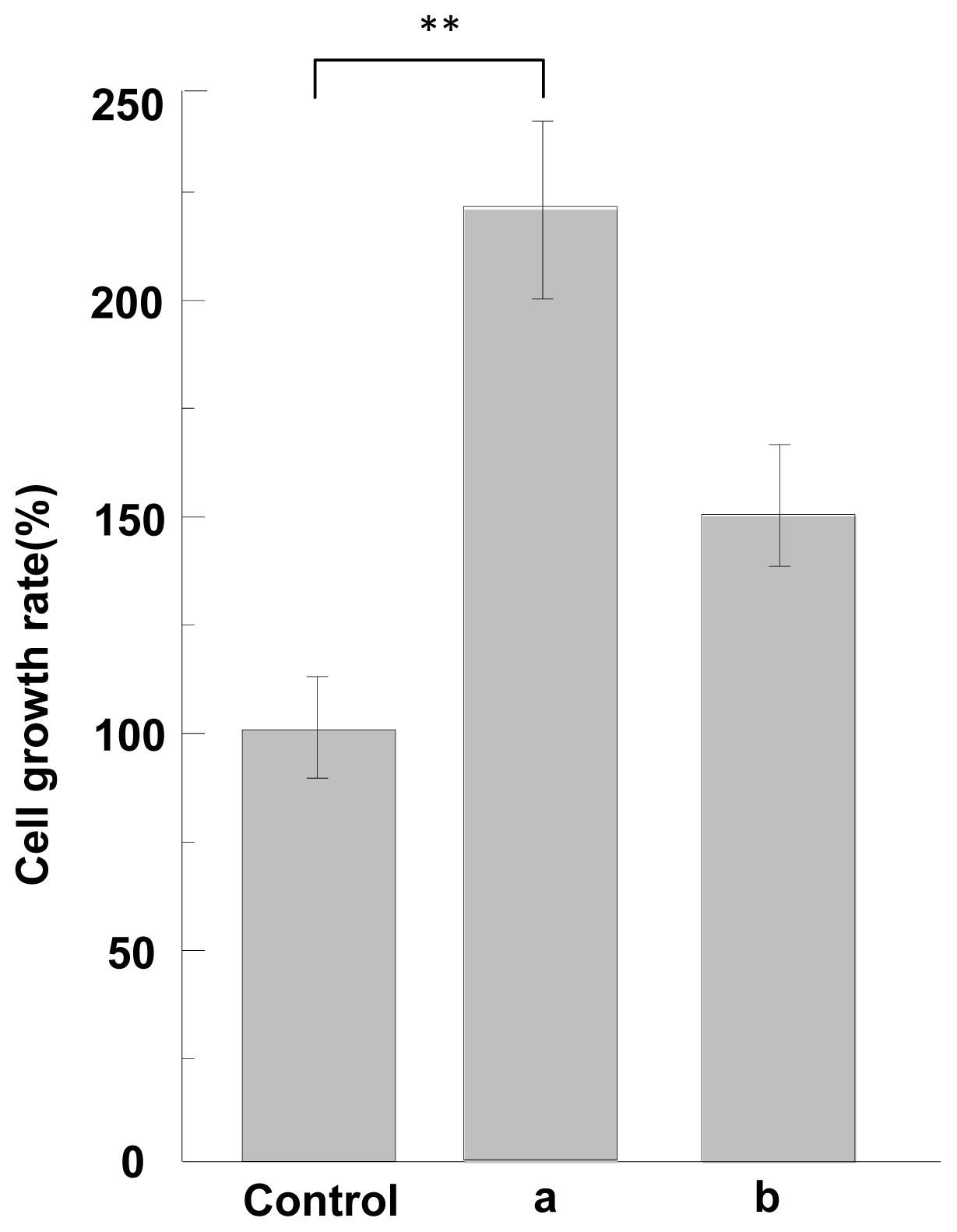
2.5. Cell Adhesion and Growth Activity of ASF
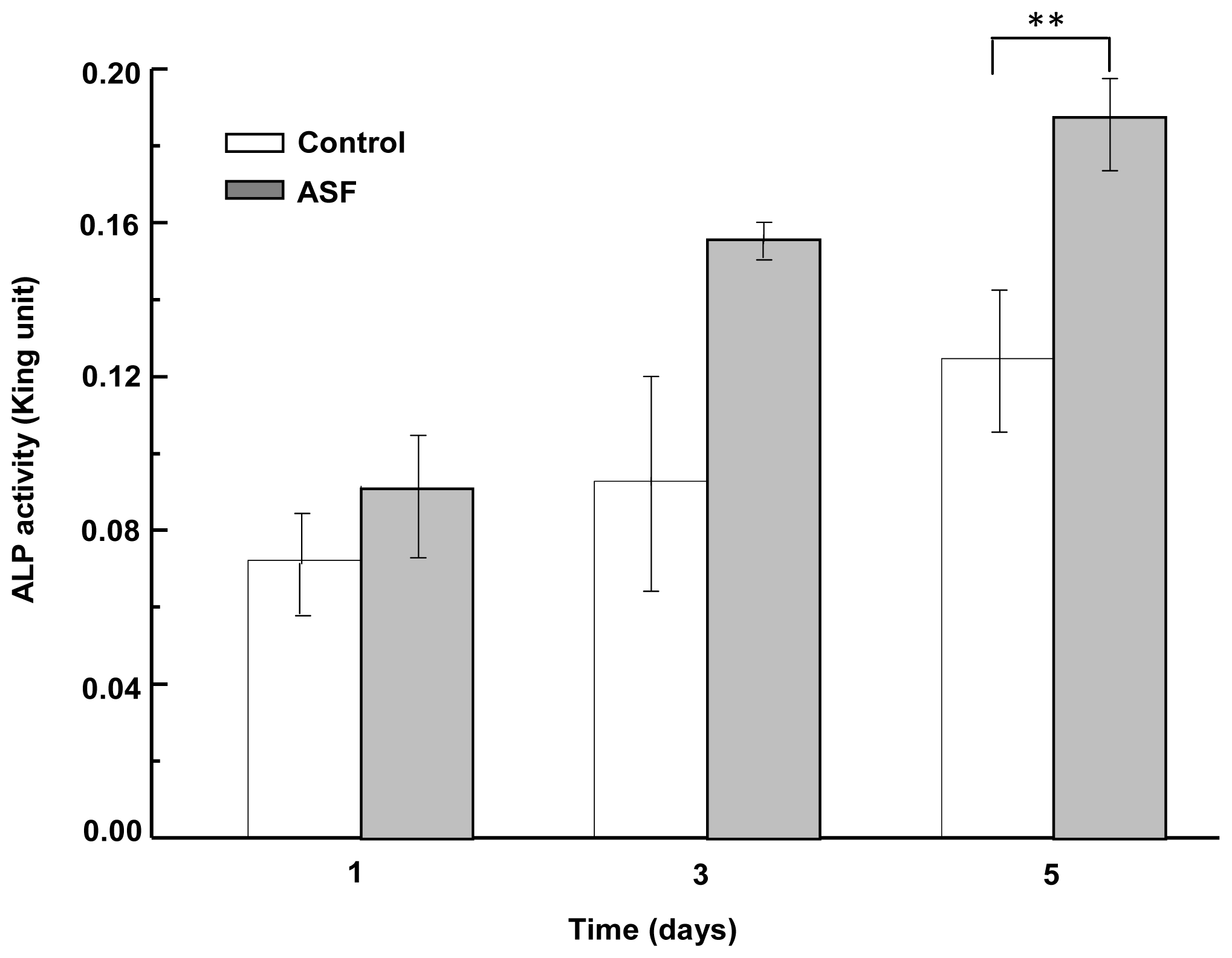
2.6. ALP Activity Assay of ASF
3. Materials and Methods
3.1. Materials
3.2. Extraction of ASF from the Silk Gland of B. mori
3.3. Amino Acid Composition Analysis
3.4. Preparation of Porous Scaffolds
3.5. Scanning Electron Microscopy
3.6. Porosity of Porous Scaffolds
3.7. Mechanical Property Test
3.8. Fourier Transformed Infrared Spectroscopy
3.9. Cell Adhesion Assay
3.10. Cell Growth Assay
3.11. ALP Activity Assay
3.12. Statistical Analysis
4. Conclusions
Acknowledgement
References
- Magoshi, J.; Magoshi, Y.; Becker, M.A.; Nakamura, S. Biospinning (silk fiber formation, multiple spinning mechanisms). In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: New York NY, USA, 1996; p. 667. [Google Scholar]
- Santin, M.; Motta, A.; Freddi, G.; Cannas, M. In vitro evaluation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res 1999, 46, 382–389. [Google Scholar]
- Minoura, N.; Tsukada, M.; Nagura, M. Fine-structure and oxygen permeability of silk fibroin membrane treated with methanol. Polymer 1990, 31, 265–269. [Google Scholar]
- Kweon, H.; Ha, H.C.; Um, I.C.; Park, Y.H. Physical properties of silk fibroin/chitosan blend films. J. Appl. Polym. Sci 2001, 80, 928–934. [Google Scholar]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar]
- Jin, H.J.; Park, J.; Karageorgiou, V.; Kim, U.J.; Valluzzi, R.; Kaplan, D.L. Water-stable silk films with reduced β-sheet content. Adv. Funct. Mater 2005, 15, 1241–1247. [Google Scholar]
- Zhang, F.; Zuo, B.; Fan, Z.; Xie, Z.; Lu, Q.; Zhang, X.; Kaplan, D.L. Mechanisms and control of silk-based electrospinning. Biomacromolecules 2012, 13, 798–804. [Google Scholar]
- Lawrence, B.D.; Omenetto, F.; Chui, K.; Kaplan, D.L. Processing methods to control silk fibroin film biomaterial features. J. Mater. Sci 2008, 43, 6967–6985. [Google Scholar]
- Hanawa, T.; Maeda, R.; Muramatsu, E.; Suzuki, M.; Sugihara, S. New oral dosage form for elderly patients. III. Stability of trichlormethiazide in silk fibroin gel and various sugar solutions. Drug Dev. Ind. Pharm 2000, 26, 1091–1097. [Google Scholar]
- Min, S.; Gao, X.; Han, C.; Chen, Y.; Yang, M.; Zhu, L.; Zhang, H.; Liu, L.; Yao, J. Preparation of a silk fibroin spongy wound dressing and its therapeutic efficiency in skin defects. J. Biomater. Sci 2012, 23, 97–110. [Google Scholar]
- Perry, H.; Gopinath, A.; Kaplan, D.L.; Negro, L.D.; Omenetto, F.G. Nano- and micropatterning of optically transparent, mechanically robust, biocompatible silk fibroin films. Adv. Mater 2008, 20, 3070–3072. [Google Scholar]
- Kim, U.J.; Park, J.; Kim, H.J.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar]
- Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Silk fibroin nanofiber. Electrospinning, properties, and structure. Polym. J 2003, 35, 185–190. [Google Scholar]
- Wang, Y.Z.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6084. [Google Scholar]
- Wang, Q.X.; Wenk, E.; Matsumoto, A.; Meinel, L.; Li, C.M.; Kaplan, D.L. Silk microspheres for encapsulation and controlled release. J. Control. Release 2007, 117, 360–370. [Google Scholar]
- Kim, H.J.; Kim, U.J.; Vunjak-Novakovic, G.; Min, B.H.; Kaplan, D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 2005, 26, 4442–4452. [Google Scholar]
- Meinel, L.; Karageorgiou, V.; Fajardo, R.; Snyder, B.; Shinde-Patil, V.; Zichner, L.; Kaplan, D.; Langer, R.; Vunjak-Novakovic, G. Bone tissue engineering using human mesenchymal stem cells: Effects of scaffold material and medium flow. Ann. Biomed. Eng 2004, 32, 112–122. [Google Scholar]
- Wang, Y.; Kim, U.J.; Blasioli, D.J.; Kim, H.J.; Kaplan, D.L. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 2005, 26, 7082–7094. [Google Scholar]
- Meinel, L.; Hofmann, S.; Karageorgiou, V.; Zichner, L.; Langer, R.; Kaplan, D.; Vunjak-Novakovic, G. Engineering cartilage-like tissue using human mesenchymal stem cells and silk protein scaffolds. Biotechnol. Bioeng 2004, 88, 379–391. [Google Scholar]
- Meinel, L.; Karageorgiou, V.; Hofmann, S.; Fajardo, R.; Snyder, B.; Li, C.M.; Zichner, L.; Langer, R.; Vunjak-Novakovic, G.; Kaplan, D.L. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. Part A 2004, 71A, 528–537. [Google Scholar]
- Meinel, L.; Betz, O.; Fajardo, R.; Hofmann, S.; Nazarian, A.; Cory, E.; Hilbe, M.; McCool, J.; Langer, R.; Vunjak-Novakovic, G.; et al. Silk based biomaterials to heal critical sized femur defects. Bone 2006, 39, 922–931. [Google Scholar]
- Kim, H.J.; Kim, H.S.; Matsumoto, A.; Chin, I.J.; Jin, H.J.; Kaplan, D.L. Processing windows for forming silk fibroin biomaterials into a 3D porous matrix. Aust. J. Chem 2005, 58, 716–720. [Google Scholar]
- Iizuka, E. Silk thread: Mechanism of spinning and its mechanical properties. J. Appl. Polym. Sci. Appl. Polym. Symp 1985, 41, 173–185. [Google Scholar]
- Tsukada, M.; Freddi, G.; Gotoh, Y.; Kasai, N. Physical and chemical properties of tussah silk fibroin films. J. Polym. Sci. Part B Polym. Phys 1994, 32, 1407–1412. [Google Scholar]
- Makaya, K.; Terada, S.; Ohgo, K.; Asakura, T. Comparative study of silk fibroin porous scaffolds derived from salt/water and sucrose/hexafluoroisopropanol in cartilage formation. J. Biosci. Bioeng 2009, 108, 68–75. [Google Scholar]
- Gil, E.; Kluge, J.; Rockwood, D.; Rajkhowa, R.; Wang, L.; Wang, X.; Kaplan, D. Mechanical improvements to reinforced porous silk scaffolds. J. Biomed. Mater. Res. A 2011, 99A, 16–28. [Google Scholar]
- Kim, U.; Park, J.; Kim, H.; Wadac, M.; Kaplan, D. Three-dimensional aqueous-derived biomaterial scaffolds. Biomaterials 2005, 26, 2775–2785. [Google Scholar]
- Nazarov, R.; Jin, H.; Kaplan, D. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 2004, 5, 718–726. [Google Scholar]
- Mandal, B.B.; Kundu, S.C. A novel method for dissolution and stabilization of non-mulberry silk gland protein fibroin using anionic surfactant sodium dodecyl sulfate. Biotechnol. Bioeng 2008, 99, 1482–1489. [Google Scholar]
- Mandal, B.B.; Kundu, S.C. Non-bioengineered silk fibroin protein 3D scaffolds for potential biotechnological and tissue engineering applications. Macromol. Biosci 2008, 8, 807–818. [Google Scholar]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar]
- Nam, J.; Park, H. Morphology of regenerated silk fibroin: Effects of freezing temperature, alcohol addition, and molecular weight. J. Appl. Poly. Sci 2001, 81, 3008–3021. [Google Scholar]
- Shimura, K. Zoku Kenshi no Kozo (structure of silk fiber); Hojyo, N., Ed.; Shinshu University: Ueda, Japan, 1980; p. 335. [Google Scholar]
- Aqida, S.N.; Ghazali, M.I.; Hashim, J. Effects of porosity on mechanical properties of metal matrix composite: An overview. J. Teknologi A 2004, 40, 17–32. [Google Scholar]
- Harris, L.D.; Kim, B.S.; Mooney, D.J. Open pore biodegradable matrices formed with gas foaming. Biomed. Mater. Res 1998, 42, 396–402. [Google Scholar]
- Yao, J.; Nakazawa, Y.; Asakura, T. Structures of Bombyx mori and Samia cynthia ricini silk fibroins studied with solid-state NMR. Biomacromolecules 2004, 5, 680–688. [Google Scholar]
- Shao, Z.; Vollrath, F. The effect of solvents on the contraction and mechanical properties of spider silk. Polymer 1999, 40, 1799–1806. [Google Scholar]
- Ha, S.W.; Tonelli, A.; Hudson, S.M. Structural Studies of Bombyx mori Silk fibroin during regeneration from solutions and wet fiber spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar]


| Amino acid | ASF | Reference [33] | Amino acid | ASF | Reference [33] |
|---|---|---|---|---|---|
| Ala | 30.0 | 30.0 | Val | 2.3 | 2.5 |
| Gly | 43.8 | 42.9 | Leu | 0.6 | 0.6 |
| Tyr | 2.5 | 4.8 | Ile | 0.6 | 0.6 |
| Ser | 11.0 | 12.2 | Phe | 1.6 | 0.7 |
| Asp | 2.1 | 1.9 | Pro | 0.8 | 0.5 |
| Arg | 0.6 | 0.5 | Thr | 1.0 | 0.9 |
| His | 0.2 | 0.2 | Met | 0 | 0.1 |
| Glu | 2.5 | 1.4 | Cys | 0 | 0 |
| Lys | 0.4 | 0.4 |
| Concentration (wt%) | Ethanol treatment | Porosity (%) | Average pore diameter (nm) | Apparent density (g/mL) | Compression (Mpa) |
|---|---|---|---|---|---|
| 2 | No | 90.9 | 24,679 | 1.1 | 1.6 ± 0.2 |
| 4 | No | 88.9 | 19,524 | 1.2 | 2.6 ± 0.1 |
| 6 | No | 85.1 | 7,736 | 1.3 | 4.5 ± 0.1 |
| 2 | Yes | 91.4 | 22,848 | 1.1 | 2.1 ± 0.2 |
| 4 | Yes | 88.4 | 19,786 | 1.4 | 3.6 ± 0.4 |
| 6 | Yes | 82.8 | 14,466 | 1.8 | 6.9 ± 0.4 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, M.; Shuai, Y.; He, W.; Min, S.; Zhu, L. Preparation of Porous Scaffolds from Silk Fibroin Extracted from the Silk Gland of Bombyx mori (B. mori). Int. J. Mol. Sci. 2012, 13, 7762-7775. https://doi.org/10.3390/ijms13067762
Yang M, Shuai Y, He W, Min S, Zhu L. Preparation of Porous Scaffolds from Silk Fibroin Extracted from the Silk Gland of Bombyx mori (B. mori). International Journal of Molecular Sciences. 2012; 13(6):7762-7775. https://doi.org/10.3390/ijms13067762
Chicago/Turabian StyleYang, Mingying, Yajun Shuai, Wen He, Sijia Min, and Liangjun Zhu. 2012. "Preparation of Porous Scaffolds from Silk Fibroin Extracted from the Silk Gland of Bombyx mori (B. mori)" International Journal of Molecular Sciences 13, no. 6: 7762-7775. https://doi.org/10.3390/ijms13067762




