Temporal and Spatial Regulation of Ezrin-Radixin-Moesin-Binding Phosphoprotein-50-kDa (EBP50) during Embryo Implantation in Mouse Uterus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
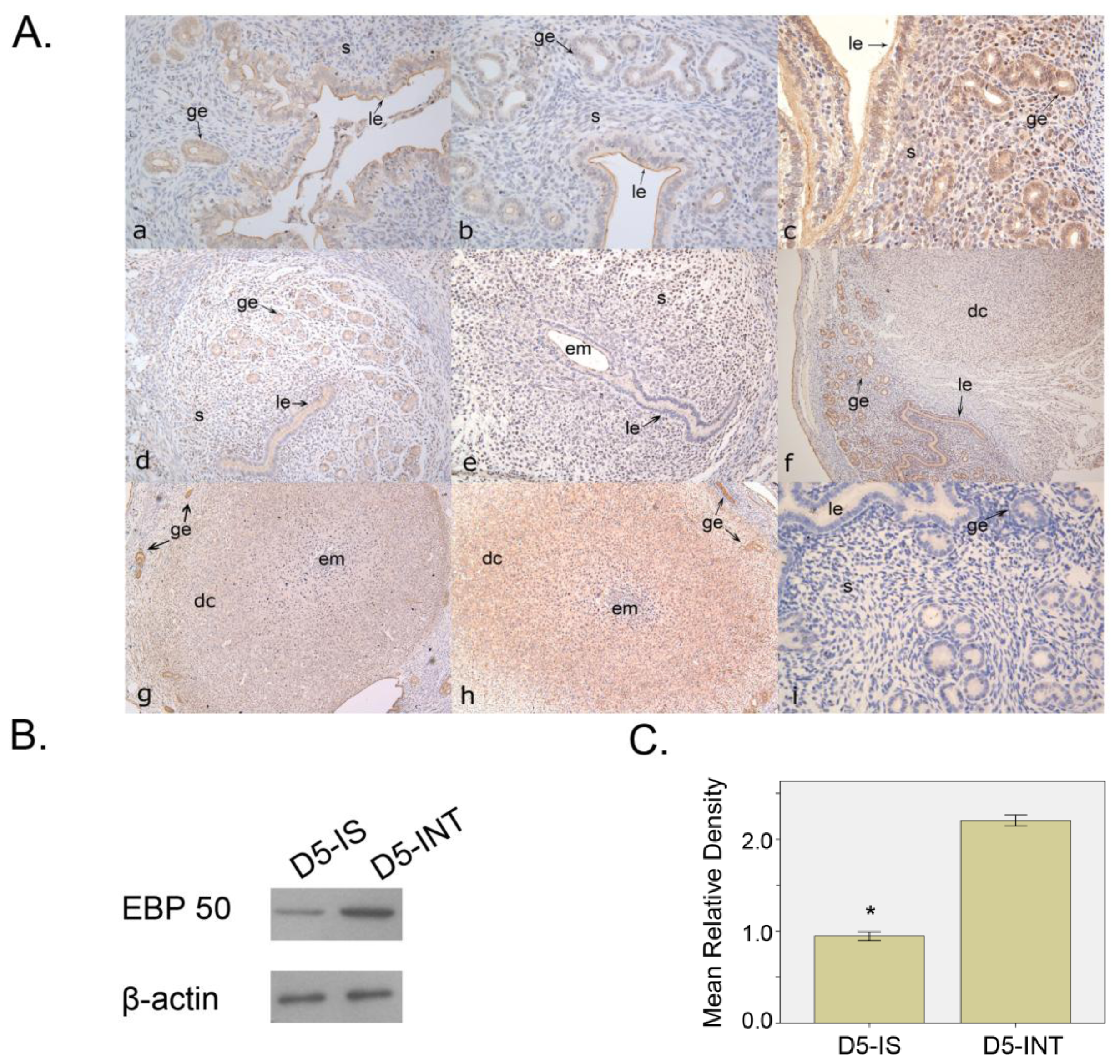
2.1.1. Expression Pattern of EBP50 in Mouse Uterus during Early Pregnancy
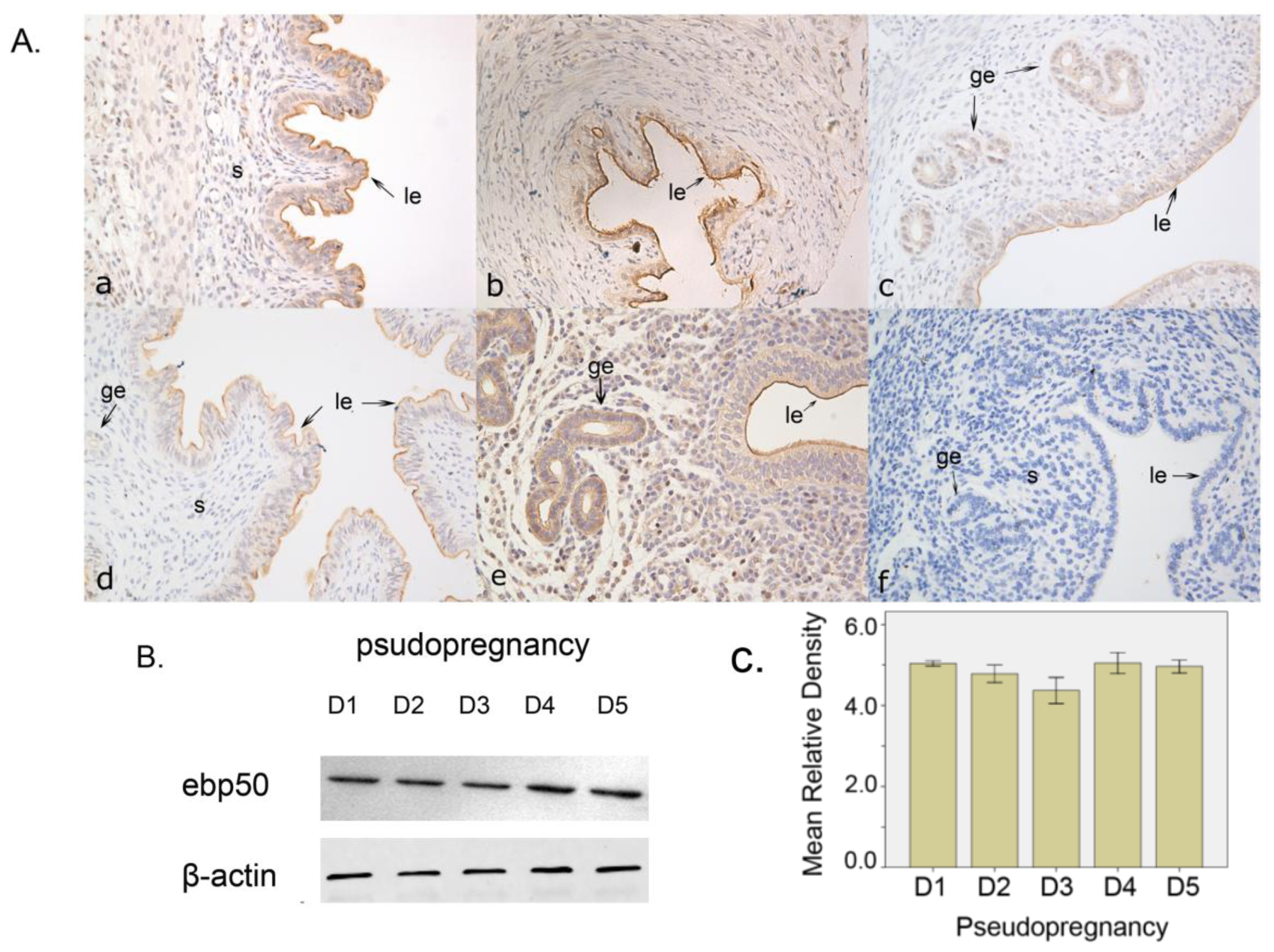
2.1.2. The Expression of EBP50 in Pseudopregnancy
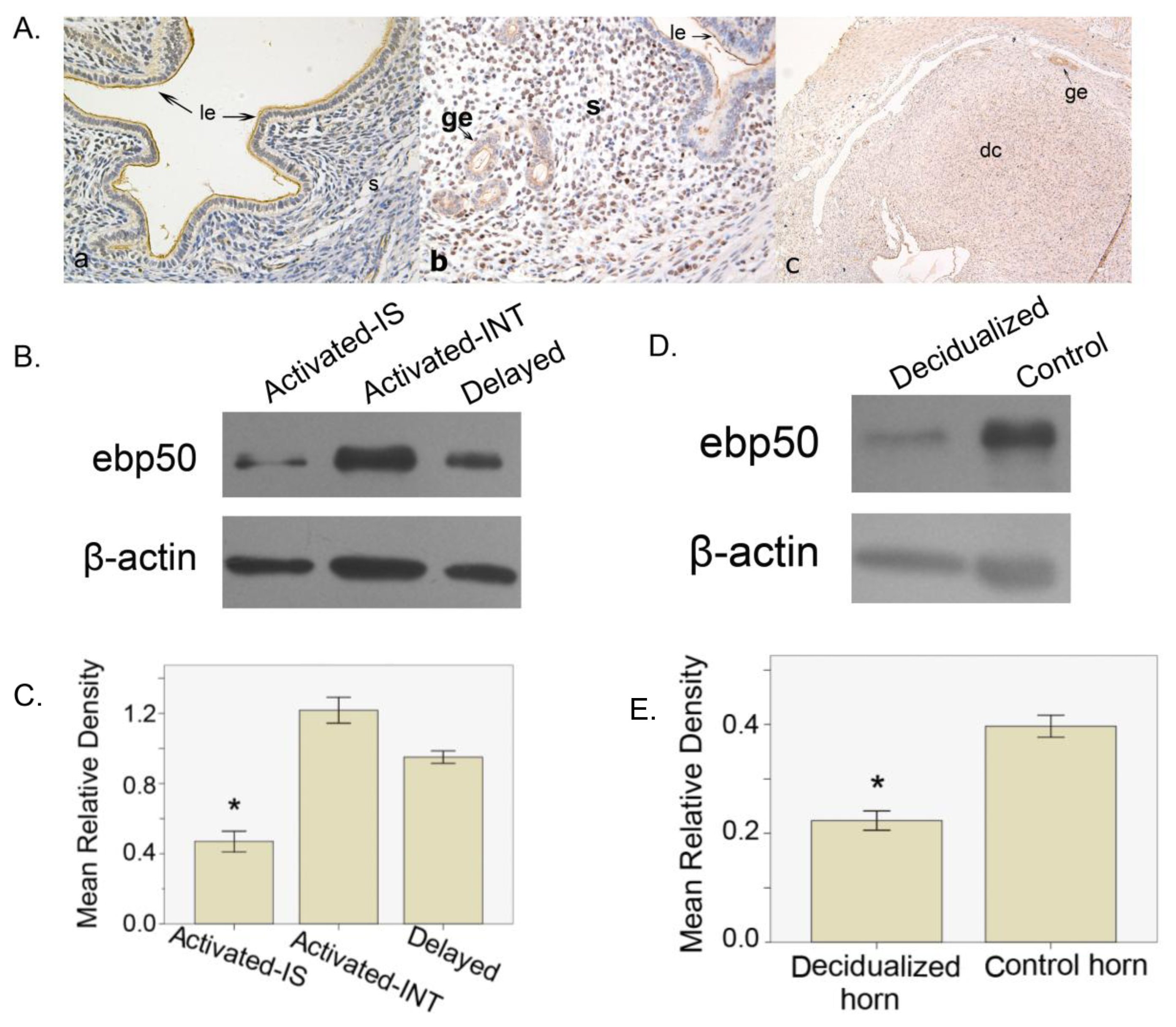
2.1.3. The Expression of EBP50 in Delayed and Activated Implantation
2.1.4. The Expression of EBP50 in Experimentally Induced Decidualization
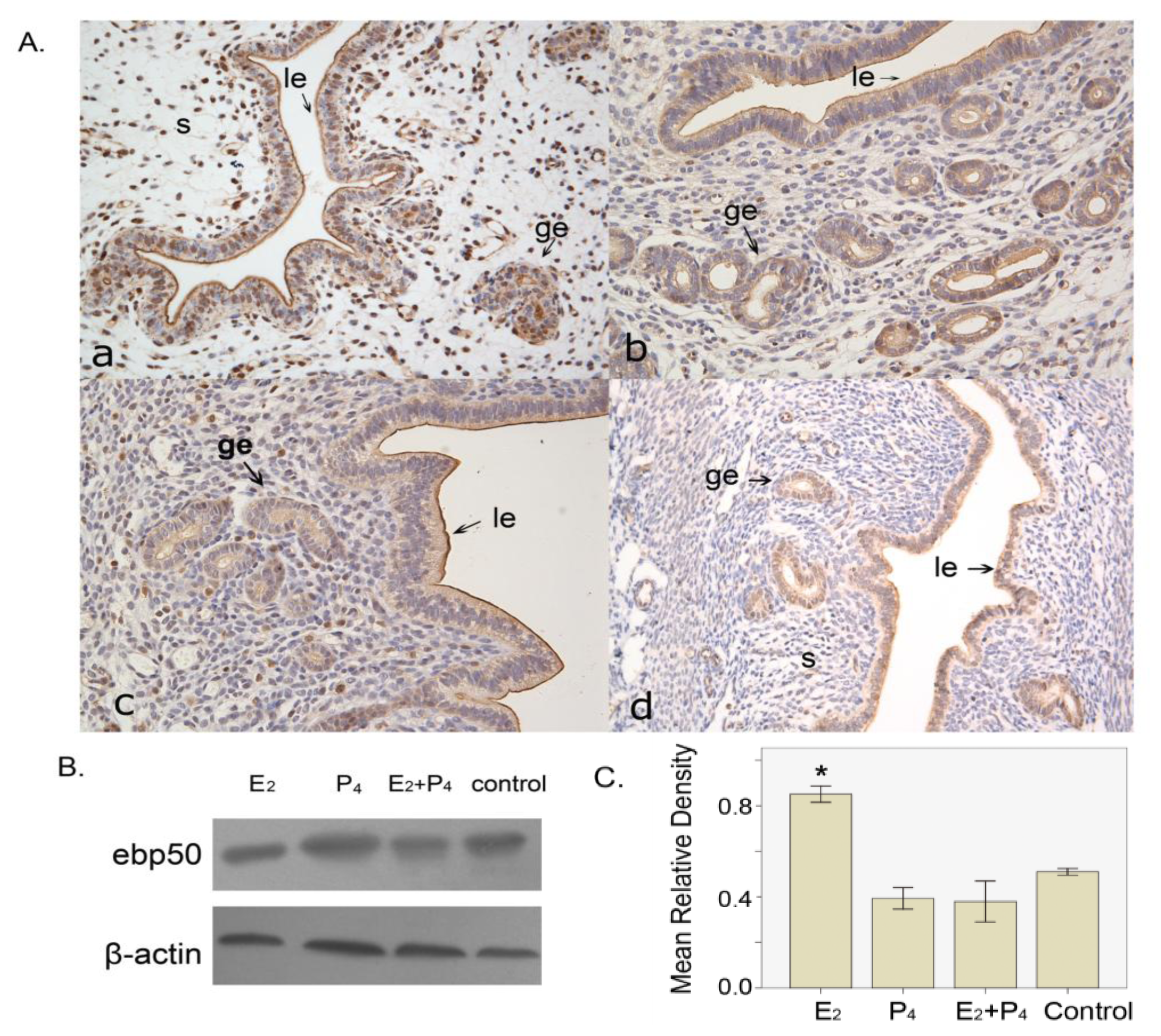
2.1.5. The Expression of EBP50 in the Ovariectomized Mouse Uterus
2.2. Discussion
3. Experimental Section
3.1. Animals and Treatments
3.2. Immunohistochemical Staining
3.3. Western Blotting
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Lessey, B.A. Assessment of endometrial receptivity. Fertil. Steril 2011, 96, 522–529. [Google Scholar]
- Lim, H.J.; Dey, S.K. HB-EGF: A unique mediator of embryo-uterine interactions during implantation. Exp. Cell Res 2009, 315, 619–626. [Google Scholar]
- Kodithuwakku, S.P.; Ng, P.Y.; Liu, Y.; Ng, E.H.; Yeung, W.S.; Ho, P.C.; Lee, K.F. Hormonal regulation of endometrial olfactomedin expression and its suppressive effect on spheroid attachment onto endometrial epithelial cells. Hum. Reprod 2011, 26, 167–175. [Google Scholar]
- Murphy, C.R.; Shaw, T.J. Plasma membrane transformation: A common response of uterine epithelial cells during the peri-implantation period. Cell Biol. Int 1994, 18, 1115–1128. [Google Scholar]
- Murphy, C.R. Uterine receptivity and the plasma membrane transformation. Cell Res 2004, 14, 259–267. [Google Scholar]
- Perry, J.K.; Lins, R.J.; Lobie, P.E.; Mitchell, M.D. Regulation of invasive growth: Similar epigenetic mechanisms underpin tumour progression and implantation in human pregnancy. Clin. Sci 2009, 118, 451–457. [Google Scholar]
- Murray, M.J.; Lessey, B.A. Embryo implantation and tumor metastasis: Common pathways of invasion and angiogenesis. Semin. Reprod. Endocrinol. 1999, 17, 275–290. [Google Scholar]
- Reczek, D.; Berryman, M.; Bretscher, A. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol 1997, 139, 169–179. [Google Scholar]
- Weinman, E.J.; Steplock, D.; Tate, K.; Hall, R.A.; Spurney, R.F.; Shenolikar, S. Structure-function of recombinant Na+/H+ exchanger regulatory factor (NHERF). J. Clin. Invest 1998, 101, 2199–2206. [Google Scholar]
- Pan, Y.; Wang, L.; Dai, J.L. Suppression of breast cancer cell growth by Na+/H+ exchanger regulatory factor 1 (NHERF1). Breast Cancer Res 2006, 8, R63. [Google Scholar]
- Mullin, J.M. Epithelial barriers, compartmentation, and cancer. Sci. STKE 2004, pe2. [Google Scholar]
- Shibata, T.; Chuma, M.; Kokubu, A.; Sakamoto, M.; Hirohashi, S. EBP50, a β-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology 2003, 38, 178–186. [Google Scholar]
- Molina, J.R.; Morales, F.C.; Hayashi, Y.; Aldape, K.D.; Georgescu, M.M. Loss of PTEN binding adapter protein NHERF1 from plasma membrane in glioblastoma contributes to PTEN inactivation. Cancer Res 2010, 70, 6697–6703. [Google Scholar]
- Hayashi, Y.; Molina, J.R.; Hamilton, S.R.; Georgescu, M.M. NHERF 1/EBP50 is a new marker in colorectal cancer. Neoplasia 2010, 12, 1013–1022. [Google Scholar]
- Zheng, J.F.; Sun, L.C.; Liu, H.; Huang, Y.; Li, Y.; He, J. EBP50 exerts tumor suppressor activity by promoting cell apoptosis and retarding extracellular signal-regulated kinase activity. Amino Acids 2010, 38, 1261–1268. [Google Scholar]
- Garbett, D.; LaLonde, D.P.; Bretscher, A. The scaffolding protein EBP50 regulates microvillar assembly in a phosphorylation-dependent manner. J. Cell Biol 2010, 191, 397–413. [Google Scholar]
- LaLonde, D.P.; Garbett, D.; Bretscher, A. A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol. Biol. Cell 2010, 21, 1519–1529. [Google Scholar]
- Lecce, L.; Lindsay, L.A.; Murphy, C.R. Ezrin and EBP50 redistribute apically in rat uterine epithelial cells at the time of implantation and in response to cell contact. Cell Tissue Res 2011, 343, 445–453. [Google Scholar]
- Weinman, E.J.; Steplock, D.; Wang, Y.; Shenolikar, S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na+-H+ exchanger. J. Clin. Invest 1995, 95, 2143–2149. [Google Scholar]
- Georgescu, M.M.; Morales, F.C.; Molina, J.R.; Hayashi, Y. Roles of NHERF1/EBP50 in cancer. Curr. Mol. Med 2008, 8, 459–468. [Google Scholar]
- Boratkó, A.; Gergely, P.; Csortos, C. Cell cycle dependent association of EBP50 with protein phosphatase 2A in endothelial cells. PLoS One 2012, 7, e35595. [Google Scholar]
- Stemmer-Rachamimov, A.O.; Wiederhold, T.; Nielsen, G.P.; James, M.; Pinney-Michalowski, D.; Roy, J.E.; Cohen, W.A.; Ramesh, V.; Louis, D.N. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am. J. Pathol 2001, 158, 57–62. [Google Scholar]
- Matsumoto, H.; Daikoku, T.; Wang, H.; Sato, E.; Dey, S.K. Differential expression of ezrin/radixin/moesin (ERM) and ERM-associated adhesion molecules in the blastocyst and uterus suggests their functions during implantation. Biol. Reprod 2004, 70, 729–736. [Google Scholar]
- Gray, C.A.; Bartol, F.F.; Tarleton, B.J.; Wiley, A.A.; Johnson, G.A.; Bazer, F.W.; Spencer, T.E. Developmental biology of uterine glands. Biol. Reprod 2001, 65, 1311–1323. [Google Scholar]
- Chen, W.; Han, B.C.; Wang, R.C.; Xiong, G.F.; Peng, J.P. Role of secretory protease inhibitor SPINK 3 in mouse uterus during early pregnancy. Cell Tissue Res 2010, 341, 441–451. [Google Scholar]
- Bulla, R.; Bossi, F.; Tedesco, F. The complement system at the embryo implantation site: Friend or foe? Front. Immunol 2012, 3, 55. [Google Scholar]
- Fouassier, L.; Rosenberg, P.; Mergey, M.; Saubaméa, B.; Clapéron, A.; Kinnman, N.; Chignard, N.; Jacobsson-Ekman, G.; Strandvik, B.; Rey, C.; et al. Ezrin-radixin-moesin-binding phosphoprotein (EBP50), an estrogen-inducible scaffold protein, contributes to biliary epithelial cell proliferation. Am. J. Pathol 2009, 174, 869–880. [Google Scholar]
- Cardone, R.A.; Bellizzi, A.; Busco, G.; Weinman, E.J.; Dell’aquila, M.E.; Casavola, V.; Azzariti, A.; Mangia, A.; Paradiso, A.; Reshkin, S.J. The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol. Biol. Cell 2007, 18, 1768–1780. [Google Scholar]
- Song, J.; Bai, J.; Yang, W.; Gabrielson, E.W.; Chan, D.W.; Zhang, Z. Expression and clinicopathological significance of oestrogen-responsive ezrin-radixin moesin-binding phosphoprotein 50 in breast cancer. Histopathology 2007, 51, 40–53. [Google Scholar]
- Voltz, J.W.; Weinman, E.J.; Shenolikar, S. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 2001, 20, 6309–6314. [Google Scholar]
- Schindelmann, S.; Windisch, J.; Grundmann, R.; Kreienberg, R.; Zeillinger, R.; Deissler, H. Expression profiling of mammary carcinoma cell lines: Correlation of in vitro invasiveness with expression of CD24. Tumour Biol 2002, 23, 139–145. [Google Scholar]
- Yu, N.; Yang, J.; Yin, T.L. Extracts from a traditional Chinese herbal remedy (Zhuyun recipe) improve endometrial receptivity in mice with embryonic implantation dysfunction and ovulation stimulation. J. Ethnopharmacol 2011, 137, 389–395. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.; Xu, W.-M.; Yin, T.-L.; Zhao, Q.-H.; Peng, L.-Y.; Yang, J. Temporal and Spatial Regulation of Ezrin-Radixin-Moesin-Binding Phosphoprotein-50-kDa (EBP50) during Embryo Implantation in Mouse Uterus. Int. J. Mol. Sci. 2012, 13, 16418-16429. https://doi.org/10.3390/ijms131216418
Li X, Xu W-M, Yin T-L, Zhao Q-H, Peng L-Y, Yang J. Temporal and Spatial Regulation of Ezrin-Radixin-Moesin-Binding Phosphoprotein-50-kDa (EBP50) during Embryo Implantation in Mouse Uterus. International Journal of Molecular Sciences. 2012; 13(12):16418-16429. https://doi.org/10.3390/ijms131216418
Chicago/Turabian StyleLi, Xing, Wang-Ming Xu, Tai-Lang Yin, Qing-Hong Zhao, Liang-Yu Peng, and Jing Yang. 2012. "Temporal and Spatial Regulation of Ezrin-Radixin-Moesin-Binding Phosphoprotein-50-kDa (EBP50) during Embryo Implantation in Mouse Uterus" International Journal of Molecular Sciences 13, no. 12: 16418-16429. https://doi.org/10.3390/ijms131216418



