Synthesis of Zinc Oxide Nanoparticles and Their Effect on the Compressive Strength and Setting Time of Self-Compacted Concrete Paste as Cementitious Composites
Abstract
:1. Introduction
2. Results and Discussion
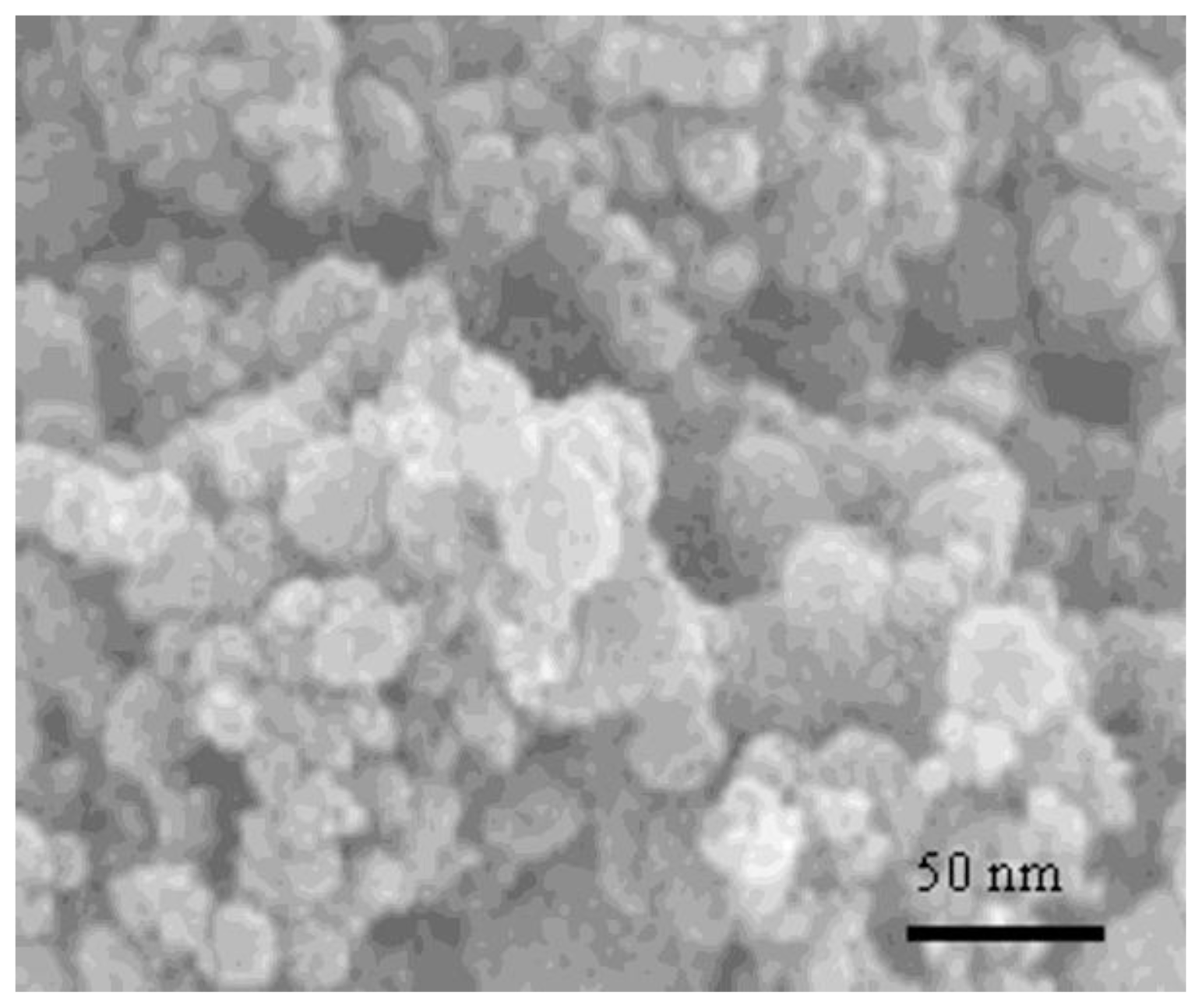
2.1. Size Distribution of Synthesized Nanoparticles by SEM and XRD
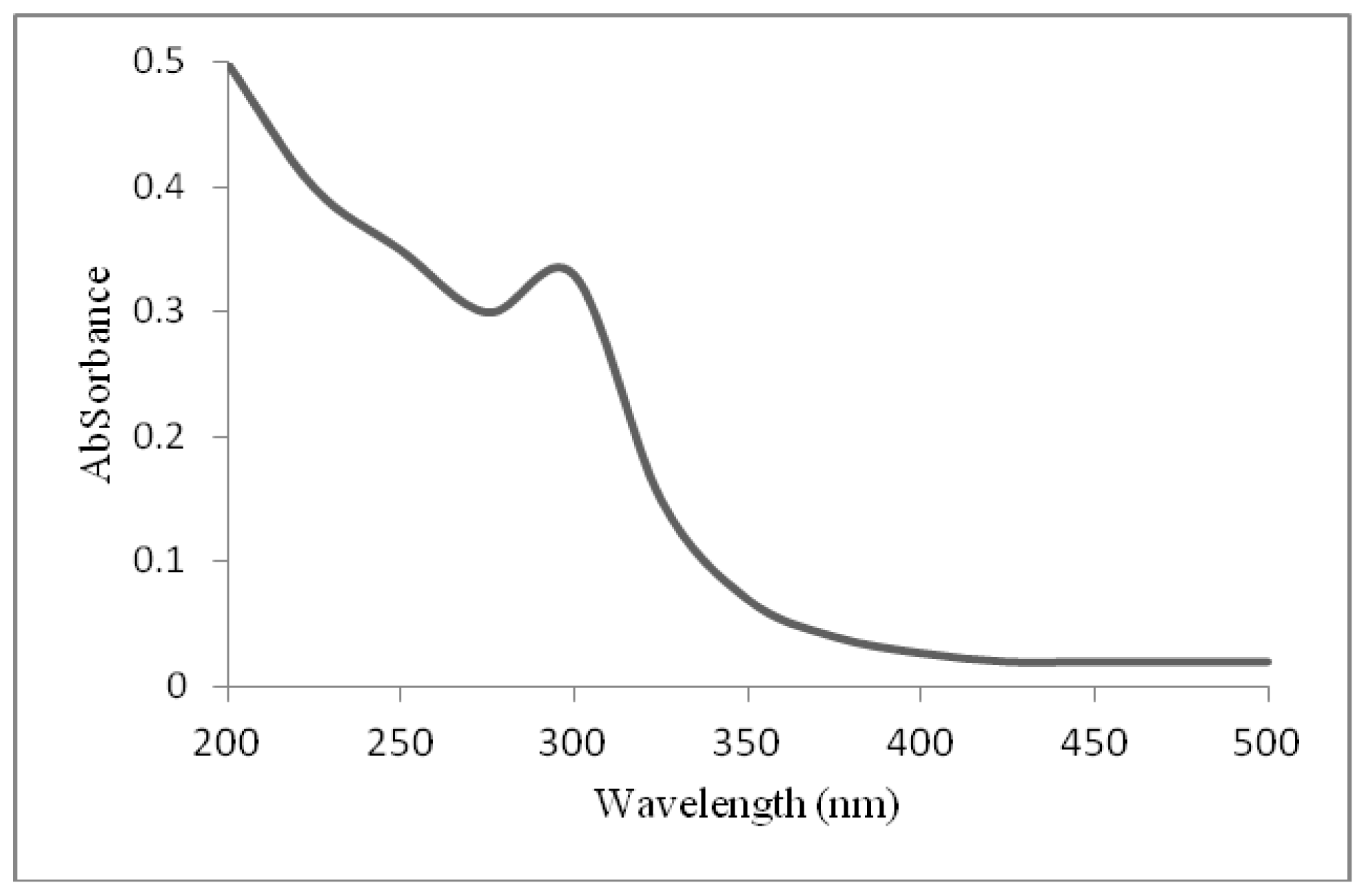
2.2. UV-Visible Absorption Spectra of ZnO Nanoparticles
2.3. Analysis of SCC Strength with and without ZnO Nanoparticles
2.4. Split Tensile Strength
2.5. Flexural Strength
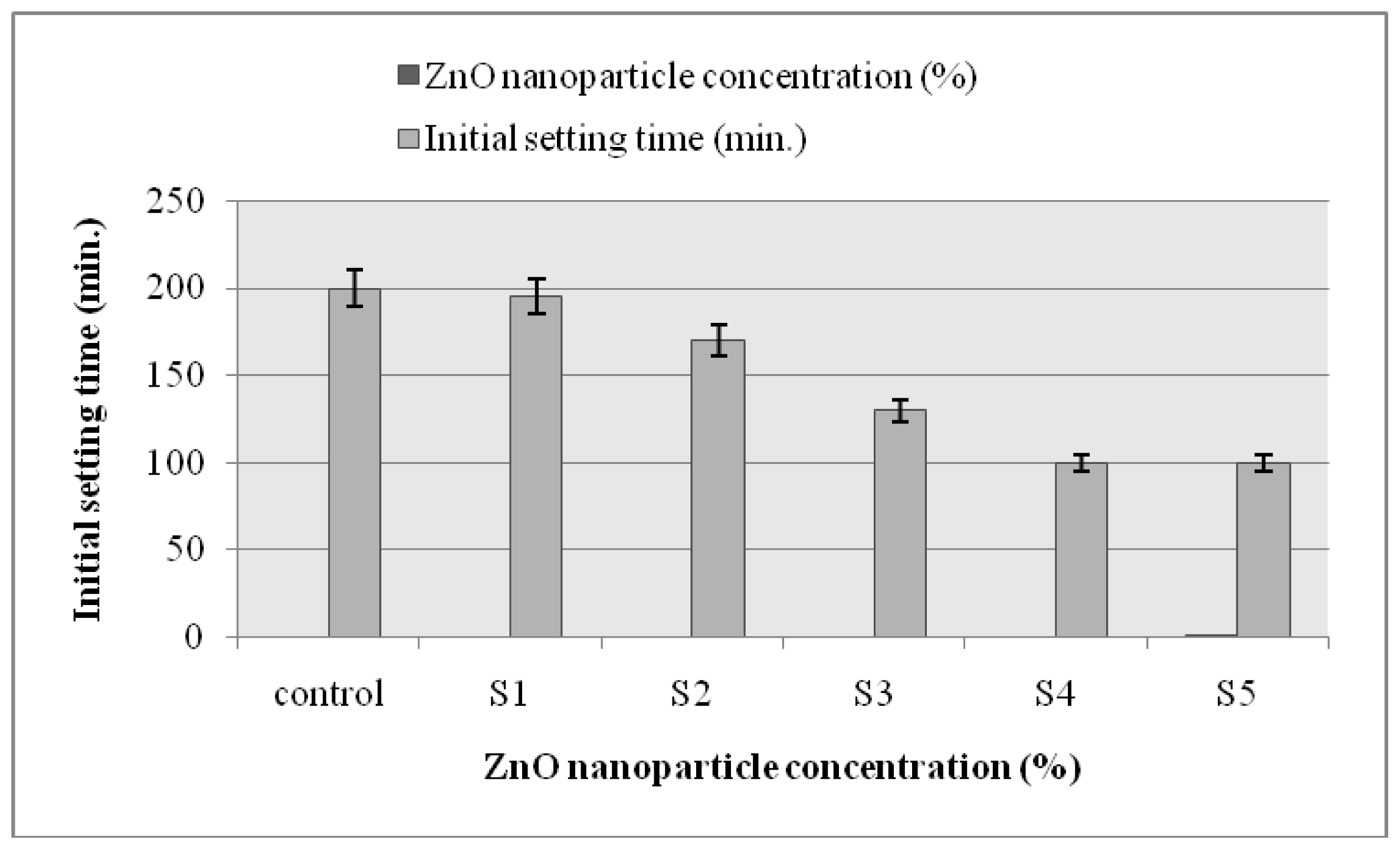
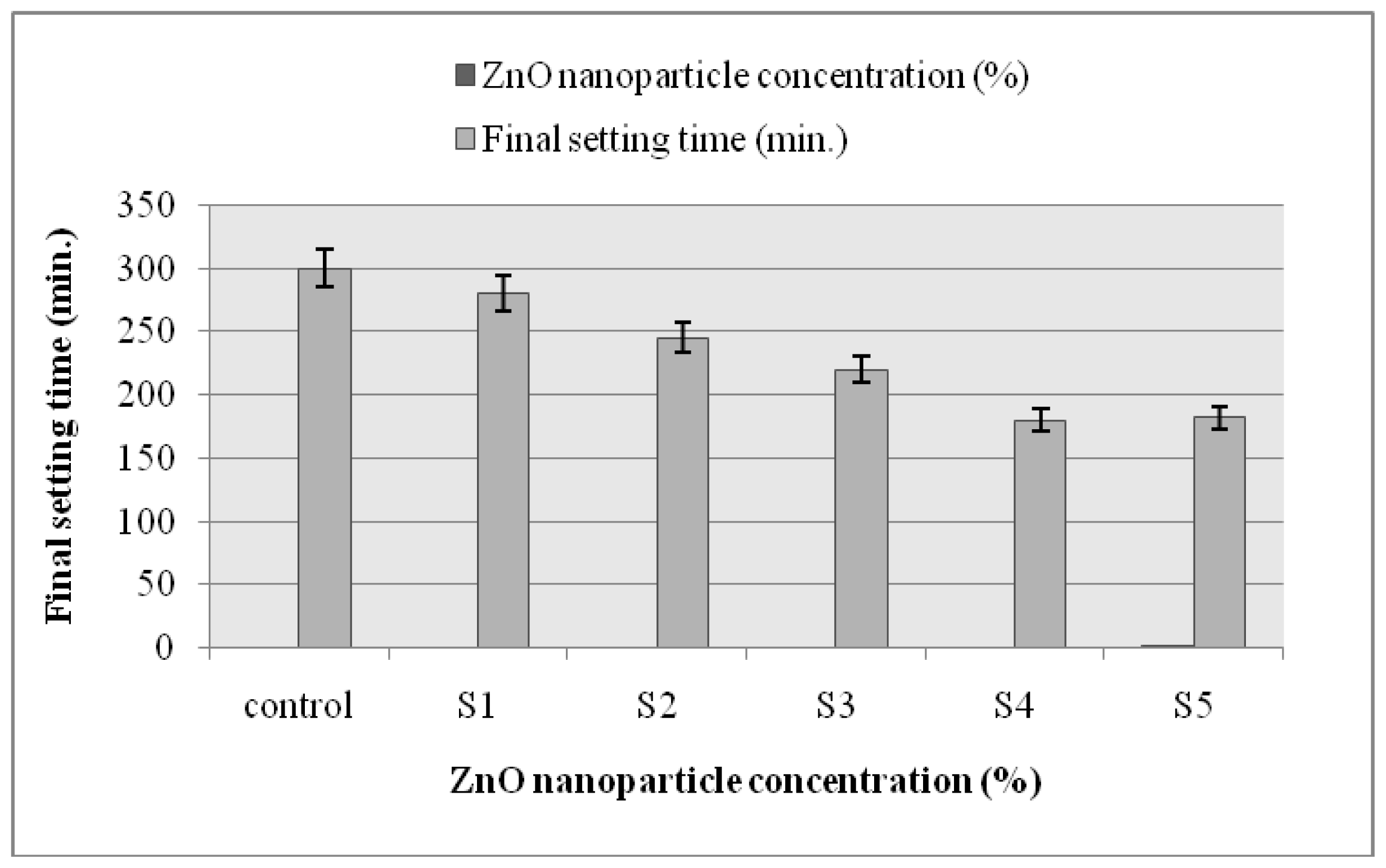
2.6. Setting Time
3. Experimental Section
3.1. Materials
3.2. Synthesis of ZnO Nanoparticles
3.3. Experimental Cement
3.4. Experimental Self-Compacted Concrete (SCC) Mixture Preparation
3.5. Preparation of Test Specimens
3.6. Split Tensile Strength of ZnO Nanoparticles Blended Self-Compacted Concrete
3.7. Flexural Strength of ZnO Nanoparticle-Modified Self-Compacted Concrete
3.8. Setting Time of ZnO Nanoparticle-Modified Self-Compacted Concrete
4. Conclusions
Acknowledgements
References
- Nazari, A.; Riahi, S. The effects of zinc dioxide nanoparticles on flexural strength of self-compacting concrete. Compos. Part B 2011, 42, 167–175. [Google Scholar]
- Sobolev, K.; Ferrada-Gutiérrez, M. How nanotechnology can change the concrete world: Part 2. Am. Ceram. Soc. Bull 2005, 84, 16–19. [Google Scholar]
- Nazari, A.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Mechanical properties of cement mortar with Al2O3 nanoparticles. J. Am. Sci 2010, 6, 94–97. [Google Scholar]
- Fava, C.; Bergol, L.; Fornasier, G.; Giangrasso, F.; Rocco, C. Fracture Behaviour of Self-Compacting Concrete. In International RILEM Symposium on Self-Compacting Concrete; Wallevik, O., Nielsson, I., Eds.; RILEM Publications SARL: Reykjavik, Iceland, 2003; pp. 628–636. [Google Scholar]
- Li, H.; Xiao, H.G.; Yuan, J.; Ou, J. Microstructure of cement mortar with nano-particles. Compos. Part B 2003, 35, 126–134. [Google Scholar]
- Ji, T. Preliminary study on the water permeability and microstructure of concrete incorporating nano-SiO2. Cem. Concr. Res 2005, 35, 1943–1947. [Google Scholar]
- Jo, B.W.; Kim, C.H.; Tae, G.H.; Park, J.B. Characteristics of cement mortar with nano-SiO2 particles. Construct. Build. Mater 2007, 21, 1351–1355. [Google Scholar]
- Li, H.; Xiao, H.G.; Ou, J.P. A study on mechanical and pressure-sensitive properties of cement mortar with nanophase materials. Cem. Concr. Res 2004, 34, 435–438. [Google Scholar]
- Li, H.; Zhang, M.H.; Ou, J.P. Abrasion resistance of concrete containing nanoparticles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Construct. Build. Mater 2007, 21, 539–545. [Google Scholar]
- Lin, K.L.; Chang, W.C.; Lin, D.F.; Luo, H.L.; Tsai, M.C. Effects of nano-SiO2 and different ash particle sizes on sludge ash-cement mortar. J. Environ. Manag 2008, 88, 708–714. [Google Scholar]
- Li, H.; Xiao, H.G.; Ou, J.P. A study on mechanical and pressure-sensitive properties of cement mortar with nanophase materials. Cem. Concr. Res 2004, 34, 435–438. [Google Scholar]
- Bui, D.D.; Hu, J.; Stroeven, P. Particle size effect on the strength of rice husk ash blended gap-graded Portland cement concrete. Cem. Concr. Compos 2005, 27, 357–366. [Google Scholar]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P.D. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar]
- Marcinek, A. Direct characterization of Hexamethyl (dewar benzene) radical cation by electronic absorption spectroscopy. J. Phys. Chem 1998, 102, 7761–7764. [Google Scholar]
- Ye, G.; Xiu, X.; De Schutter, G.; Poppe, A.M.; Taerwe, L. Influence of limestone powder as filler in SCC on hydration and microstructure of cement pastes. Cem. Concr. Compos 2007, 29, 94–102. [Google Scholar]
- Naji, G.A.; AbdulRashid, S.; Aziz, F.N.A.; Salleh, M.A.M. Contribution of rice husk ash to the properties of mortar and concrete: A review. J. Am. Sci 2010, 6, 157–165. [Google Scholar]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Mechanical properties of cement mortar with Al2O3 nanoparticles. J. Am. Sci 2010, 6, 94–97. [Google Scholar]
- Qing, Y.; Zenan, Z.; Li, S.; Rongshen, C. A comparative study on the pozzolanic activity between nano-SiO2 and silica fume. J. Wuhan Univ. Technol. Mater. Sci 2008, 21, 153–157. [Google Scholar]
- AI-Khalaf, M.N.; Yousif, H.A. Use of rice husk ash in concrete. Int. J. Cem. Compos. Lightweight Concr 1984, 6, 241–248. [Google Scholar]
- Lee, S.J.; Kriven, W.M. Synthesis and hydration study of Portland cement components prepared by the organic steric entrapment method. Mater. Struct 2005, 38, 87–92. [Google Scholar]
- Li, H.; Zhang, M.; Ou, J. Flexural fatigue performance of concrete containing nanoparticles for pavement. Int. J. Fatigue 2007, 29, 1292–1301. [Google Scholar]
- Ye, Q. The study and development of the nano-composite cement structure materials. New Build. Mater 2001, 1, 4–6. [Google Scholar]
- Kuo, W.T.; Lin, K.L.; Chang, W.C.; Luo, H.L. Effects of nano-materials on properties of waterworks sludge ash cement paste. J. Ind. Eng. Chem 2000, 12, 702–709. [Google Scholar]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. The effects of incorporation Fe2O3 nanoparticles on tensile and flexural strength of concrete. J. Am. Sci 2010, 6, 90–93. [Google Scholar]

| Sample Designation | Total Specific Pore Volume (mL/g) | Most Probable Pore Diameter (nm) |
|---|---|---|
| S0-SCC (Control) | 0.040 | 67 |
| S1-SCC (0.05% ZnO) | 0.038 | 51 |
| S2-SCC (0.1% ZnO) | 0.0356 | 43 |
| S3-SCC (0.2% ZnO) | 0.0321 | 32 |
| S4-SCC (0.5% ZnO) | 0.0301 | 21 |
| S5-SCC (0.1% ZnO) | 0.0296 | 14 |
| Sample Designation | Porosity (%) | Average Diameter (nm) | Median Diameter, Volume (nm) |
|---|---|---|---|
| S0-SCC (Control) | 10.2 | 31.56 | 45.00 |
| S1-SCC (0.05% ZnO) | 9.66 | 29.63 | 37.5 |
| S2-SCC (0.1% ZnO) | 9.02 | 26.81 | 31.5 |
| S3-SCC (0.2% ZnO) | 8.51 | 21.30 | 26.0 |
| S4-SCC (0.5% ZnO) | 7.93 | 17.63 | 21.0 |
| S5-SCC (0.1% ZnO) | 6.34 | 14.97 | 19.50 |
| Sample Designation | ZnO Nanoparticles (%) | Split Tensile Strength (MPa) | |||
|---|---|---|---|---|---|
| 7 Days | 14 Day | 21 Days | 28 Days | ||
| Control | 0 | 4.7 | 5.0 | 5.1 | 5.0 |
| S1 | 0.05 | 5.2 | 5.4 | 5.3 | 5.1 |
| S2 | 0.1 | 5.8 | 5.7 | 5.3 | 5.0 |
| S3 | 0.2 | 6.5 | 6.55 | 5.4 | 5.2 |
| S4 | 0.5 | 7.0 | 6.6 | 5.2 | 5.3 |
| S5 | 1.0 | 6.6 | 6.5 | 5.3 | 5.2 |
| Sample Designation | ZnO Nanoparticles (%) | Split Tensile Strength (MPa) | |||
|---|---|---|---|---|---|
| 7 Days | 14 Day | 21 Days | 28 Days | ||
| Control | 0 | 2.0 | 2.3 | 2.1 | 2.0 |
| S1 | 0.05 | 2.3 | 2.4 | 2.3 | 2.1 |
| S2 | 0.1 | 2.8 | 2.7 | 2.3 | 2.0 |
| S3 | 0.2 | 3.1 | 3.2 | 2.4 | 2.2 |
| S4 | 0.5 | 4.0 | 3.6 | 2.2 | 2.3 |
| S5 | 1.0 | 3.0 | 3.5 | 2.3 | 2.2 |
| Chemical Properties | |||||
|---|---|---|---|---|---|
| Material | SiO2 | Al2O3 | Fe2O3 | CaO MgO | |
| Cement | 21.89 | 5.3 | 3.34 | 53.27 | 6.45 |
| Material | SO3 | Na2O | K2O | Loss On Ignition | |
| Cement | 3.67 | 0.18 | 0.98 | 3.21 | |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arefi, M.R.; Rezaei-Zarchi, S. Synthesis of Zinc Oxide Nanoparticles and Their Effect on the Compressive Strength and Setting Time of Self-Compacted Concrete Paste as Cementitious Composites. Int. J. Mol. Sci. 2012, 13, 4340-4350. https://doi.org/10.3390/ijms13044340
Arefi MR, Rezaei-Zarchi S. Synthesis of Zinc Oxide Nanoparticles and Their Effect on the Compressive Strength and Setting Time of Self-Compacted Concrete Paste as Cementitious Composites. International Journal of Molecular Sciences. 2012; 13(4):4340-4350. https://doi.org/10.3390/ijms13044340
Chicago/Turabian StyleArefi, Mohammad Reza, and Saeed Rezaei-Zarchi. 2012. "Synthesis of Zinc Oxide Nanoparticles and Their Effect on the Compressive Strength and Setting Time of Self-Compacted Concrete Paste as Cementitious Composites" International Journal of Molecular Sciences 13, no. 4: 4340-4350. https://doi.org/10.3390/ijms13044340




