Enhancing Osteoconduction of PLLA-Based Nanocomposite Scaffolds for Bone Regeneration Using Different Biomimetic Signals to MSCs
Abstract
:1. Introduction
2. Results and Discussion
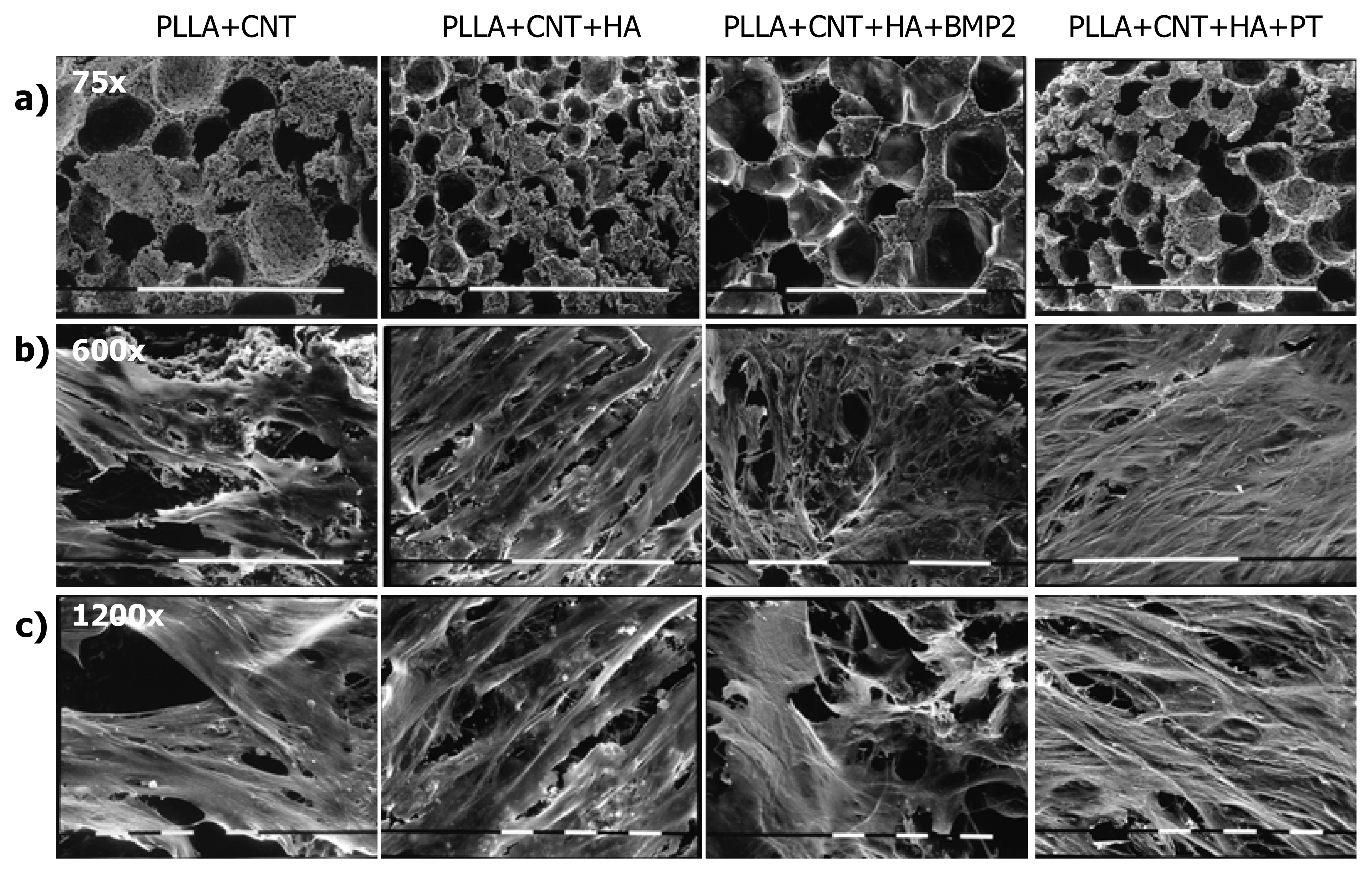
2.1. Cell Culture Characterization
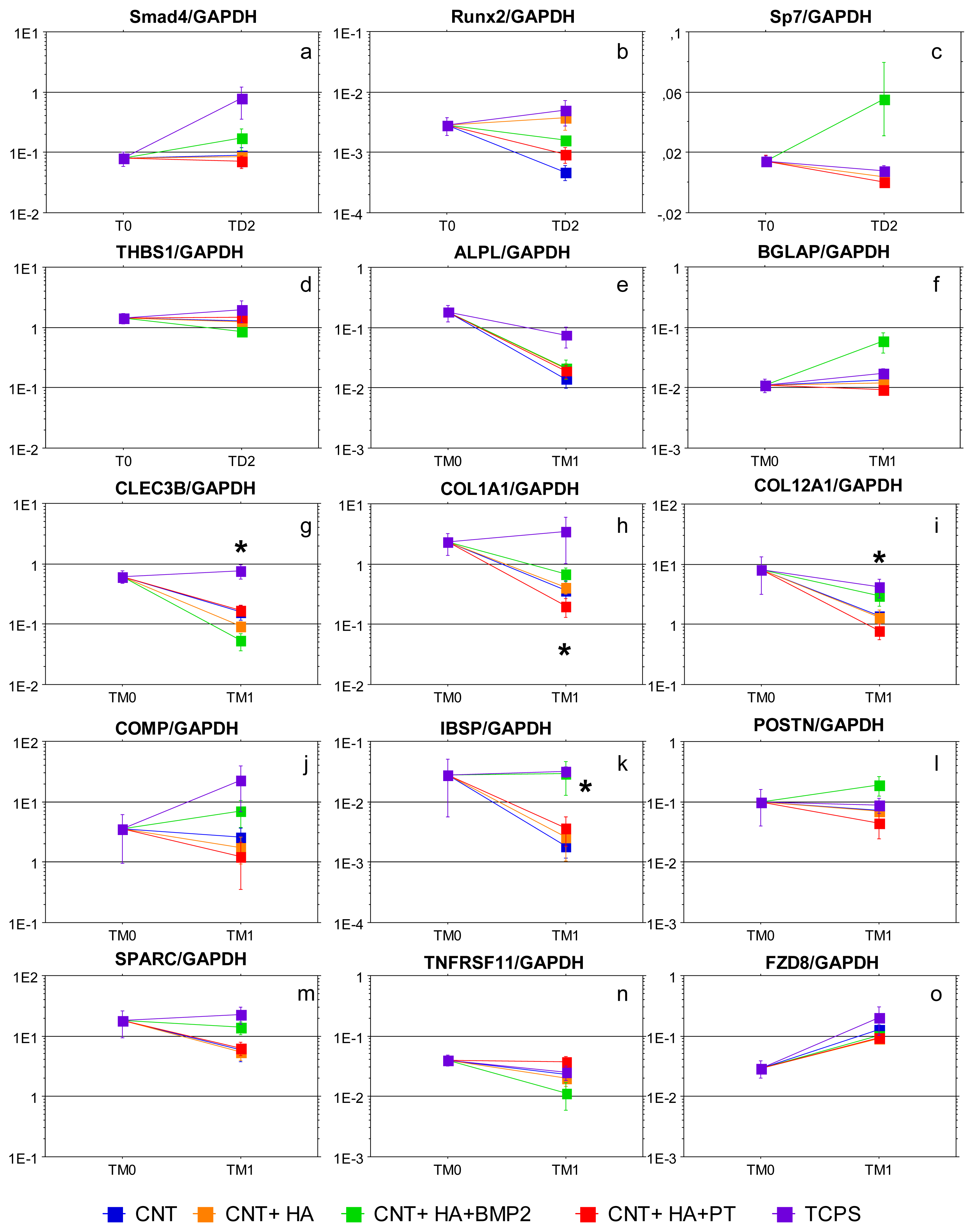
2.2. Gene Expression Analysis
- 3 = scaffold - to - TCPS equal or higher than 1.1;
- 2 = scaffold - to - TCPS from 0.9 to 1.1;
- 1 = scaffold - to - TCPS from 0.1 to 0.9;
- 0 = scaffold - to - TCPS from −0.1 to 0.1;
- −1 = scaffold - to - TCPS from −0.9 to −0.1;
- −2 = scaffold - to - TCPS from −1.1 to −0.9
- −3 = scaffold - to - TCPS equal or lower than −1.1.
3. Experimental Section
3.1. Scaffold Preparation
3.2. Cell Culture
3.3. Morphological Assays
3.4. Biochemical Assays
3.5. Gene Expression Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Fröhlich, M.; Grayson, W.L.; Wan, L.Q.; Marolt, D.; Drobnic, M.; Vunjak-Novakovic, G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr. Stem Cell Res. Ther 2008, 3, 254–264. [Google Scholar]
- Baldini, N.; Cenni, E.; Ciapetti, G.; Granchi, D.; Savarino, L. Bone Repair Biomaterials. In Bone Repair and Regeneration, 1st ed; Planell, J.A., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 69–105. [Google Scholar]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: material and matrix consideration. J. Bone Joint Surg. Am 2008, 90, S36–S42. [Google Scholar]
- Ciapetti, G.; Ambrosio, L.; Marletta, G.; Baldini, N.; Giunti, A. Human bone marrow stromal cells: in vitro expansion and differentiation for bone engineering. Biomaterials 2006, 27, 6150–6160. [Google Scholar]
- Patterson, T.E.; Kumagai, K.; Griffith, L.; Muschler, G.F. Cellular strategies for enhancement of fracture repair. J. Bone Joint Surg. Am 2008, 90, S111–S119. [Google Scholar]
- Tabata, Y. Biomaterial technology for tissue engineering applications. J. R. Soc. Interface 2009, 6, S311–S324. [Google Scholar]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In vitro differentiation potential of mesenchymal stem cells. Transfus. Med. Hemother 2008, 35, 228–238. [Google Scholar]
- Burdick, J.A.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell. Differ. Tissue Eng 2009, 15, 205–219. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blazer, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/ inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar]
- Yanoso-Scholl, L.; Jacobson, J.A.; Bradica, G.; Lerner, A.L.; O’Keefe, R.J.; Schwarz, E.M.; Zuscik, M.J.; Awad, H.A. Evaluation of dense polylactic acid/beta-tricalcium phosphate scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2010, 95, 717–726. [Google Scholar]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate micro- and nanotopography to control cell function. Angew. Chem. Int. Ed. Engl 2009, 48, 5406–5415. [Google Scholar]
- Damadzadeh, B.; Labari, H.; Skrifvars, M.; Aiola, K.; Moritz, N.; Vallittu, P.K. Effect of ceramic filler content on the mechanical and thermal behaviour of poly-L-lactic acid and poly-L-lactic-co-glycolic acid composites for medical applications. J. Mater. Sci. Mater. Med 2010, 21, 2523–2531. [Google Scholar]
- Vagaská, B.; Bacáková, L.; Filová, E.; Balík, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res 2010, 59, 309–322. [Google Scholar]
- Hanson, A.D.; Wall, M.E.; Pourdeyhimi, B.; Loboa, E.G. Effects of oxygen plasma treatment on adipose-derived human mesenchymal stem cell adherence to poly(L-lactic acid) scaffolds. J. Biomater. Sci. Polym. Ed 2007, 18, 1387–1400. [Google Scholar]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar]
- Khan, S.N.; Lane, J.M. The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in orthopaedic applications. Expert Opin. Biol. Ther 2004, 4, 741–748. [Google Scholar]
- Valdes, M.A.; Thakur, N.A.; Namdari, S.; Ciombor, D.M.; Palombo, M. Recombinant bone morphogenic protein-2 in orthopaedic surgery: A review. Arch. Orthop. Trauma Surg 2009, 129, 1651–1657. [Google Scholar]
- Chou, Y.F.; Zuk, P.A.; Chang, T.L.; Benhaim, P.; Wu, B.M. Adipose-derived stem cells and BMP2: part 1. BMP2-treated adipose-derived stem cells do not improve repair of segmental femoral defects. Connect. Tissue Res 2011, 52, 109–118. [Google Scholar]
- Singh, M.; Berkland, C.; Detamore, M.S. Strategies and applications for incorporatine physical and chemical signal gradients in tissue engineering. Tissue Eng: Part B 2008, 14, 341–366. [Google Scholar]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar]
- Kallai, I.; van Lente, G.H.; Buffoni, D.; Zilberman, Y.; Müller, R.; Pelled, G.; Gazit, D. Quantitative, structural, and image-based mechanical analysis of nonunion fracture repaired by genetically engineered mesenchymal stem cells. J. Biomech 2010, 43, 2315–2320. [Google Scholar]
- Jorgensen, C.; Djouad, F.; Bouffi, C.; Frugala, D.; Noel, D. Multipotent mesenchymal stromal cells in articular diseases. Best Pract. Res. Clin. Rheumat 2008, 22, 269–284. [Google Scholar]
- Abdallah, B.M.; Kassem, M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. J. Cell. Physiol 2008, 218, 9–12. [Google Scholar]
- Huang, N.F.; Chu, J.; Lee, R.J.; Li, S. Biophysical and chemical effects of fibrin on mesenchymal stromal cell gene expression. Acta Biomater 2010, 6, 3947–3956. [Google Scholar]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-dimensional cell culture matrices: State of the art. Tissue Eng. Part B Rev 2008, 14, 61–86. [Google Scholar]
- Dutta, R.C.; Dutta, A.K. Cell-interactive 3D-scaffold; advances and applications. Biotechnol. Adv 2009, 27, 334–339. [Google Scholar]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater 2010, 6, 3824–3846. [Google Scholar]
- Sitharaman, B.; Shi, X.; Walboomers, X.F.; Liao, H.; Cuijpers, V.; Wilson, L.J.; Mikos, A.G.; Jansen, J.A. In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering. Bone 2008, 43, 362–370. [Google Scholar]
- Bhattacharyya, S.; Guillot, S.; Dabboue, H.; Tranchant, J.F.; Salvetat, J.P. Carbon nanotubes as structural nanofibers for hyaluronic acid hydrogel scaffolds. Biomacromolecules 2008, 9, 505–509. [Google Scholar]
- Cheung, W.; Pontoriero, F.; Taratula, O.; Chen, A.M.; He, H. DNA and carbon nanotubes as medicine. Adv. Drug Deliv. Rev 2010, 62, 633–649. [Google Scholar]
- Bianco, A.; Del Gaudio, C.; Baiguera, S.; Armentano, I.; Bertarelli, C.; Dottori, M.; Bultrini, G.; Lucotti, A.; Kenny, J.M.; Folin, M. Microstructure and cytocompatibility of electrospun nanocomposites based on poly(epsilon-caprolactone) and carbon nanostructures. Int. J. Artif. Organs 2010, 33, 271–282. [Google Scholar]
- Holt, B.D.; Short, P.A.; Rape, A.D.; Wang, Y.L.; Islam, M.F.; Dahl, K.N. Carbon nanotubes reorganize actin structures in cells and ex vivo. ACS Nano 2010, 4, 4872–4878. [Google Scholar]
- Guarino, V.; Taddei, P.; Di Foggia, M.; Fagnano, C.; Ciapetti, G.; Ambrosio, L. The influence of hydroxyapatite particles on in vitro degradation behavior of poly -epsilon-caprolactone based composite scaffolds. Tissue Eng. Part A 2009, 15, 3655–3668. [Google Scholar]
- Tami, A.E.; Leitner, M.M.; Baucke, M.G.; Mueller, T.L.; van Lente, G.H.; Müller, R.; Ito, K. Hydroxyapatite particles maintain peri-implant bone mantle during osseointegration in osteoporotic bone. Bone 2009, 45, 1117–1124. [Google Scholar]
- Rehfeldt, F.; Engler, A.J.; Eckhardt, A.; Ahmed, F.; Discher, D.E. Cell responses to the mechanochemical microenvironment-Implications for regenerative medicine and drug delivery. Adv. Drug Deliv. Rev 2007, 59, 1329–1339. [Google Scholar]
- Poulsson, A.H.; Mitchell, S.A.; Davidson, M.R.; Johnstone, A.J.; Emmison, N.; Bradley, R.H. Attachment of human primary osteoblast cells to modified polyethylene surfaces. Langmuir 2009, 25, 3718–3727. [Google Scholar]
- Marletta, G.; Ciapetti, G.; Satriano, C.; Perut, F.; Salerno, M.; Baldini, N. Improved osteogenic differentiation of human marrow stromal cells cultured on ion-induced chemically structured poly-epsilon-caprolactone. Biomaterials 2007, 28, 1132–1140. [Google Scholar]
- Osycza, A.M.; Diefenderfer, D.L.; Bhargave, G.; Leboy, P.S. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs 2004, 176, 109–119. [Google Scholar]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar]
- Fu, K.; Xu, Q.; Czernuszka, J.; McKenna, C.E.; Ebetino, F.H.; Russell, R.G.; Triffitt, J.T.; Xia, Z. Prolonged osteogenesis from human mesenchymal stem cells implanted in immunodeficient mice by using coralline hydroxyapatite incorporating rhBMP2 microspheres. J. Biomed. Mater. Res. A 2010, 92, 1256–1264. [Google Scholar]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett 2009, 31, 1825–1835. [Google Scholar]
- Granchi, D.; Ochoa, G.; Leonardi, E.; Devescovi, V.; Baglio, S.R.; Osaba, L.; Baldini, N.; Ciapetti, G. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during ex vivo expansion. Tissue Eng. Part C Methods 2010, 16, 511–524. [Google Scholar]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci 2007, 12, 3068–3092. [Google Scholar]
- Lai, C.F.; Cheng, S.L. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-beta in normal human osteoblastic cells. J. Biol. Chem 2002, 277, 15514–15522. [Google Scholar]
- Ueno, A.; Miwa, Y.; Miyoshi, K.; Horiguchi, T.; Inoue, H.; Ruspata, I.; Abe, K.; Yamashita, K.; Hayashi, E.; Noma, T. Constitutive expression of thrombospondin 1 in MC3T3-E1 osteoblastic cells inhibits mineralization. J. Cell. Physiol 2006, 209, 322–332. [Google Scholar]
- Landis, W.J.; Silver, F.H. Mineral deposition in the extracellular matrices of vertebrate tissues: Identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs 2009, 189, 20–24. [Google Scholar]
- Raymond, M.H.; Schutte, B.C.; Torner, J.C.; Burns, T.L.; Willing, M.C. Osteocalcin: genetic and physical mapping of the human gene BGLAP and its potential role in postmenopausal osteoporosis. Genomics 1999, 60, 210–217. [Google Scholar]
- Wewer, U.M.; Ibaraki, K.; Schjørring, P.; Durkin, M.E.; Young, M.F.; Albrechtsen, R. A potential role for tetranectin in mineralization during osteogenesis. J. Cell Biol 1994, 127, 1767–1775. [Google Scholar]
- Ogata, Y. Bone sialoprotein and its transcriptional regulatory mechanism. J. Periodontal Res 2008, 43, 127–135. [Google Scholar]
- Motamed, K. SPARC (osteonectin/BM-40). Int. J. Biochem. Cell Biol 1999, 31, 1363–1366. [Google Scholar]
- Coutu, D.L.; Wu, J.H.; Monette, A.; Rivard, G.E.; Blostein, M.D.; Galipeau, J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J. Biol. Chem 2008, 283, 17991–18001. [Google Scholar]
- Kong, L.; Tian, Q.; Guo, F.; Mucignat, M.T.; Perris, R.; Sercu, S.; Merregaert, J.; Di Cesare, P.E.; Liu, C.J. Interaction between cartilage oligomeric matrix protein and extracellular matrix protein 1 mediates endochondral bone growth. Matrix Biol 2010, 29, 276–286. [Google Scholar]
- Iba, K.; Sawada, N.; Chiba, H.; Wewer, U.M.; Ishii, S.; Mori, M. Transforming growth factor beta 1 downregulates dexamethasone-induced tetranectin gene expression during the in vitro mineralization of the human osteoblastic cell line SV-HFO. FEBS Lett 1995, 373, 1–4. [Google Scholar]
- Gori, F.; Hofbauer, L.C.; Dunstan, C.R.; Spelsberg, T.C.; Khosla, S.; Riggs, B.L. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology 2000, 41, 4768–4776. [Google Scholar]
- Gregory, C.A.; Gunn, W.G.; Reyes, E.; Smolarz, A.J.; Munoz, J.; Spees, J.L.; Prockop, D.J. How Wnt signaling affects bone repair by mesenchymal stem cells from the bone marrow. Ann. N. Y. Acad. Sci 2005, 1049, 97–106. [Google Scholar]
- van der Zande, M.; Walboomers, X.F.; Brännvall, M.; Olalde, B.; Jurado, M.J.; Alava, J.L.; Jansen, J.A. Genetic profiling of osteoblast-like cells cultured on a novel bone reconstructive material.; consisting of poly-l-lactide.; carbon nanotubes and microhydroxyapatite.; in the presence of bone morphogenetic protein-2. Acta Biomater 2010, 6, 4352–4360. [Google Scholar]
- Lian, J.B.; Stein, G.S. Concepts of Osteoblast Growth and Differentiation: Basis for Modulation of Bone Cell Development and Tissue Formation. Crit. Rev. Oral Biol. Med 1992, 3, 369–305. [Google Scholar]
- Schop, D.; Janssen, F.W.; van Rijn, L.D.S.; Fernandes, H.; Bloem, R.M.; de Bruijn, J.D.; van Dijkhuizen-Radersma, R. Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 1877–1886. [Google Scholar]
- Osyczka, A.M.; Diefenderfer, D.L.; Bhargave, G.; Leboy, P.S. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs 2004, 176, 109–119. [Google Scholar]
- Wang, A.; Ding, X.; Sheng, S.; Yao, Z. Bone morphogenetic protein receptor in the osteogenic differentiation of rat bone marrow stromal cells. Yonsei Med. J 2010, 51, 740–745. [Google Scholar]
- Sung Nam, Y.; Gwan Park, T. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mater. Res 1999, 47, 8–17. [Google Scholar]
- Armentano, I.; Ciapetti, G.; Pennacchi, M.; Dottori, M.; Devescovi, V.; Granchi, D.; Baldini, N.; Olalde, B.; Jurado, M.J.; Marquinez Alava, J.I.; Kenny, J.M. Role of PLLA plasma surface modification in the interaction with human marrow stromal cells. J. Appl. Pol. Sci 2009, 114, 3602–3611. [Google Scholar]
- Leonardi, E.; Ciapetti, G.; Baglio, S.R.; Devescovi, V.; Baldini, N.; Granchi, D. Osteogenic properties of late adherent subpopulations of human bone marrow stromal cells. Histochem. Cell Biol 2009, 132, 547–557. [Google Scholar]
- Mouritzen, P.; Noerholm, M.; Nielsen, P.S.; Jacobsen, N.; Lomholt, C.; Pfundheller, H.M.; Tolstrup, N. ProbeLibrary: A new method for faster design and execution of quantitative real-time PCR. Nat. Methods 2005, 12, 313–316. [Google Scholar]
- Roche Applied Science. Available online: https://www.roche-applied-science.com/sis/rtpcr/upl accessed on 20 February 2012.

| Gene Symbol | Gene | Function | Expression in TCPS cultures [43] |
|---|---|---|---|
| ALPL | Alkaline phosphatase liver/bone/kidney | Membrane bound glycosylated enzyme involved in matrix mineralization. | ↑ TD2 |
| BGLAP | Bone gamma-carboxyglutamate protein (Osteocalcin) | Noncollagenous matrix protein is associated the calcium phosphate mineral phase of bone. BGLAP is the only gene that is expressed in osteoblasts but not in other cells. | ↑ TD2 |
| CLEC3B | Tetranectin | Matrix protein (plasminogen-binding) involved in mineralization process. | ↑ TD2 |
| COL12A1 | Type 12 collagen, alpha 1 chain | Type 12 collagen is found in association with type 1 collagen, an association that is thought to modify the interactions between collagen 1 and the surrounding matrix. | ↑ TD1, ↑ TD2 |
| COL1A1 | Type 1 collagen, alpha 1 chain | Type 1 collagen is a fibril-forming collagen found in most connective tissues and is abundant in bone, cornea, dermis and tendon. It comprises two α1 chains and one α2 chain. | ↑ TD1, ↑ TD2 |
| COMP | Cartilage oligomeric matrix protein | Noncollagenous ECM protein; it is expressed in the hypertrophic chondrocytes and in osteoblasts around developing bone. | ↑ TD1, ↑ TD2, ↑ TM1 |
| FZD8 | Frizzled homolog 8 | Receptor for the Wingless type MMTV integration site family of signaling proteins. | ↑ TM1 |
| IBSP | Bone sialoprotein | Noncollagenous glycoprotein expressed in mineralized tissues; it mediates cell-to-matrix attachment and binds to calcium and HA. | ↑ TD1, ↑ TD2, ↑ TM1 |
| POSTN | Periostin | Secreted protein expressed during osteoblastic differentiation and maturation and abundantly found in mineralized bone nodules in vitro. | ↑ TD1, ↑ TD2 |
| Runx2 | Runt-related transcription factor 2 | Trascription factor belonging to the TGFβ signaling pathway; it is considered a master regulatory switch to address the commitment of MSC to osteoblastic differentiation and skeletal morphogenesis. | ↑ TD1, ↑ TD2 |
| Smad4 | Mothers against decapentaplegic homolog 4 | Smad 4 is a common partner of BMP- and TGFβ-receptor Smads; Smad4 induces expression of Runx2 and Osterix in osteoprogenitor cells. | ↑ TD1 |
| SP7 | Sp7 transcription factor (Osterix) | SP7 is a transcription factor which acts downstream of Runx2 to induce osteoblastic differentiation in osteochondroprogenitor cells. Sp7 is responsible for the activation of BGLAP and COLA1 genes. | ↑ TD1 |
| SPARC | Osteonectin | Matrix-associated protein expressed in bone remodeling areas; it regulates angiogenesis and cell-matrix interactions. | ↑ TD1, ↑ TD2 |
| THBS1 | Thrombospondin 1 | THBS1 is a negative regulator of TGFβ signaling. It co-localizes with TGFβ and mediates cell-to-cell and cell-to-matrix interactions. | ↑ TD1, ↑ TD2 |
| TNFRSF11B | Tumor necrosis factor receptor superfamily, member 11b (osteoprotegerin) | Osteoblast-secreted decoy receptor that functions as a negative regulator of bone resorption. | ↑ TD2 |
| Process | Gene | PLLA+CNT | PLLA+CNT+HA | PLLA+CNT+HA+BMP | PLLA+CNT+HA+PT |
|---|---|---|---|---|---|
| Differentiation | |||||
| ALP | −2.00 | 0.00 | −1.33 | −1.33 | |
| BGLAP | 0.67 | 0.33 | 1.33 | 1.00 | |
| CLEC3B | 0.33 | −0.33 | −0.33 | −0.33 | |
| COL12A1 | −0.33 | −0.33 | 0.33 | −0.67 | |
| COL1A1 | 0.33 | 0.33 | −0.33 | 0.33 | |
| COMP | 1.33 | 0.33 | 0.00 | −0.33 | |
| IBSP | −0.33 | −0.33 | 0.00 | −0.67 | |
| Osx | −2.00 | −0.67 | −0.33 | −2.00 | |
| POSTN | 0.33 | 0.33 | 0.33 | 0.00 | |
| RUNX2 | −2.67 | 0.33 | 0.67 | −0.67 | |
| Smad4 | 0.00 | 0.33 | 0.33 | 1.00 | |
| SPARC | 0.00 | −0.33 | −0.33 | −0.33 | |
| THS1 | 0.33 | 0.33 | 0.33 | 0.33 | |
| TNFRS11 | 0.33 | 0.33 | −0.33 | 1.00 | |
| Differentiation Score a | −3.67 | 0.67 | 0.33 | −2.67 | |
| Mineralization | |||||
| ALP | −1.67 | −0.67 | −1.67 | −1.00 | |
| BGLAP | −0.33 | −0.33 | 0.67 | −0.33 | |
| CLEC3B | −1.00 | −1.33 | −1.67 | −0.67 | |
| COL12A1 | −1.00 | −1.00 | −0.33 | −1.00 | |
| COL1A1 | −1.67 | −1.33 | −1.00 | −1.33 | |
| COMP | −2.00 | −1.67 | −0.33 | −2.00 | |
| FZD8 | 0.33 | −0.67 | −0.33 | −0.33 | |
| IBSP | −2.00 | −2.00 | −0.33 | −1.67 | |
| POSTN | −0.33 | −0.67 | 1.00 | −1.00 | |
| SPARC | −1.33 | −1.00 | −0.33 | −1.00 | |
| TNFRS11 | −0.33 | −1.00 | −1.00 | 1.00 | |
| Mineralization Score b | −11.33 | −11.67 | −5.33 | −9.33 | |
| Total scorec | −15.00 | −11.00 | −5.00 | −12.00 | |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ciapetti, G.; Granchi, D.; Devescovi, V.; Baglio, S.R.; Leonardi, E.; Martini, D.; Jurado, M.J.; Olalde, B.; Armentano, I.; Kenny, J.M.; et al. Enhancing Osteoconduction of PLLA-Based Nanocomposite Scaffolds for Bone Regeneration Using Different Biomimetic Signals to MSCs. Int. J. Mol. Sci. 2012, 13, 2439-2458. https://doi.org/10.3390/ijms13022439
Ciapetti G, Granchi D, Devescovi V, Baglio SR, Leonardi E, Martini D, Jurado MJ, Olalde B, Armentano I, Kenny JM, et al. Enhancing Osteoconduction of PLLA-Based Nanocomposite Scaffolds for Bone Regeneration Using Different Biomimetic Signals to MSCs. International Journal of Molecular Sciences. 2012; 13(2):2439-2458. https://doi.org/10.3390/ijms13022439
Chicago/Turabian StyleCiapetti, Gabriela, Donatella Granchi, Valentina Devescovi, Serena R. Baglio, Elisa Leonardi, Desirèe Martini, Maria Jesus Jurado, Beatriz Olalde, Ilaria Armentano, Josè M. Kenny, and et al. 2012. "Enhancing Osteoconduction of PLLA-Based Nanocomposite Scaffolds for Bone Regeneration Using Different Biomimetic Signals to MSCs" International Journal of Molecular Sciences 13, no. 2: 2439-2458. https://doi.org/10.3390/ijms13022439






